Plant Adaptogens—History and Future Perspectives
Abstract
:1. Introduction
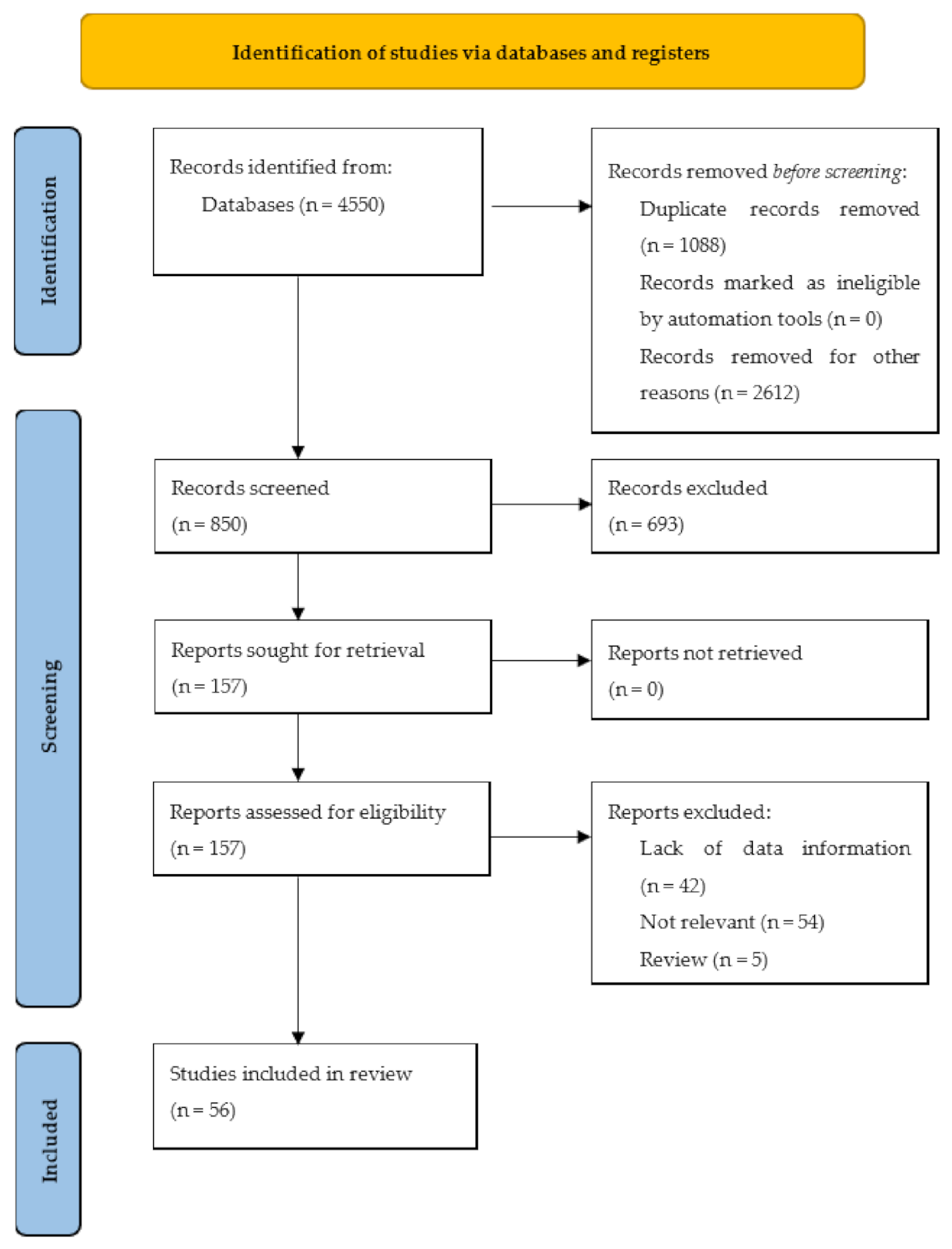
2. Materials and Methods
3. Results and Discussion
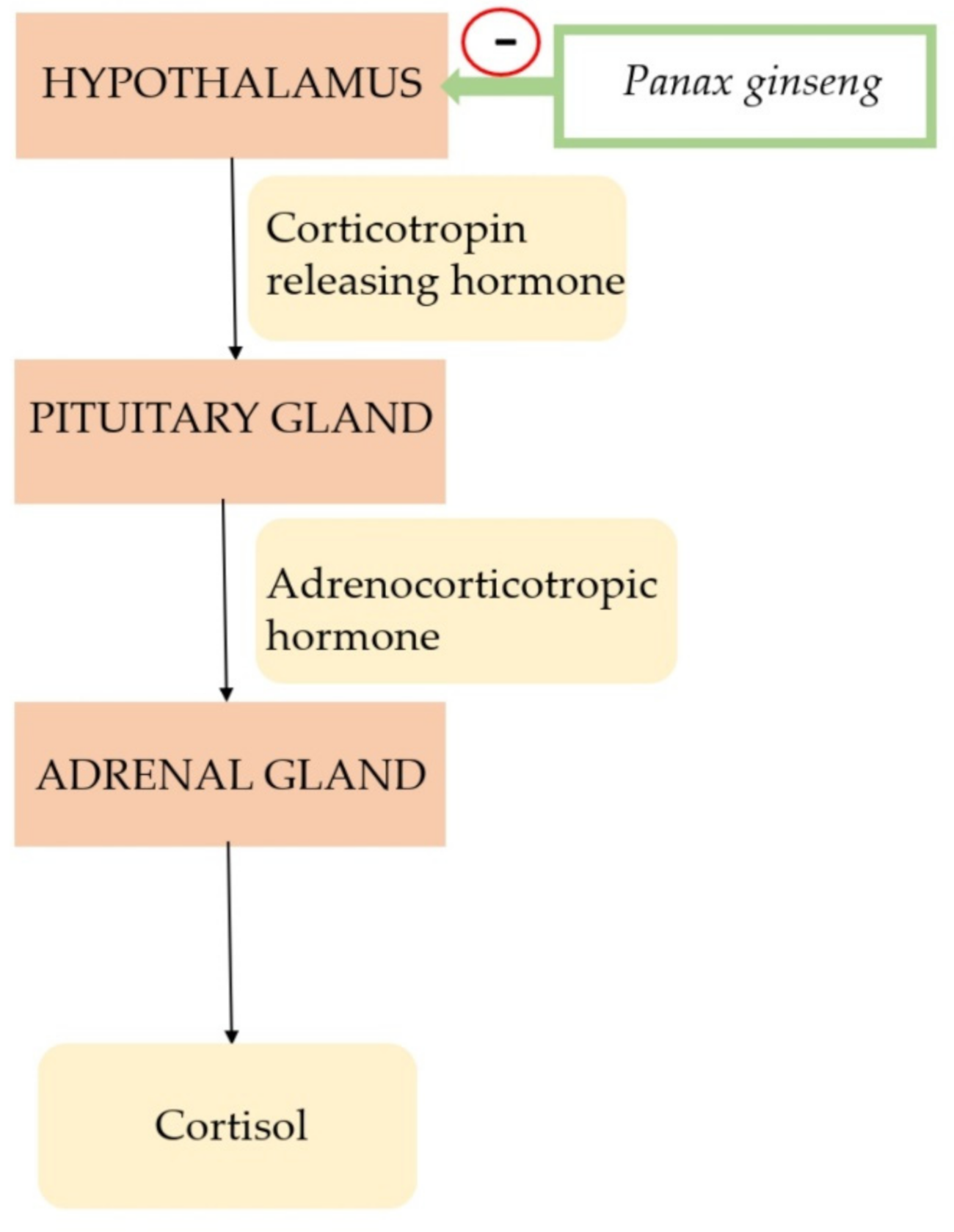
3.1. Panax Ginseng
3.2. Eleutherococcus Senticosus
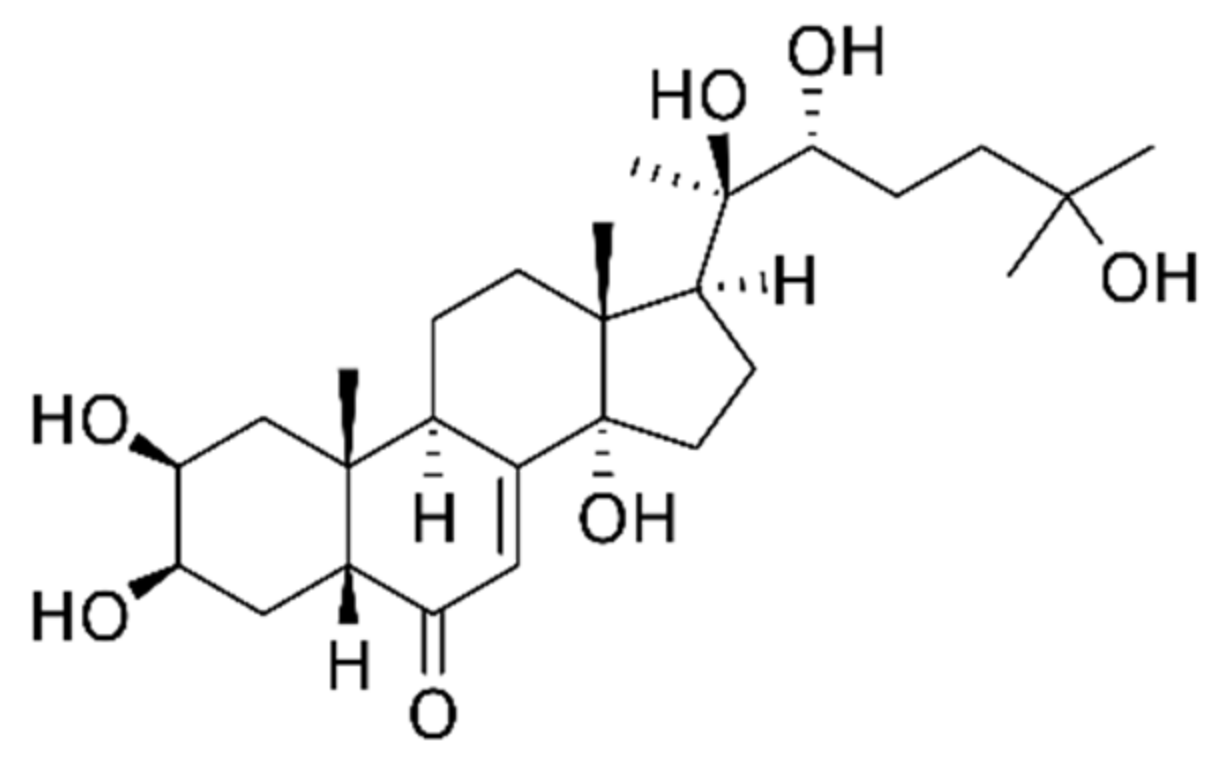
3.3. Rhaponticum Carthmoides
3.4. Rhodiola rosea
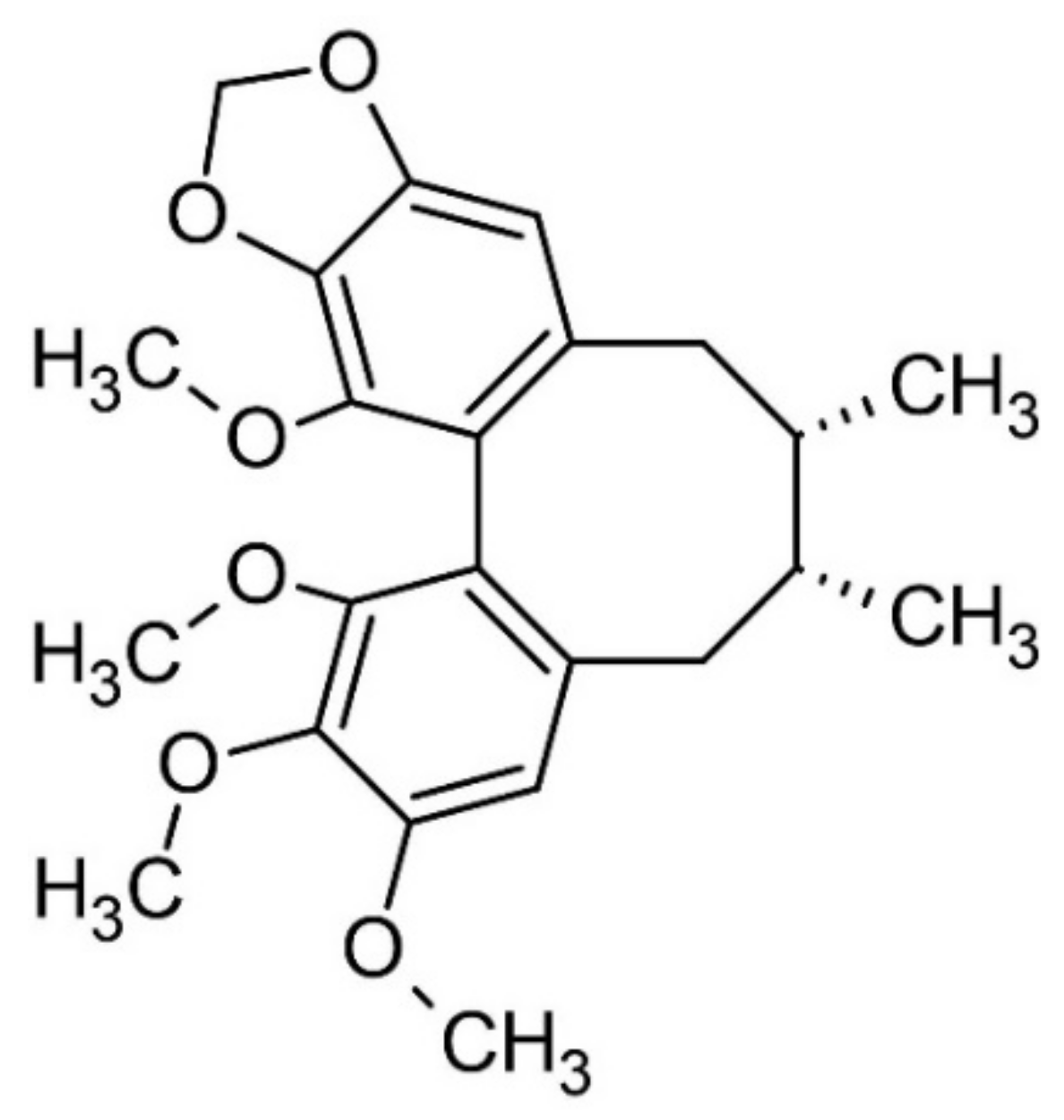
3.5. Schisandra chinensis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wagner, H.; Nörr, H.; Winterhoff, H. Plant adaptogens. Phytomedicine 1994, 1, 63–76. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Wagner, H. Plant adaptogens III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef]
- Oliynyk, S.; Oh, S.-K. The pharmacology of Actoprotectors: Practical application for improvement of mental and physical performance. Biomol. Ther. 2012, 20, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress and aging related diseases. Med. Res. Rev. 2020, 41, 630–703. [Google Scholar] [CrossRef]
- The World Anti-Doping Agency—WADA. Executive Committee Approved the List of Prohibited Substances and Methods for 2009. Available online: https://www.wada-ama.org/en/media/news/2008-09/wada-executive-committee-approves-2009-prohibited-list-new-delhi-laboratory-0 (accessed on 1 May 2021).
- The World Anti-Doping Agency—WADA. Prohibited List. 2018. Available online: https://www.wada-ama.org/sites/default/files/prohibited_list_2018_en.pdf (accessed on 1 May 2021).
