Maternal High-Fat Diet Modulates Cnr1 Gene Expression in Male Rat Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. The Forced Swimming Test
2.3. Brain Structure Isolation
2.4. Molecular Analyses
2.4.1. RNA/DNA Isolation
2.4.2. RT-qPCR Analyses
2.4.3. Analyses of miRNA Expression
2.4.4. Methylation of CpG Islands at the Cnr1 Promoter
2.5. Statistical Analyses
3. Results
3.1. Behavioral Analyses
The Forced Swimming Test
3.2. Molecular Analyses
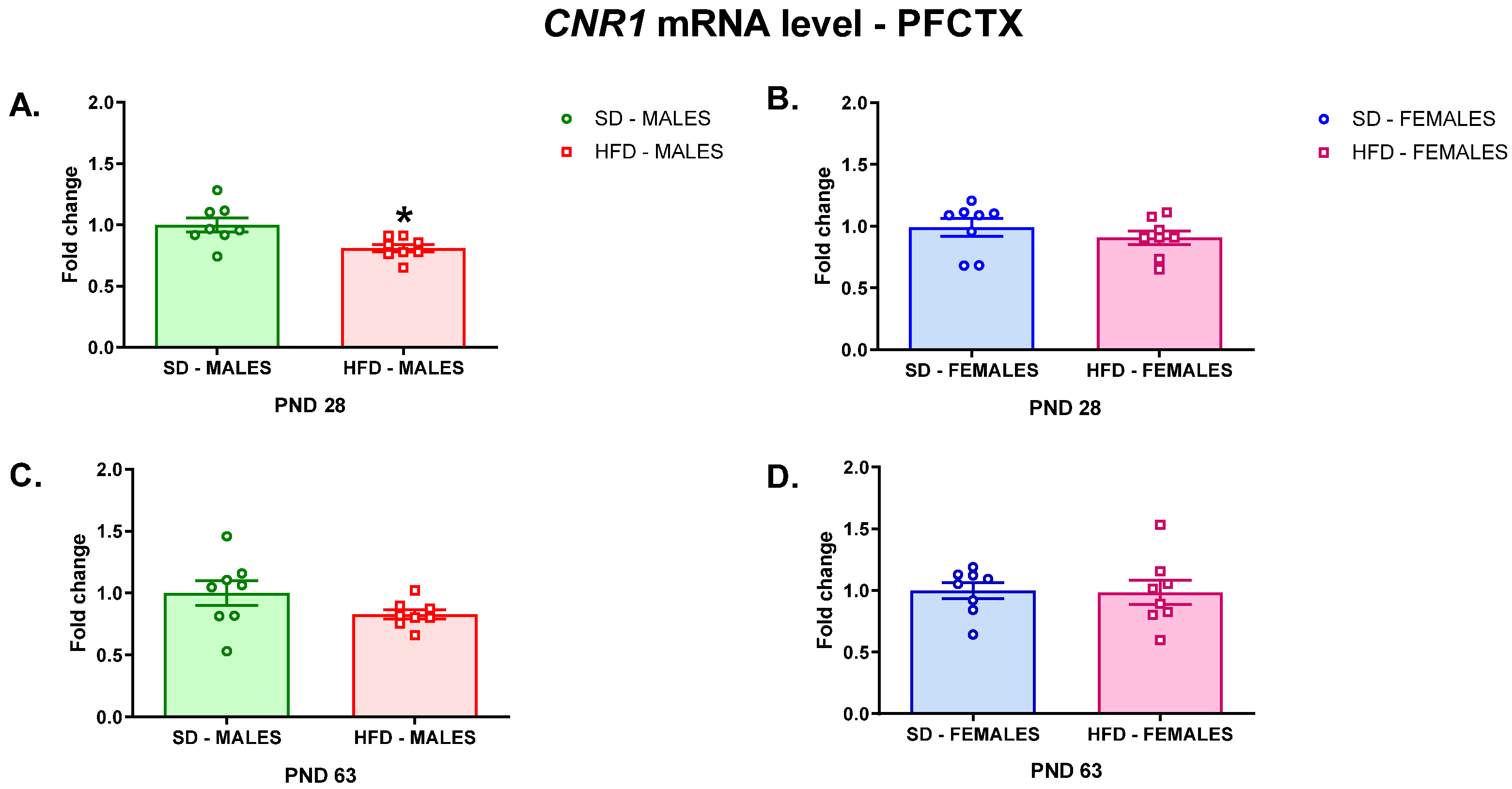
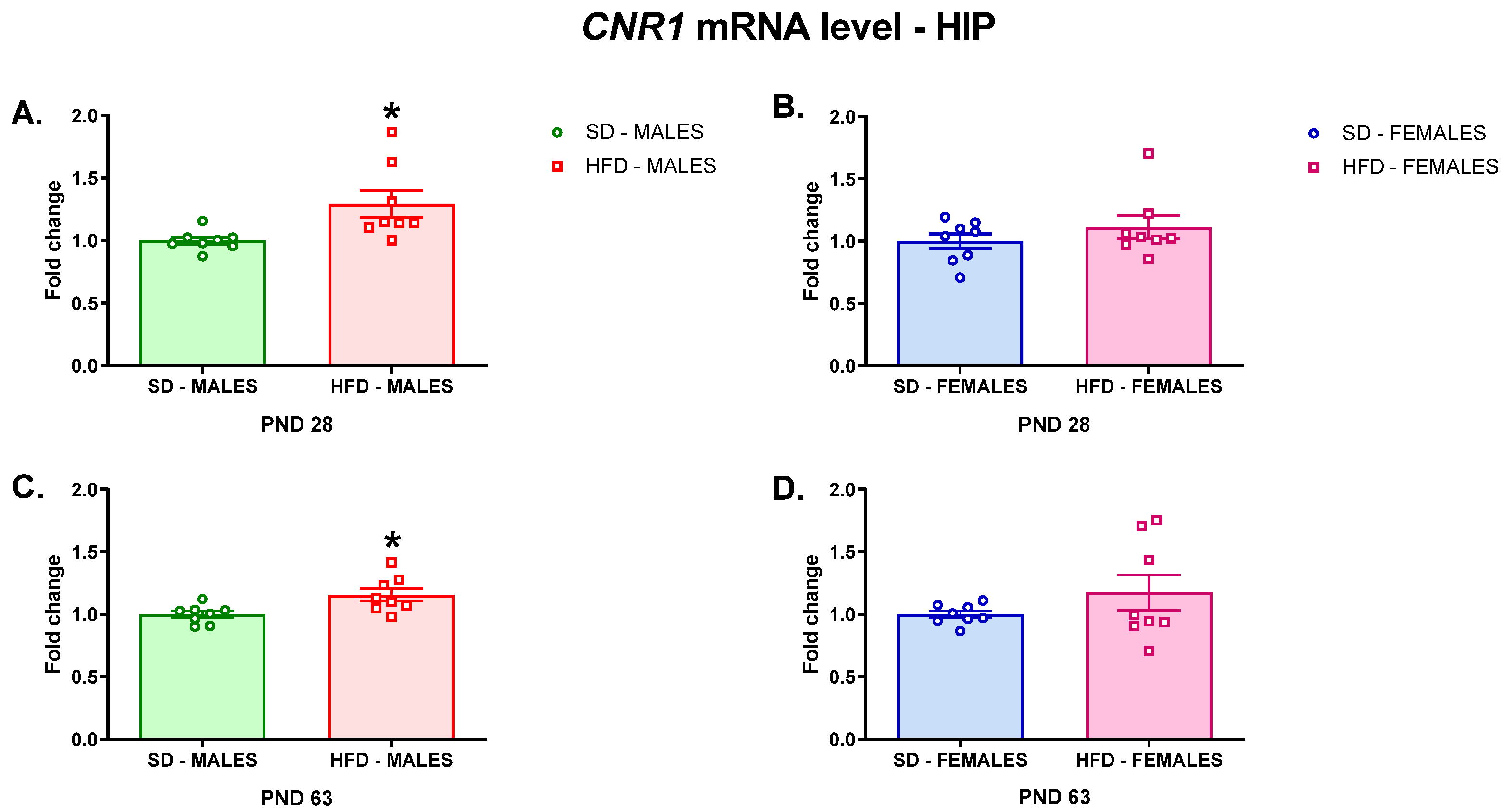
3.2.1. RT-qPCR Analyses
3.2.2. miRNA Analyses
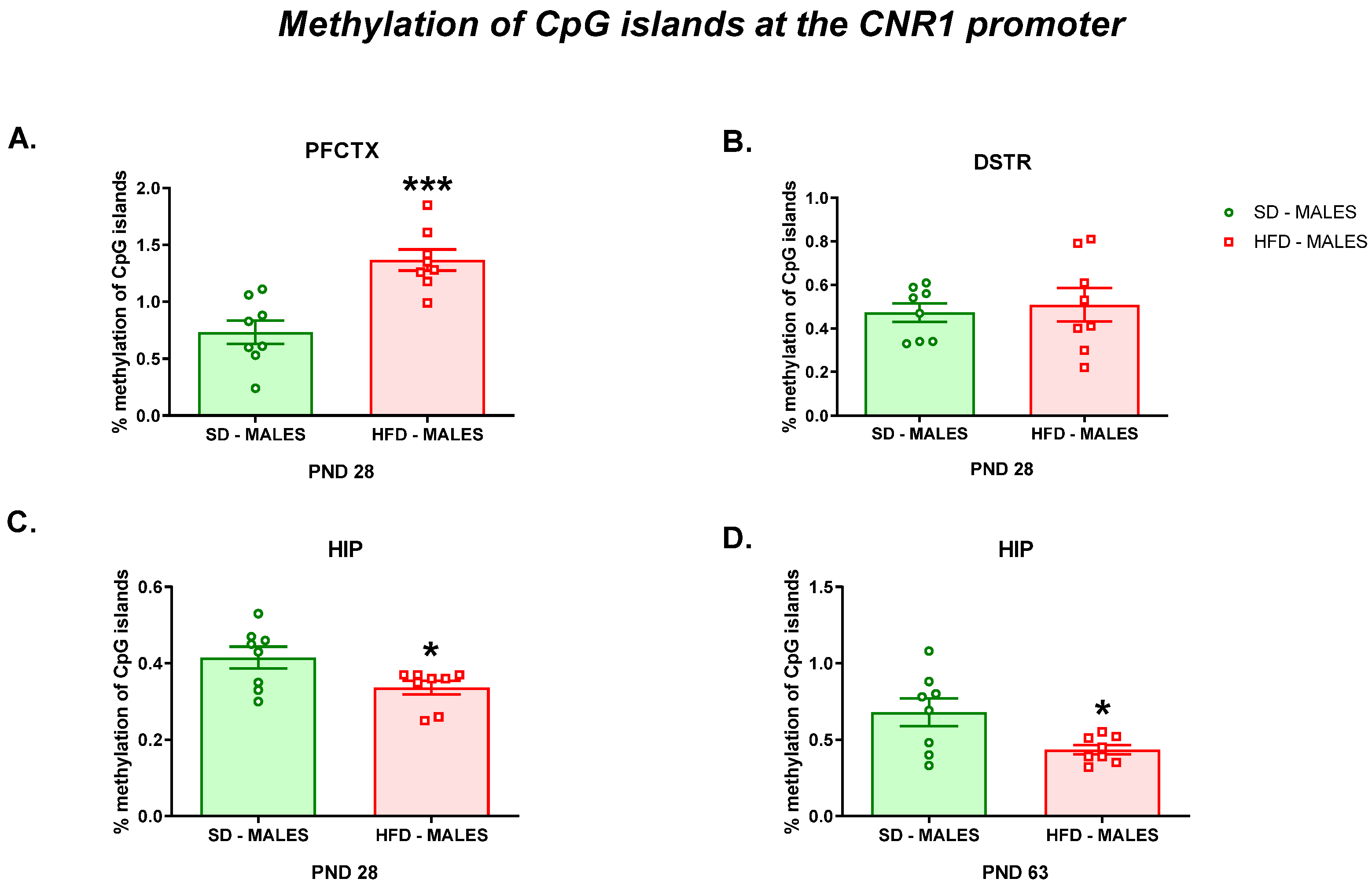
3.2.3. Methylation of CpG Islands at the Cnr1 Promoter
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, M.; Jiang, M.; Yang, C.; Wu, Y.; Liu, Y.; Cui, Y.; Huang, G. Maternal high-fat diet affects Msi/Notch/Hes signaling in neural stem cells of offspring mice. J. Nutr. Biochem. 2014, 25, 227–231. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Przegaliński, E.; Filip, M. Maternal high-fat diet during pregnancy and lactation provokes depressive-like behavior and influences the irisin/brain-derived neurotrophic factor axis and inflammatory factors in male and female offspring in rats. J. Physiol. Pharmacol. 2019, 70, 407–417. [Google Scholar]
- Shook, L.L.; Kislal, S.; Edlow, A.G. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 2020, 40, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, H.L.; Kesmodel, U.S.; Underbjerg, M.; Kilburn, T.R.; Bertrand, J.; Mortensen, E.L. Predictors of intelligence at the age of 5: Family, pregnancy and birth characteristics, postnatal influences, and postnatal growth. PLoS ONE 2013, 8, e79200. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Minabe, Y.; Takagai, S.; Ogai, M.; Matsumoto, H.; Mori, N.; Takei, N. Poor maternal care and high maternal body mass index in pregnancy as a risk factor for schizophrenia in offspring. Acta Psychiatr. Scand. 2004, 110, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.; Zubrick, S.R.; Pennell, C.E.; Van Lieshout, R.J.; Jacoby, P.; Beilin, L.J.; Mori, T.A.; Stanley, F.J.; Newnham, J.P.; Oddy, W.H. Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. J. Dev. Orig. Health Dis. 2013, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, R.J.; Robinson, M.; Boyle, M.H. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can. J. Psychiatry 2013, 58, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Räikkönen, K.; Minnis, H.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol. Med. 2017, 47, 353–362. [Google Scholar] [CrossRef]
- Dunn, G.A.; Bale, T.L. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 2011, 152, 2228–2236. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.M.; Walker, D.M.; Nestler, E.J. Paternal transgenerational epigenetic mechanisms mediating stress phenotypes of offspring. Eur. J. Neurosci. 2021, 53, 271–280. [Google Scholar] [CrossRef]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef]
- Fusco, S.; Spinelli, M.; Cocco, S.; Ripoli, C.; Mastrodonato, A.; Natale, F.; Rinaudo, M.; Livrizzi, G.; Grassi, C. Maternal insulin resistance multigenerationally impairs synaptic plasticity and memory via gametic mechanisms. Nat. Commun. 2019, 10, 4799. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.N.; Gorzalka, B.B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur. Neuropsychopharmacol. 2005, 15, 593–599. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Smaga, I.; Jastrzebska, J.; Zaniewska, M.; Bystrowska, B.; Gawlinski, D.; Faron-Gorecka, A.; Broniowska, Ż.; Miszkiel, J.; Filip, M. Changes in the Brain Endocannabinoid System in Rat Models of Depression. Neurotox. Res. 2017, 31, 421–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolato, M.; Mangieri, R.A.; Fu, J.; Kim, J.H.; Arguello, O.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; Piomelli, D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry 2007, 62, 1103–1110. [Google Scholar]
- Reich, C.G.; Taylor, M.E.; McCarthy, M.M. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 2009, 203, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N.; Carrier, E.J.; McLaughlin, R.J.; Morrish, A.C.; Meier, S.E.; Hillard, C.J.; Gorzalka, B.B. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J. Neurochem. 2008, 106, 2322–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N.; Patel, S.; Carrier, E.J.; Rademacher, D.J.; Ormerod, B.K.; Hillard, C.J.; Gorzalka, B.B. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 2005, 30, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Mikics, E.; Vas, J.; Aliczki, M.; Halasz, J.; Haller, J. Interactions between the anxiogenic effects of CB1 gene disruption and 5-HT3 neurotransmission. Behav. Pharmacol. 2009, 20, 265–272. [Google Scholar] [CrossRef]
- Sanchis-Segura, C.; Cline, B.H.; Marsicano, G.; Lutz, B.; Spanagel, R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology 2004, 176, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Aso, E.; Ozaita, A.; Valdizán, E.M.; Ledent, C.; Pazos, A.; Maldonado, R.; Valverde, O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J. Neurochem. 2008, 105, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, M.A.; Marsicano, G.; Nestler, E.J.; Holsboer, F.; Lutz, B.; Wotjak, C.T. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology 2008, 33, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Valverde, O.; Torrens, M. CB1 receptor-deficient mice as a model for depression. Neuroscience 2012, 204, 193–206. [Google Scholar] [CrossRef]
- Smaga, I.; Bystrowska, B.; Gawlinski, D.; Przegalinski, E.; Filip, M. The endocannabinoid/endovanilloid system and depression. Curr. Neuropharmacol. 2014, 12, 462–474. [Google Scholar] [CrossRef] [Green Version]
- Smaga, I.; Bystrowska, B.; Gawliński, D.; Pomierny, B.; Stankowicz, P.; Filip, M. Antidepressants and changes in concentration of endocannabinoids and N-acylethanolamines in rat brain structures. Neurotox. Res. 2014, 26, 190–206. [Google Scholar] [CrossRef] [Green Version]
- Smaga, I.; Zaniewska, M.; Gawliński, D.; Faron-Górecka, A.; Szafrański, P.; Cegła, M.; Filip, M. Changes in the cannabinoids receptors in rats following treatment with antidepressants. Neurotoxicology 2017, 63, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Research 2020, 48, D127–D131. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci. Biobehav. Rev. 2008, 32, 1293–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, O.J.; Cools, R.; Carlisi, C.O.; Sahakian, B.J.; Drevets, W.C. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am. J. Psychiatry 2012, 169, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawliński, D.; Gawlińska, K.; Frankowska, M.; Filip, M. Maternal Diet Influences the Reinstatement of Cocaine-Seeking Behavior and the Expression of Melanocortin-4 Receptors in Female Offspring of Rats. Nutrients 2020, 12, 1462. [Google Scholar] [CrossRef] [PubMed]
- Smaga, I.; Pomierny, B.; Krzyżanowska, W.; Pomierny-Chamioło, L.; Miszkiel, J.; Niedzielska, E.; Ogórka, A.; Filip, M. N-acetylcysteine possesses antidepressant-like activity through reduction of oxidative stress: Behavioral and biochemical analyses in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Gawlińska, K.; Gawliński, D.; Korostyński, M.; Borczyk, M.; Frankowska, M.; Piechota, M.; Filip, M.; Przegaliński, E. Maternal dietary patterns are associated with susceptibility to a depressive-like phenotype in rat offspring. Dev. Cogn. Neurosci. 2021, 47, 100879. [Google Scholar] [CrossRef]
- Giriko, C.; Andreoli, C.A.; Mennitti, L.V.; Hosoume, L.F.; Souto Tdos, S.; Silva, A.V.; Mendes-da-Silva, C. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int. J. Dev. Neurosci. 2013, 31, 731–739. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Schmidt, P.J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019, 44, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Eid, R.S.; Gobinath, A.R.; Galea, L.A.M. Sex differences in depression: Insights from clinical and preclinical studies. Prog. Neurobiol. 2019, 176, 86–102. [Google Scholar] [CrossRef]
- Dalla, C.; Pitychoutis, P.M.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2010, 106, 226–233. [Google Scholar] [CrossRef]
- Rivera, P.; Tovar, R.; Ramírez-López, M.T.; Navarro, J.A.; Vargas, A.; Suárez, J.; Fonseca, F.R. Sex-Specific Anxiety and Prefrontal Cortex Glutamatergic Dysregulation Are Long-Term Consequences of Pre-and Postnatal Exposure to Hypercaloric Diet in a Rat Model. Nutrients 2020, 12, 1829. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.M.; Jiang, M.T.; Lin, P.; Yang, H.; Dang, Y.W.; Yu, Q.; Liao, D.Y.; Luo, D.Z.; Chen, G. Potential ceRNA networks involved in autophagy suppression of pancreatic cancer caused by chloroquine diphosphate: A study based on differentially-expressed circRNAs, lncRNAs, miRNAs and mRNAs. Int. J. Oncol. 2019, 54, 600–626. [Google Scholar] [PubMed]
