A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health
Abstract
1. Introduction
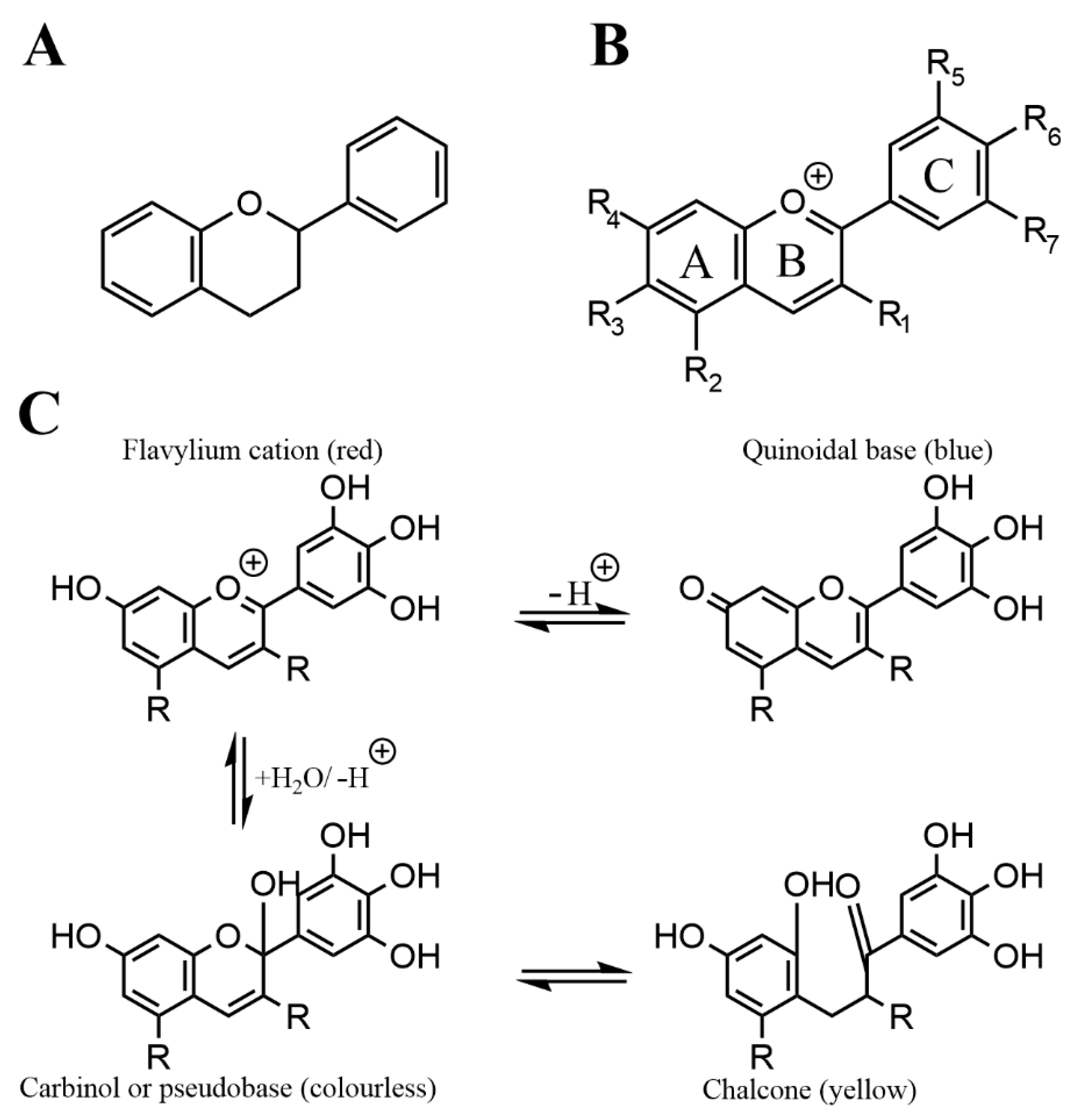