- Brekhman, A.I.; Dardymov, I.V. New substances of plant origin which increase nonspecific resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef]
- Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar]
- Kamal, M.; Arif, M.; Jawaid, T. Adaptogenic medicinal plants utilized for strengthening the power of resistance during chemotherapy—A review. Orient. Pharm. Exp. Med. 2017, 17, 1–18. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine 2009, 16, 617–622. [Google Scholar] [CrossRef]
- Pawar, V.S.; Shivakumar, H. A current status of adaptogens: Natural remedy to stress. Asian Pac. J. Trop. Dis. 2012, 2, S480–S490. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef]
- Hikino, H.; Takahashi, M.; Otake, K.; Konno, C. Isolation and Hypoglycemic Activity of Eleutherans A, B, C, D, E, F, and G: Glycans of Eleutherococcus senticosus Roots. J. Nat. Prod. 1986, 49, 293–297. [Google Scholar] [CrossRef]
- Kokoska, L.; Janovska, D. Chemistry and pharmacology of Rhaponticum carthamoides: A review. Phytochemistry 2009, 70, 842–855. [Google Scholar] [CrossRef]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef]
- Mendes, F.R.; Carlini, E. Brazilian plants as possible adaptogens: An ethnopharmacological survey of books edited in Brazil. J. Ethnopharmacol. 2007, 109, 493–500. [Google Scholar] [CrossRef]
- Ajala, T.O. The effects of adaptogens on the physical and psychological symptoms of chronic stress. DISCOV. Ga. State Honor. Coll. Undergrad. Res. J. 2017, 4, 2. [Google Scholar] [CrossRef]
- Domene, A.M. Effects of adaptogen supplementation on sport performance. A recent review of published studies. J. Hum. Sport Exerc. 2013, 8, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Krasutsky, A.G.; Cheremisinov, V.N. The use of Levzey’s extract to increase the efficiency of the training process in fitness clubs students. In Proceedings of the Actual Problems of Biochemistry and Bioenergy of Sport of the XXI Century, Moscow, Russia, 10–26 April 2017; pp. 382–388. [Google Scholar]
- Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Nylander, M.; Wikman, G.; Panossian, A. Double-blind, placebo-controlled, randomised study of single dose effects of ADAPT-232 on cognitive functions. Phytomedicine 2010, 17, 494–499. [Google Scholar] [CrossRef]
- Reay, J.L.; Scholey, A.; Kennedy, D. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 462–471. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Baeg, I.-H.; So, S.-H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, D.S.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Patel, S.; Rauf, A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed. Pharmacother. 2016, 85, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shergis, J.; Zhang, A.L.; Zhou, W.; Xue, C.C. Panax ginseng in randomised controlled trials: A systematic review. Phytother. Res. 2012, 27, 949–965. [Google Scholar] [CrossRef]
- Nocerino, E.; Amato, M.; Izzo, A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71, S1–S5. [Google Scholar] [CrossRef]
- Mahady, G.B.; Gyllenhaal, C.; Fong, H.H.; Farnsworth, N.R. Ginsengs: A review of safety and efficacy. Nutr. Clin. Care 2000, 3, 90–101. [Google Scholar] [CrossRef]
- Wilson, L. Review of adaptogenic mechanisms: Eleuthrococcus senticosus, panax ginseng, rhodiola rosea, schisandra chinensis and withania somnifera. Aust. J. Med. Herbal. 2007, 19, 126–138. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides: Chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2008, 55, 1–99. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. Ginseng radix. In European Pharmacopoeia, Monograph 07/2019:1523; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2019. [Google Scholar]
- Kim, S.H.; Park, K.S. Effects of panax ginseng extract on lipid metabolism in humans. Pharmacol. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Bhattacharjee, I.; Bandyopadhyay, A. Effects of acute supplementation of panax ginseng on endurance performance in healthy adult males of Kolkata, India. Int. J. Clin. Exp. Physiol. 2020, 7, 63–68. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sayahi, F.; Abtahi, S.-H.; Shemshaki, H.; Dorooshi, G.-A.; Goodarzi, M.; Akbari, M.; Fereidan-Esfahani, M. Ginseng in the treatment of fatigue in multiple sclerosis: A randomized, placebo-controlled, double-blind pilot study. Int. J. Neurosci. 2013, 123, 480–486. [Google Scholar] [CrossRef]
- Engels, H.-J.; Said, J.M.; Wirth, J.C. Failure of chronic ginseng supplementation to affect work performance and energy metabolism in healthy adult females. Nutr. Res. 1996, 16, 1295–1305. [Google Scholar] [CrossRef]
- Perazzo, F.F.; Fonseca, F.L.; Souza, G.H.B.; Maistro, E.L.; Rodrigues, M.; Carvalho, J.C. Double-blind clinical study of a multivitamin and polymineral complex associated with panax ginseng extract (Gerovital®). Open Complement. Med. J. 2010, 2, 100–104. [Google Scholar]
- Ziemba, A.W. The effect of ginseng supplementation on psychomotor performance, indices of physical capacity and plasma concentration of some hormones in young well fit men. In Proceedings of the Ginseng Society Conference, Seoul, Korea, 1 October 2002; pp. 145–158. [Google Scholar]
- Zarabi, L.; Arazi, H.; Izadi, M. The effects of panax ginseng supplementation on growth hormone, cortisol and lactate response to high-intensity resistance exercise. Biomed. Hum. Kinet. 2018, 10, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Kang, S.G.; Lee, H.J.; Jung, K.Y.; Kim, L. Effect of Korean red ginseng on sleep: A randomized, placebo-controlled Trial. Sleep Med. Psychophysiol. 2010, 17, 85–90. [Google Scholar]
- Kim, H.-G.; Cho, J.-H.; Yoo, S.-R.; Lee, J.-S.; Han, J.-M.; Lee, N.-H.; Ahn, Y.-C.; Son, C.-G. Antifatigue Effects of Panax ginseng CA Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e61271. [Google Scholar] [CrossRef] [Green Version]
- Ping, F.W.C.; Keong, C.C.; Bandyopadhyay, A. Effects of acute supplementation of Panax ginseng on endurance running in a hot & humid environment. Indian J. Med. Res. 2011, 133, 96–102. [Google Scholar]
- Davydov, M.; Krikorian, A. Eleutherococcus senticosus (Rupr. & Maxim.) maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmacol. 2000, 72, 345–393. [Google Scholar] [CrossRef]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- Bleakney, T.L. Deconstructing an adaptogen: Eleutherococcus Senticosus. Holist. Nurs. Pract. 2008, 22, 220–224. [Google Scholar] [CrossRef]
- Jia, A.; Zhang, Y.; Gao, H.; Zhang, Z.; Zhang, Y.; Wang, Z.; Zhang, J.; Deng, B.; Qiu, Z.; Fu, C. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2020, 268, 113586. [Google Scholar] [CrossRef]
- Asano, K.; Takahashi, T.; Miyashita, M.; Matsuzaka, A.; Muramatsu, S.; Kuboyama, M.; Kugo, H.; Imai, J. Effect of eleutheroccocus senticosus extract on human physical working capacity. Planta Med. 1986, 52, 175–177. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. Eleutherococci radix. In European Pharmacopoeia, Monograph 01/2008:1419; Corrected 7.0; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2016. [Google Scholar]
- Dowling, E.A.; Redondo, D.R.; Branch, J.D.; Jones, S.; McNabb, G.; Williams, M.H. Effect of Eleutherococcus senticosus on submaximal and maximal exercise performance. Med. Sci. Sports Exerc. 1996, 28, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.; Chen, K.W.; Cheng, I.S.; Tsai, P.H.; Lu, Y.J.; Lee, N.Y. The effect of eight weeks of supplementation with Eleutherococcus senticosus on endurance capacity and metabolism in human. Chin. J. Physiol. 2010, 53, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.; DeRosa, G.; Brillante, R.; Bernardi, R.; Nascetti, S.; Gaddi, A. effects of siberian ginseng (eleutherococcus senticosus maxim.) on elderly quality of life: A randomized clinical trial. Arch. Gerontol. Geriatr. 2004, 38, 69–73. [Google Scholar] [CrossRef]