- Ushijima, T.; Nakajima, T.; Maekita, T. DNA methylation as a marker for the past and future. J. Gastroenterol. 2006, 41, 401–407. [Google Scholar] [CrossRef]
- McKee, S.E.; Zhang, S.; Chen, L.; Rabinowitz, J.D.; Reyes, T.M. Perinatal high fat diet and early life methyl donor supplementation alter one carbon metabolism and DNA methylation in the brain. J. Neurochem. 2018, 145, 362–373. [Google Scholar] [CrossRef]
- D’Addario, C.; Micale, V.; Di Bartolomeo, M.; Stark, T.; Pucci, M.; Sulcova, A.; Palazzo, M.; Babinska, Z.; Cremaschi, L.; Drago, F.; et al. A preliminary study of endocannabinoid system regulation in psychosis: Distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr. Res. 2017, 188, 132–140. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, C.G.; Mihalik, G.R.; Iskander, A.N.; Seckler, J.C.; Weiss, M.S. Adolescent chronic mild stress alters hippocampal CB1 receptor-mediated excitatory neurotransmission and plasticity. Neuroscience 2013, 253, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, C.G.; Iskander, A.N.; Weiss, M.S. Cannabinoid modulation of chronic mild stress-induced selective enhancement of trace fear conditioning in adolescent rats. J. Psychopharmacol. 2013, 27, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Segev, A.; Rubin, A.S.; Abush, H.; Richter-Levin, G.; Akirav, I. Cannabinoid Receptor Activation Prevents the Effects of Chronic Mild Stress on Emotional Learning and LTP in a Rat Model of Depression. Neuropsychopharmacology 2013, 39, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, S.A.; Clapper, J.R.; Holmes, P.V.; Piomelli, D.; Hohmann, A.G. A role for 2-arachidonoylglycerol and endocannabinoid signaling in the locomotor response to novelty induced by olfactory bulbectomy. Pharmacol. Res. 2010, 61, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.P.; Wu, X.; Meng, J.H.; Yao, J.; Wang, B.J. Functional Analysis of the 3’ Untranslated Region of the Human GRIN1 Gene in Regulating Gene Expression in vitro. Neuropsychiatr. Dis. Treat. 2020, 16, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Han, X.; Li, X.; Song, Q.; Lu, M.; Jia, M.; Ding, J.; Hu, G. MicroRNA-212-5p Prevents Dopaminergic Neuron Death by Inhibiting SIRT2 in MPTP-Induced Mouse Model of Parkinson’s Disease. Front. Mol. Neurosci. 2018, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.V. Olfactory bulbectomy increases prepro-enkephalin mRNA levels in the ventral striatum in rats. Neuropeptides 1999, 33, 206–211. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 2005, 25, 10537–10545. [Google Scholar] [CrossRef] [PubMed]
- Masini, C.V.; Holmes, P.V.; Freeman, K.G.; Maki, A.C.; Edwards, G.L. Dopamine overflow is increased in olfactory bulbectomized rats: An in vivo microdialysis study. Physiol. Behav. 2004, 81, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rademacher, D.J.; Hillard, C.J. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J. Pharmacol. Exp. Ther. 2003, 306, 880–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, J.; Boureau, P.; Moyon, T.; Nicklaus, S.; Parnet, P.; Paillé, V. Perinatal Western Diet Consumption Leads to Profound Plasticity and GABAergic Phenotype Changes within Hypothalamus and Reward Pathway from Birth to Sexual Maturity in Rat. Front Endocrinol. (Lausanne) 2017, 8, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias-Rocha, C.P.; Almeida, M.M.; Santana, E.M.; Costa, J.C.B.; Franco, J.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet induces sex-specific endocannabinoid system changes in newborn rats and programs adiposity, energy expenditure and food preference in adulthood. J. Nutr. Biochem. 2018, 51, 56–68. [Google Scholar] [CrossRef]
- Almeida, M.M.; Dias-Rocha, C.P.; Reis-Gomes, C.F.; Wang, H.; Atella, G.C.; Cordeiro, A.; Pazos-Moura, C.C.; Joss-Moore, L.; Trevenzoli, I.H. Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats. Psychoneuroendocrinology 2019, 103, 306–315. [Google Scholar] [CrossRef]
- Vinod, K.Y.; Xie, S.; Psychoyos, D.; Hungund, B.L.; Cooper, T.B.; Tejani-Butt, S.M. Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS ONE 2012, 7, e36743. [Google Scholar]