- Szołomicki, S.; Samochowiec, L.; Wójcicki, J.; Droździk, M. The influence of active components of eleutherococcus senticosus on cellular defence and physical fitness in man. Phytother. Res. 2000, 14, 30–35. [Google Scholar] [CrossRef]
- Schaffler, K.; Wolf, O.; Burkart, M. No Benefit Adding Eleutherococcus senticosus to Stress Management Training in Stress-Related Fatigue/Weakness, Impaired Work or Concentration, A Randomized Controlled Study. Pharmacopsychiatry 2013, 46, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, L.C.; Webster, M.J.; Boyd, J.C.; McArthur, P.D.; Evetovich, T.K. The Effect of Siberian Ginseng (Eleutherococcus Senticosus) on Substrate Utilization and Performance during Prolonged Cycling. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 444–451. [Google Scholar] [CrossRef]
- Krasutsky, A.G.; Cheremisinov, V.N. Research of the influence of adaptogens on increasing the efficacy of the training process in fitness clubs. In Proceedings of the Current Problems of Biochemistry and Bioenergy Sport of the XXI Centyry, Moscow, Russia, 10–12 April 2018; pp. 267–282. [Google Scholar]
- Jacquet, A.; Grolleau, A.; Jove, J.; Lassalle, R.; Moore, N. Burnout: Evaluation of the efficacy and tolerability of TARGET 1® for professional fatigue syndrome (burnout). J. Int. Med. Res. 2015, 43, 54–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buděšínský, M.; Vokáč, K.; Harmatha, J.; Cvačka, J. Additional minor ecdysteroid components of Leuzea carthamoides. Steroids 2008, 73, 502–514. [Google Scholar] [CrossRef]
- Timofeev, N.P. Leuzea Carthamoides DC: Application prospects as pharmpreparations and biologically active components. In Functional Foods for Chronic Diseases; Martirosyan, D.M., Ed.; Richardson: Texas, TX, USA, 2006; pp. 105–120. [Google Scholar]
- Bathori, M.; Toth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and anabolic-androgenic steroids—Structure and effects on humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as non-conventional anabolic agent: Performance enhancement by ecdysterone supplementation in humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef]
- The World Anti-Doping Agency—WADA. The 2020 Monitoring Program. Available online: https://www.wada-ama.org/sites/default/files/resources/files/wada_2020_english_monitoring_program_pdf (accessed on 6 May 2021).
- Vanyuk, A.I. Evaluation of the effectivnness of rehabilitation measures among female volleyball players 18-22 years old in the competitive period of the annual training cycle. Slobozhanskiy Sci. Sports Visnik. 2012, 5, 95–98. [Google Scholar]
- Timofeev, N.P.; Koksharov, A.V. Study of Leuzea from leaves: Results of 15 years of trials in athletics. New Unconv. Plants Prospect. Use 2016, 12, 502–505. [Google Scholar]
- Wilborn, C.D.; Taylor, L.W.; Campbell, B.I.; Kerksick, C.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Effects of Methoxyisoflavone, ecdysterone, and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males. J. Int. Soc. Sports Nutr. 2006, 3, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.D.; Gerstner, G.R.; Mota, J.A.; Trexler, E.T.; Giuliani, H.K.; Blue, M.N.M.; Hirsch, K.R.; Smith-Ryan, A.E. The acute effects of a multi-ingredient herbal supplement on performance fatigability: A double-blind, randomized, and placebo-controlled trial. J. Diet. Suppl. 2020, 1–10. [Google Scholar] [CrossRef]
- Selepcova, L.; Sommer, A.; Vargova, M. Effect of feeding on a diet containing varying amounts of rhaponticum car-thamoides hay meal on selected morphological parameters in rats. Eur. J. Entornol. 2013, 92, 391–397. [Google Scholar]
- Plotnikov, M.B.; Aliev, O.I.; Vasil’Ev, A.S.; Andreeva, V.Y.; Krasnov, E.A.; Kalinkina, G.I. Effect of Rhaponticum carthamoides extract on structural and metabolic parameters of erythrocytes in rats with cerebral ischemia. Bull. Exp. Biol. Med. 2008, 146, 45–48. [Google Scholar] [CrossRef]
- Wu, J.; Gao, L.; Shang, L.; Wang, G.; Wei, N.; Chu, T.; Chen, S.; Zhang, Y.; Huang, J.; Wang, J.; et al. Ecdysterones from Rhaponticum carthamoides (Willd.) Iljin reduce hippocampal excitotoxic cell loss and upregulate mTOR signaling in rats. Fitoter 2017, 119, 158–167. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic effects of 20-OH-Ecdysone in ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Koudela, K.; Tenora, J.; Bajer, J.; Mathova, A.; Slama, K. Stimulation of growth and development in Japanase quails after oral administration of ecdysteroid-containing diet. Eur. J. Entomol. 1995, 92, 349. [Google Scholar]
- Sláma, K.; Koudela, K.; Tenora, J.; Maťhová, A. Insect hormones in vertebrates: Anabolic effects of 20-hydroxyecdysone in Japanese quail. Experientia 1996, 52, 702–706. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Q.; Liu, R.; Wang, Z.; Tang, N.; Liu, F.; Huang, G.; Jiang, X.; Gui, G.; Wang, L.; et al. Effects of 20-hydroxyecdysone on improving memory deficits in streptozotocin-induced type 1 diabetes mellitus in rat. Eur. J. Pharmacol. 2014, 740, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Roumanille, R.; Vernus, B.; Brioche, T.; Descossy, V.; Van Ba, C.T.; Campredon, S.; Philippe, A.G.; Delobel, P.; Bertrand-Gaday, C.; Chopard, A.; et al. Acute and chronic effects of Rhaponticum carthamoides and Rhodiola rosea extracts supplementation coupled to resistance exercise on muscle protein synthesis and mechanical power in rats. J. Int. Soc. Sports Nutr. 2020, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea: A phytomedicinal overview. Herbal. Gram. 2002, 56, 40–52. [Google Scholar]
- Pu, W.-L.; Zhang, M.-Y.; Bai, R.-Y.; Sun, L.-K.; Li, W.-H.; Yu, Y.-L.; Zhang, Y.; Song, L.; Wang, Z.-X.; Peng, Y.-F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Khanum, F.; Bawa, A.S.; Singh, B. Rhodiola rosea: A versatile adaptogen. Compr. Rev. Food Sci. Food Saf. 2005, 4, 55–62. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.-J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Ballmann, C.G.; Maze, S.B.; Wells, A.C.; Marshall, M.R.; Rogers, R.R. Effects of short-term Rhodiola Rosea (golden root extract) supplementation on anaerobic exercise performance. J. Sports Sci. 2019, 37, 998–1003. [Google Scholar] [CrossRef]
- Jówko, E.; Sadowski, J.; Długołęcka, B.; Gierczuk, D.; Opaszowski, B.; Cieśliński, I. Effects of Rhodiola rosea supplementation on mental performance, physical capacity, and oxidative stress biomarkers in healthy men. J. Sport Health Sci. 2018, 7, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Abidov, M.; Grachev, S.; Seifulla, R.D.; Ziegenfuss, T.N. Extract of Rhodiola rosea radix reduces the level of c-reactive protein and creatinine kinase in the blood. Bull. Exp. Biol. Med. 2004, 138, 63–64. [Google Scholar] [CrossRef]
- Edwards, D.; Heufelder, A.; Zimmermann, A. Therapeutic effects and safety of Rhodiola rosea extract WS® 1375 in subjects with life-stress symptoms—Results of an open-label study. Phytother. Res. 2012, 26, 1220–1225. [Google Scholar] [CrossRef]
- Shevtsov, V.; Zholus, B.; Shervarly, V.; Vol’Skij, V.; Korovin, Y.; Khristich, M.; Roslyakova, N.; Wikman, G. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine 2003, 10, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2007, 61, 343–348. [Google Scholar] [CrossRef]
- Shanely, R.A.; Nieman, D.C.; Zwetsloot, K.A.; Knab, A.M.; Imagita, H.; Luo, B.; Davis, B.; Zubeldia, J.M. Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav. Immun. 2013, 39, 204–210. [Google Scholar] [CrossRef]
- Stejnborn, A.S.; Pilaczyńska-Szcześniak, S.; Basta, P.; Deskur-Śmielecka, E. The influence of supplementation with Rhodiola rosea L. Extract on selected redox parameters in professional rowers. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Parisi, A.; Tranchita, E.; Duranti, G.; Ciminelli, E.; Quaranta, F.; Ceci, R.; Sabatini, S. Effects of chronic Rhodiola Rosea sup-plementation on sport performance and antioxidant capacity in trained male: Preliminary results. J. Sports Med. Phys. Fit. 2010, 50, 57. [Google Scholar]