- D’Asti, E.; Long, H.; Tremblay-Mercier, J.; Grajzer, M.; Cunnane, S.C.; Di Marzo, V.; Walker, C.D. Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology 2010, 151, 1685–1694. [Google Scholar] [CrossRef] [Green Version]
- DiNicolantonio, J.J.; O’Keefe, J.H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Wanet, A.; Tacheny, A.; Arnould, T.; Renard, P. miR-212/132 expression and functions: Within and beyond the neuronal compartment. Nucleic Acids Res. 2012, 40, 4742–4753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadakierska-Chudy, A.; Frankowska, M.; Miszkiel, J.; Wydra, K.; Jastrzebska, J.; Filip, M. Prolonged Induction of miR-212/132 and REST Expression in Rat Striatum Following Cocaine Self-Administration. Mol. Neurobiol. 2017, 54, 2241–2254. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Jiang, Y.; Liang, W.; Wang, Y.; Cao, S.; Yan, H.; Gao, L.; Zhang, L. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol. Brain 2019, 12, 78. [Google Scholar] [CrossRef] [PubMed]


| Group | Sex | Immobility Time (s) | Statistical Analyses | |
|---|---|---|---|---|
| SD | HFD | |||
| adolescent | male | 173.5 ± 11.47 | 209.8 ± 2.88 ** | t = 3.073; df = 14; p = 0.0083 |
| female | 151.9 ± 11.15 | 197.0 ± 9.86 ** | t = 3.033; df = 14; p = 0.0089 | |
| “young” adult | male | 220.6 ± 5.22 | 246.5 ± 5.44 ** | t = 3.440; df = 14; p = 0.004 |
| female | 226.7 ± 9.23 | 260.5 ± 4.63 ** | t = 3.277; df = 14; p = 0.0055 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawliński, D.; Gawlińska, K.; Smaga, I. Maternal High-Fat Diet Modulates Cnr1 Gene Expression in Male Rat Offspring. Nutrients 2021, 13, 2885. https://doi.org/10.3390/nu13082885
Gawliński D, Gawlińska K, Smaga I. Maternal High-Fat Diet Modulates Cnr1 Gene Expression in Male Rat Offspring. Nutrients. 2021; 13(8):2885. https://doi.org/10.3390/nu13082885
Chicago/Turabian StyleGawliński, Dawid, Kinga Gawlińska, and Irena Smaga. 2021. "Maternal High-Fat Diet Modulates Cnr1 Gene Expression in Male Rat Offspring" Nutrients 13, no. 8: 2885. https://doi.org/10.3390/nu13082885
APA StyleGawliński, D., Gawlińska, K., & Smaga, I. (2021). Maternal High-Fat Diet Modulates Cnr1 Gene Expression in Male Rat Offspring. Nutrients, 13(8), 2885. https://doi.org/10.3390/nu13082885