- Bystritsky, A.; Kerwin, L.; Feusner, J.D. A pilot study of Rhodiola rosea (Rhodax®) for generalized anxiety disorder (GAD). J. Altern. Complement. Med. 2008, 14, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Hancke, J.; Burgos, R.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, D.-F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Slanina, J.; Táborská, E.; Lojková, L. Lignans in the seeds and fruits of Schisandra chinensis cultured in Europe. Planta Med. 1997, 63, 277–280. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. Schisandrae chinensis fructus. In European Pharmacopoeia, Monograph 07/2016:2428; Corrected 9.1, Corrected 7.0; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2016. [Google Scholar]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current findings and future applications. Phytochem. Rev. 2019, 18, 109–128. [Google Scholar] [CrossRef] [Green Version]
- Kochetkov, N.; Khorlin, A.; Chizhov, O.; Sheichenko, V. Schizandrin—Lignan of unusual structure. Tetrahedron Lett. 1961, 2, 730–734. [Google Scholar] [CrossRef]
- Chen, D.-F.; Zhang, S.-X.; Kozuka, M.; Sun, Q.-Z.; Feng, J.; Wang, Q.; Mukainaka, T.; Nobukuni, Y.; Tokuda, H.; Nishino, H.; et al. Interiotherins C and D, two new lignans from Kadsurainteriorand antitumor-promoting effects of related neolignans on Epstein−Barr Virus Activation. J. Nat. Prod. 2002, 65, 1242–1245. [Google Scholar] [CrossRef]
- Yoo, H.H.; Lee, M.; Lee, M.W.; Lim, S.Y.; Shin, J.; Kim, D.-H. Effects of Schisandra lignans on P-Glycoprotein-mediated drug efflux in human intestinal Caco-2 Cells. Planta Med. 2007, 73, 444–450. [Google Scholar] [CrossRef]
- Fong, W.-F.; Wan, C.-K.; Zhu, G.-Y.; Chattopadhyay, A.; Dey, S.; Zhao, Z.; Shen, X.-L. Schisandrol A from Schisandra chinensis reverses P-Glycoprotein-mediated multidrug resistance by affecting Pgp-substrate complexes. Planta Med. 2007, 73, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Kilgore, N.; Lee, K.-H.; Chen, D.-F. Rubrisandrins A and B, lignans and related anti-HIV compounds from Schisandra rubriflora. J. Nat. Prod. 2006, 69, 1697–1701. [Google Scholar] [CrossRef]
- Chen, D.-F.; Zhang, S.-X.; Xie, L.; Xie, J.-X.; Chen, K.; Kashiwada, Y.; Zhou, B.-N.; Wang, P.; Cosentino, L.; Lee, K.-H. Anti-aids agents—XXVI. Structure-activity correlations of Gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorganic Med. Chem. 1997, 5, 1715–1723. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; Zhang, J.; Liang, Q.; Li, D. Chemical composition and antioxidant activity of essential oil from berries of Schisandra chinensis(Turcz.) Baill. Nat. Prod. Res. 2012, 26, 2199–2203. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zu, Y.; Yang, L. Chemical composition and antioxidant activity of the essential oil of Schisandra chinensisfruits. Nat. Prod. Res. 2012, 26, 842–849. [Google Scholar] [CrossRef]
- Xu, M.; Yan, T.; Gong, G.; Wu, B.; He, B.; Du, Y.; Xiao, F.; Jia, Y. Purification, structural characterization, and cognitive improvement activity of a polysaccharides from Schisandra chinensis. Int. J. Biol. Macromol. 2020, 163, 497–507. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.-T.; Wang, Z.-B.; Li, Z.-Y.; Zheng, G.-X.; Xia, Y.-G.; Yang, B.-Y.; Kuang, H.-X. Aromatic monoterpenoid glycosides from rattan stems of Schisandra chinensis and their neuroprotective activities. Fitoterapia 2019, 134, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crișan, G.; Vlase, L.; Crișan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.-M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.-Y.; Guo, J.-T.; Li, Z.-Y.; Wang, C.-F.; Wang, Z.-B.; Wang, Q.-H.; Kuang, H.-X. New Thymoquinol Glycosides and Neuroprotective Dibenzocyclooctane Lignans from the Rattan Stems ofSchisandra chinensis. Chem. Biodivers. 2016, 13, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, S.; Park, H. Effect of Schisandra chinensis extract supplementation on quadriceps muscle strength and fatigue in adult women: A randomized, double-blind, placebo-controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 2475. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Kim, K.H. A randomized, double-blind, placebo-controlled trial of Schisandra chinensis for menopausal symptoms. Climacteric 2016, 19, 574–580. [Google Scholar] [CrossRef]
- Song, M.-Y.; Wang, J.; Eom, T.; Kim, H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef]
- Cao, S.; Shang, H.; Wu, W.; Du, J.; Putheti, R. Evaluation of anti-athletic fatigue activity of Schizandra chinensis aqueous extracts in mice. Afr. J. Pharm. Pharmacol. 2009, 3, 593–597. [Google Scholar]
- Li, J.; Wang, J.; Shao, J.-Q.; Du, H.; Wang, Y.-T.; Peng, L. Effect of Schisandra chinensis on interleukins, glucose metabolism, and pituitary-adrenal and gonadal axis in rats under strenuous swimming exercise. Chin. J. Integr. Med. 2014, 21, 43–48. [Google Scholar] [CrossRef]
- Chen, X.; Cao, J.; Sun, Y.; Dai, Y.; Zhu, J.; Zhang, X.; Zhao, X.; Wang, L.; Zhao, T.; Li, Y.; et al. Ethanol extract of Schisandrae chinensis fructus ameliorates the extent of experimentally induced atherosclerosis in rats by increasing antioxidant capacity and improving endothelial dysfunction. Pharm. Biol. 2018, 56, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-H.; Liu, X.; Cong, L.-X.; Li, H.; Zhang, C.-Y.; Chen, J.-G.; Wang, C.-M. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans in diet-induced hyperlipidemia mice. Lipids Health Dis. 2017, 16, 227. [Google Scholar] [CrossRef] [Green Version]
- Ip, S.-P.; Poon, M.; Wu, S.; Che, C.; Ng, K.; Kong, Y.; Ko, K. Effect of Schisandrin B on hepatic glutathione antioxidant system in mice: Protection against carbon tetrachloride toxicity. Planta Med. 1995, 61, 398–401. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free. Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Ku, S.-K.; Lee, K.-W.; Song, C.-H.; An, W.G. Muscle-protective effects of Schisandrae fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018, 212, 175–187. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Wang, X.; Sun, F.; Liang, S. An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz.) Baill. Int. J. Biol. Macromol. 2011, 50, 844–848. [Google Scholar] [CrossRef]
- Zhao, T.; Mao, G.-H.; Zhang, M.; Li, F.; Zou, Y.; Zhou, Y.; Zheng, W.; Zheng, D.-H.; Yang, L.-Q.; Wu, X.-Y. Anti-diabetic effects of polysaccharides from ethanol-insoluble residue of Schisandra chinensis (Turcz.) baill on alloxan-induced diabetic mice. Chem. Res. Chin. Univ. 2012, 29, 99–102. [Google Scholar] [CrossRef]
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Evaluation of the effects on subjective mood and memory of a single and sub-chronic Panax ginseng dose. | Thirty adults, aged 22.87 ± 4.01 years, participated in the study. They received a placebo, 200 or 400 mg Panax ginseng extract per day for 3 treatments—8 days with 6 days washout. Period of the study—32 days. | Improved calmness, mood, and mental health. | [21] |
| Examine the effects of Panax ginseng on the lipid profile. | Eight men, aged 21.1 ± 2.1 years, participated in the study. All participants received 2 g of ginseng extract 3 times per day. Period of the study—8 weeks. | Decreased levels of serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), plasma malondialdehyde (MDA), and an increase in high-density lipoprotein (HDL). | [32] |
| Examine the effects on endurance performance of acute supplementation of Panax ginseng. | Twelve men, aged 20–24 years, participated in the study. All participants received 200 mg ginseng extract or a placebo one hour before the exercise. | Increased endurance time, blood glucose and insulin levels, catalase, superoxide-dismutase, and total thiol. | [33] |
| Evaluation of benefits on fatigue in multiple sclerosis with Panax ginseng treatment. | Fifty-two women, aged 18–50 years, participated in the study. There were 26 participants who received 500 mg daily of Korean ginseng tablets and 26 were in the placebo group.Period of the study—3 months. | Reduced fatigue. Improved quality of life. | [34] |
| Examine the effects of Panax ginseng on work performance. | Nineteen women, 21–35 years of age, participated in the study. There were 10 participants who received 200 mg of Panax ginseng extract daily, and nine of them were in the placebo group. Period of the study—2 months. | No change in maximal work performance, oxygen consumption (VO2), respiratory exchange ratio, minute ventilation, heart rate, and blood lactic acid levels. | [35] |
| Evaluation of the efficacy of a combination of Panax ginseng and vitamins on physical and mental stress. | One-hundred and fourteen women and men, aged 30–60 years, participated in the study. There were 59 participants who received 200 mg daily of ginseng dried extract and vitamins; 55 were in the placebo group. Period of the study—8 weeks. | Increased quality of life, without a difference in blood pressure and heart rate. | [36] |
| Evaluation of the efficacy of Panax ginseng extract on physical and mental performance. | Fifteen men, aged 19.07 ± 0.62 years, participated in the study. Seven of them received 200 mg daily of ginseng extract; eight of the participants were in the placebo group. Period of study—6 weeks. | Increased lactate levels. No change in VO2 and heart rate. Decreased cortisone levels and no change in testosterone levels. | [37] |
| The influence of Panax ginseng on cortisol, growth hormone, and lactate. | Ten women, aged 23.4 ± 0.69 years, participated in the study. In the first 4 weeks, five received 100 mg per day and five were in the placebo group. In the second 4 weeks, they were switched.Period of study—8 weeks. | Increased cortisol. No change in growth hormone and lactate. | [38] |
| Evaluation of the effects of Panax ginseng on sleep. | Fifteen men, aged 19–25 years, participated in the study. There were eight participants who received 4.5 g of ginseng extract daily; seven participants were in the placebo group. Period of the study—2 weeks. | Increased deep sleep. Decreased shallow sleep. | [39] |
| Evaluation of anti-fatigue effects of Panax ginseng. | Eighty-eight men and women 20–60 years of age participated in the study. There were 30 participants who received 1 g of ginseng extract daily; 29 participants had an intake of 2 g of ginseng extract daily. There were29 participants who were in the placebo group. Period of study—2 months. | Reduced the severity of fatigue. Increased glutathione reductase and total glutathione. | [40] |
| Assessment of an ergogenic effect on Malaysian population of Panax ginseng in humid and hot conditions. | Nine men, 25.4 ± 6.9 years of age, participated in the study. In the first trial, they had an intake of 200 mg of Panax ginseng one hour before an exercise test and in the second trial, they had an intake placebo. | Decreased lactate, plasma glucose, plasma insulin. Increased free fatty acids. No change in heart rate, VO2, skin, and body temperature. | [41] |
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Examine the effects of Eleutherococcus senticosus extract on physical working capacity. | Six men, aged 21–22 years, participated in the study. They were in the control group, placebo group, and the group that received 2 mL ethanol extract (125 mg dried extract) Eleutherococcus senticosus twice daily. Period of study—8 days. | Increased maximal oxygen uptake, oxygen pulse, total work, and exhaustion time. | [46] |
| Examine the effects of Eleutherococcus senticosus during maximal and submaximal aerobic exercise. | Twenty men and women, aged 37 ± 8 years, participated in the study. There were 10 participants who received 3.4 mL Eleutherococcus senticosus extract; 10 participants were in the placebo group. Period of study—8 weeks. | No changes in heart rate, VO2, lactate. | [48] |
| Assessment of the effects of Eleutherococcus senticosus on metabolism, endurance capacity, and cardiovascular functions. | Nine men, aged 19 ± 2.1 years, participated in the study. The participants received 800 mg daily of Eleutherococcus senticosus or placebo. Period of study—8 weeks. | Decreased blood glucose levels. Increased VO2, endurance time, heart rate, and free fatty acids. | [49] |
| Assessment of the impact of Eleutherococcus senticosus on quality of life. | Twenty volunteers, aged over 65 years, participated in the study. There were 10 participants who received 300 mg/day Siberian ginseng extract and 10 participants who were in placebo group. Period of study—2 months. | Improved mental health and social functioning, but prolonged use decreased these improvements. Blood pressure was not affected. | [50] |
| Assessment of the influence of Eleutherococcus senticosus on physical fitness and cellular defence. | Forty-six men and women, aged 23–73 years, participated in the study. There were 31 participants who received 75 drops of Eleutherococcus senticosus extract daily and 15 participants who received 120 drops of Echinacea extract daily for one month. | Decreased total cholesterol, LDL, triglycerides, free fatty acids, and glucose. Increased maximal oxygen consumption (VO2max). | [51] |
| Examine the effects of a dietary supplement containing Eleutherococcus senticosus extract on burnout symptoms. | Eighty-seven volunteers, aged 27–63 years, participated in the study. There were 44 participants who had an intake of 100 mg dry extract from Eleutherococcus senticosus; 43 were in the placebo group. Period of study—12 weeks. | Decreased fatigue score and Beck depression. | [52] |
| Evaluate the effects of Eleutherococcus senticosus extract on stress. | One-hundred and thirty women and men, aged 30–50 years, participated in the study. There were 49 participants who received 120 mg/day of dry Eleutherococcus senticosus extract, 40 participants who worked out, and 41 who worked out and took 120 mg/day dry extract. Period of study—2 months. | Improvement in fatigue, exhaustion, sleep, and restlessness. | [52] |
| Examine physiological reactions from the intake of Eleutherococcus senticosus on cyclists. | Nine men, aged 28 ± 2 years, participated in the study. The participants received 1200 mg per day of Eleutherococcus senticosus extract for 7 days and the placebo for 7 days. | No difference in respiratory exchange ratio, oxygen consumption, heart rate, perceived exertion, plasma lactate, plasma glucose. | [53] |
| Evaluate the effects of Eleutherococcus senticosus during the training process in fitness. | In the second series of study, 17 men and women participated; 10 of them received Eleutherococcus senticosus extract, and 7 participants were in the controlled group. | Decreased body weight. Increased physical endurance and performance. | [54] |
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Evaluation of the effects of an increased dose of Rhaponticum carthamoides during the training process. | Twenty women, aged 25–40 years, participated in the study. There were 12 of them whoreceived 5–15 mg/kg/day ecdysterone; 8 were in controlled group. | Decreased body weight. Increased physical endurance and performance. Improvement of cardiac and cognitive function. | [19] |
| Examine the effect of ecdysterone-containing products on sport physical exercises. | Forty-six men, aged 25.6 ± 3.7 years, participated in the study. There were 12 participants whohad an intake of 200 mg ecdysterone; 10 participants received 800 mg ecdysterone, 12 participants received the placebo, and 12 of the participants were in the control group—they had an intake of 200 mg ecdysterone without training. Period of study—10 weeks. | Ecdysterone increased body weight, muscle mass. Increased power and strength of performance. Without negative effects on creatinine, glutamate–oxaloacetate transaminase, gamma-glutamyl transferase, and glutamate–pyruvate transaminase. Did not affect steroid profile. | [59] |
| Evaluation of the effectiveness of ecdysterone in athletes. | Twenty-six women aged 18–22 years participated in the study. There were 12 participants who received ecdysterone from 37.5 to 50 mg; 14 participants were in the controlled group. Period of study—9 moths. | Increased VO2 lactate, performance activity. | [61] |
| Evaluation of the effectiveness of ecdysterone from Rhaponticum carthamoides leaves in athletes. | No information—number of participants. The age of the participants ranged between 27–58 years. Participants received 2–3 g Rhaponticum carthamoides tea, infusion, tincture, fermented tea without bitterness. Period of study—15 years. | Increased resistance to disease, physical, and mental endurance. | [62] |
| Assessment of effects of methoxyisoflavone, 20-hydroxyecdysone, and sulfopolysaccharides intake on training adaptation and markers of muscle anabolism and catabolism. | Forty-five men, aged 20.5 ± 3 years, participated in the study. The participants were divided randomly into four groups: the placebo group, the group that received methoxyisoflavone—800 mg daily, the group that received 20-hydroxyecdysone—200 mg/day, and the group that received sulfo-polysaccharides—1000 mg daily. Period of study—8 weeks. | No change in training adaptation and in anabolic and catabolic effect in training. | [63] |
| Evaluation of the effects of the combination of Rhaponticum carthamoides and Rhodiola rosea on performance fatigability and reactions before and after training. | Twenty-seven men, aged 22.3 ± 4.1 years, participated in the study. The participants received a 350 mg tablet which contains 70:30 Rhaponticum carthamoides extract and Rhodiola rosea extract, or a tablet containing 175 mg maltodextrin, and 175 mg Rhaponticum carthamoides and Rhodiola rosea extract in ratio 70:30 or placebo. | No change in muscle strength and total work. | [64] |
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Evaluating Rhaponticum carthamoides effects—growth, increased body weight, and behaviour on rats. | For study, 60 rats were used, divided into 10 groups with six rats in group. Females were fed with 5% Raponticum hay meal, males were fed with 10–20% Raponticum hay meal. Period of study—21 days. | Increased growth and body weight. | [65] |
| Evaluating Rhaponticum carthamoides effects on lipid profile on rats with cerebral ischemia. | For the study, 18 rats were used and received 150 mg/kg Rhaponticum carthamoides extract or placebo. Period of study—5 days. | Decreased lysophospholipids in erythrocyte membrane. Increased total lipids and phospholipids. | [66] |
| Examine ecdysterone neuroprotective mechanism of action. | For the study, 35 rats were used, aged 6–8 weeks. They were divided into seven groups: non-operated, two controlled groups, and three experimental groups. In the experimental groups, the rats were administrated ecdysterone in 5 mg/kg, 10 mg/kg, 20 mg/kg. Period of study—7 days. | Removed glutamatergic excitotoxicity. Neuroprotective effect. | [67] |
| Examine the anti-obesity effect of 20-hydroxyecdysone. | For the study, 60 rats were used, aged 3 months. They were divided into 12 rats in each group and three of the groups were treated with 18, 56, and 116 mg per day 20-hydroxyedysone. Period of study—3 months. | Decreased LDL. Increased muscle mass. No changes in thyroid-stimulating hormone (TSH), tetraiodothyronine (T4), and triiodothyronine (T3). | [68] |
| Examine anabolic effects of Leuzea carthamoides in quails. | For the study, 1000 quails were used. They were divided into six groups—the control group, four groups that had received a standard diet combined with Leuzea seed 0.2—5%, and the other two groups. Period of study—50 days. | Increased body mass and growth. | [69] |
| Examine anabolic effects of 20-hydroxyecdysone in quails. | For the study, 200 quails were used; 160 were in the control group. There were 10 that received 20 mg/kg, 10 that received 100 mg/kg, and 10 that received 500 mg/kg 20-hydroxyecdysone isolated from Leuzea carthamoides. Period of study—4 weeks. | Increased growth. Anabolic effect. | [70] |
| Examine the improving memory effect of 20-hydroxyecdysone. | For the study, 80 rats were used, divided into two groups: 10 were in the control group, 70 in the experimental group. They received 1, 10, and 100 mg/kg 20-hydroxyecdysone per day. Period of study—12 weeks. | Induced superoxide dismutase (SOD), catalase, glutathione peroxidase (GSH-Px), and glutathione reductase (GR). Decreased glucose levels, nuclear factor-kB (NF-kB). | [71] |
| Examine the effects of Rhapoticum carthamoides, Rhodiola rosea, and their combination on resistance exercise and mechanical power. | For the study, 56 rats were used. Rats were divided into seven groups, eight rats in each group: control group, a group that received only Rhodiola rosea extract, a group that received only Rhaponticum carthamoides extract, and four groups that received a combination of Rhaponticum carthamoides and Rhodiola rosea extracts in different quantitative ratios. | Rhaponticum carthamoides extract increased muscle protein synthesis. The combination of Rhaponticum carthamoides and Rhodiola rosea increased muscle protein synthesis and mean power performance. | [72] |
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Studying the effects of short-term supplementation with Rhodiola rosea. | Eleven women, aged 19.4 ± 0.8 years, participated in the study. They had an intake of 1.5 g/day Rhodiola rosea extract or placebo for 3 days. A 500 mg additional dose of Rhodiola rosea extract was taken before each trial. | Increased anaerobic capacity, anaerobic power, and total work. No change in fatigue index. | [77] |
| Examine hormonal and oxidative stress of Rhodiola rosea supplementation and the effects on mental and physical performance. | Twenty-six men participated in the study. Thirteen of them had an intake of 600 mg/day extract of Rhodiola rosea and 13 were in placebo group. Period of study—4 weeks. | Improved reaction and response time. Increased antioxidant capacity. Without changes in hormone profile and endurance exercise capacity. | [78] |
| Examine the levels of inflammatory C-reactive protein and creatinine kinase in blood after intake of Rhodiola rosea. | Thirty-six volunteers aged 21–24 years participated in the study. Twelve of them had an intake of 340 mg Rhodiola rosea extract twice a day, 12 participants were in the placebo group, and 12 participants were in the control group. Period of study—36 days. | Increased levels of C-reactive protein and creatinine kinase. | [79] |
| Examine the effects and safety of Rhodiola rosea extract for 4 weeks of treatment. | There were 101 women and men, aged 30–60 years, who participated in study. All participants had an intake of Rhodiola rosea extract 400 mg/day. Period of the study—1 month. | Improved mood, stress symptoms, and quality of life. | [80] |
| Examine the effects of a single dose of standardized Rhodiola rosea extract. | There were 121 men aged 19–21 years participated in the study; 41 participants received 370 mg dry extract Rhodiola rosea, 20 participants received 555 mg dry extract of Rhodiola rosea before test, 40 of participants were in the placebo group, 20 participants were in the controlled group. | Improvement in the anti-fatigue index. | [81] |
| Examine the effects of standardized Rhodiola rosea extract in patients suffering from depression. | Eighty-nine women and men, aged 18–70 years, participated in the study. Thirty-one participants received 340 mg/day extract of Rhodiola rosea, 29 participants received 680 mg/day extract of Rhodiola rosea, and 29 participants were in the placebo group. Period of study—42 days. | Improved in overall depression, insomnia, somatization, and emotional instability. No improvements in self-belief. | [82] |
| Evaluating the changes of Rhodiola rosea supplementation on muscle damage and inflammation. | There were 48 men and women, aged 25–60 years, who participated in the study. Twenty-four participants received a 300 mg capsule per day containing Rhodiola rosea extract, and 24 participants were in the placebo group. Period of study—38 days. | Increased myoglobin, creatine phosphokinase, aspartate aminotransferase, alanine aminotransferase, and interleukin (IL-6, IL-8, IL-10) without a difference in both groups. | [83] |
| Examine the effects of Rhodiola rosea supplementation on selected redox parameters in athletes. | Twenty-two men aged 20.4 ± 1.2 participated in the study. Eleven of them had an intake of 200 mg/day Rhodiola rosea extract, and 11 were in the placebo group.Period of study—4 weeks. Decreased levels of superoxide dismutase. Increased total antioxidant capacity. | Decreased levels of superoxide dismutase. Increased total antioxidant capacity. | [84] |
| Examine the effects of chronic intake of Rhodiola rosea on physical performance and antioxidant capacity during exercise in athletes. | Fourteen men, aged 25 ± 5 years, participated in the study. All of the participants received a placebo; after that, all of them received 170 mg R. rosea extract for 1 month. | Decreased free fatty acids levels, blood lactate, and creatinine kinase levels. No change in VO2max. | [85] |
| The efficacy of Rhodiola rosea in generalized anxiety disorder. | Ten men and women, aged 34–55 years, participated in the study. All participants had intake 340 mg Rhodiola rosea extract per day for 10 weeks. | Decreased scores in Hamilton Anxiety Rating Scale and Hamilton Depression Rating Scale. | [86] |
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Examine the effects of Schisandra chinensis extract on muscle strength and lactate. | Forty-five volunteers aged 61.9 ± 8.4 years participated in the study. Twenty-four participants received 1000 mg/day extract of Schisandra chinensis and 21 participants were in the placebo group. Period of study—3 months. | Decreased lactate levels. Increased quadriceps and muscle strength. | [105] |
| Examine the effects of Schisandra chinensis extract for menopausal symptoms. | Thirty-six women aged 40–70 years participated in the study. Eighteen participants received Schisandra chinensis extract 748 mg twice a day for 6 weeks; 18 were in the placebo group. Period of study—12 weeks. | Decreased hot flushes, sweating, and heart rate. | [106] |
| Examine the effects of Schisandra chinensis fruit on gut microbiota. | Twenty-eight women participated in the study. Thirteen participants received 6.7 g/day dried Schisandra chinensis fruits, and 15 participants were in placebo group. Period of study—12 weeks. | Decreased blood glucose, triglycerides, alanine aminotransferase, aspartate aminotransferase, and fat mass. Increased Bacteroides and Bacteroidetes. | [107] |
| Study Objectives | Study Design | Main Results | References |
|---|---|---|---|
| Examine the anti-athletics fatigue effects of Schisandra chinensis. | For the study, eight mice were used, divided into five groups—the control group, low-dose group—treated with 15 mg/kg Schisandra chinensis aqueous extract; the medium-dose group—treated with 30 mg/kg Schisandra chinensis aqueous extract; intermediate-high group—treated with 50 mg/kg Schisandra chinensis aqueous extract; and high-group—treated with 80 mg/kg Schisandra chinensis aqueous extract for 28 days. | Prevented an increase of lactate levels and blood urea nitrogen. Increased blood hemoglobin levels. | [108] |
| Examine Schisandra chinensis effects on pituitary-adrenal and gonadal axis, interleukins, and blood glucose levels. | For the study, 45 rats were used, aged 6 weeks old. There were 15 mice in the control group, 15 in the stress group, and 15 in the group that received 5 g/kg/day Schisandra chinensis and exercise. Period of study—11 days. | Decreased blood glucose levels, cortisol, and interleukins (IL-1 and IL-2). | [109] |
| Examine Schisandra chinensis effects on atherosclerosis in rats. | For the study, there were 60 rats used, aged 4 weeks, and 20 mice, aged 6 weeks. They were divided into five groups—normal, model, simvastatin (received 4 mg/kg/day), and low-dose group—which received an extract of Schisandra chinensis, 0.35 mg/kg day; medium-dose group—received extract of Schisandra chinensis 0.7 mg/kg/day; and high-dose group—received 1.4 mg/kg/day Schisandra chinensis extract for 3 weeks. Period of study—12 weeks. | Decreased TG and LDL levels. Increased HDL. | [110] |
| Examine the effect of Schisandra chinensis on mice with hyperlipidemia. | For the study, 48 mice were used. There were 24 mice in the control group and 24 received 100 mg/kg/day Schisandra chinensis lignans for 4 weeks. | Decreased TC, TG, LDL. Increased HDL, inhibited the mRNA expression of liver X receptor alfa and the mRNA expression level of hepatic lipogenesis. | [111] |
| Examine Schisandrine B effects on hepatic glutathione antioxidant system. | For the study, mice were used, with no information for number of mice. They were divided into five groups. Control group and the rest had received Schisandrine B 1 to 4 mmol/kg for 3 days; after that, they were threatened with CCl4 0.1 mL/kg. | Decreased glucose-6-phosphate dehydrogenase, gama-glutamilcysteine synthetase, and Se-glutathione peroxidase. Increased hepatic gluthatione S-transferase and glutathione reductase. | [112] |
| Examine the preventive effect of Schisandrine B on scopolamine-induced dementia in mice. | For the study, 30 mice were used, aged 12–14 weeks. They were divided into groups—the control group, three groups that had received Schisandrine B, respectively, 10, 25, or 50 mg/kg/daily before treatment with scopolamine, and a group that had received 10 mg/kg/day tacrine. Period of study—7 days. | Prevented scopolamine-induced oxidative stress. Prevented the decrease of acetylcholine levels. | [113] |
| Evaluated muscle-protective effects of Schisandra chinensis extract in mice, after exercise. | For the study, 48 mice were used, aged 10 months. They were divided into six groups with eight mice in each group. The first group received distilled water. The second group received distilled water with exercise control. The third group received 50 mg/kg oxymetholone. The rest of the three groups received, respectively, 125, 250, or 500 mg/kg per day of Schisandra chinensis extract and exercises. Period of study—28 days. | Decreased creatine, creatine kinase, and lactate dehydrogenase. Increased myofibre diameter. Inhibited lipid peroxidation, reactive oxygen species. | [114] |
| Examine the immunostimulatory effect of polysaccharides from Schisandra chinensis. | For the study, 50 mice were used, divided into five groups. Three of the groups were administered with 50, 100, or 200 mg/kg/day Schisandra chinensis polysaccharides IIa; the other two groups were the control and model groups. Period of study—10 days. | Increased the phagocytic activity of peritoneal macrophages and lymphocyte transformation. | [115] |
| Examine the anti-diabetic effect of polysaccharides from Schisandra chinensis. | For the study, 60 mice were used, divided into six groups: control group, control group with diabetes, alloxan-induced diabetic mice treated with sodium chloride solution, and three groups treated with 162, 324, or 648 mg/kg Schisandra chinensis polysaccharides, and a placebo group. Period of study—21 days. | Decreased blood glucose levels. Improved lipid metabolism. | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. https://doi.org/10.3390/nu13082861
Todorova V, Ivanov K, Delattre C, Nalbantova V, Karcheva-Bahchevanska D, Ivanova S. Plant Adaptogens—History and Future Perspectives. Nutrients. 2021; 13(8):2861. https://doi.org/10.3390/nu13082861
Chicago/Turabian StyleTodorova, Velislava, Kalin Ivanov, Cédric Delattre, Vanya Nalbantova, Diana Karcheva-Bahchevanska, and Stanislava Ivanova. 2021. "Plant Adaptogens—History and Future Perspectives" Nutrients 13, no. 8: 2861. https://doi.org/10.3390/nu13082861