2. Overview
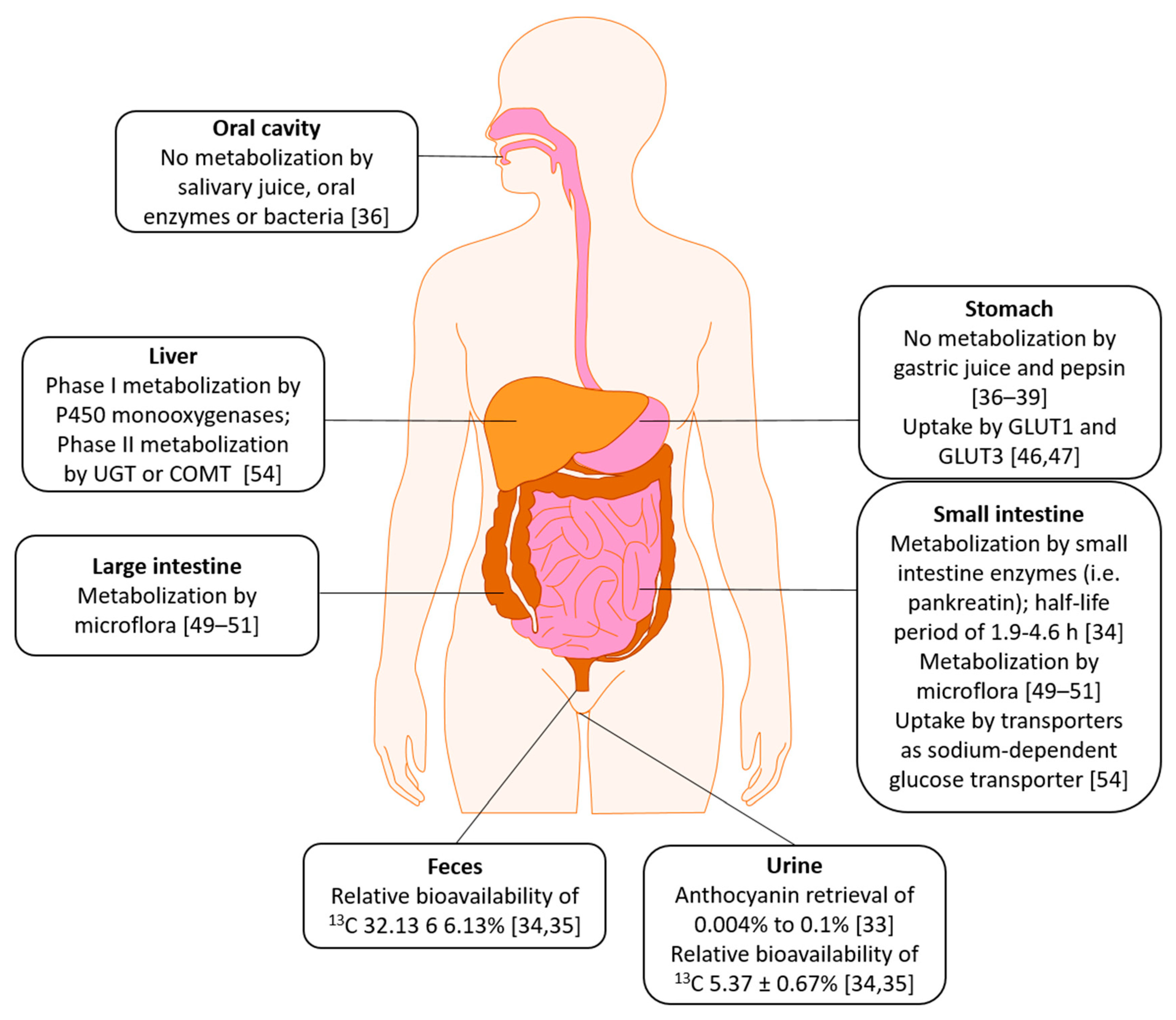
3. Absorption, Distribution, Metabolism, Excretion (ADME)
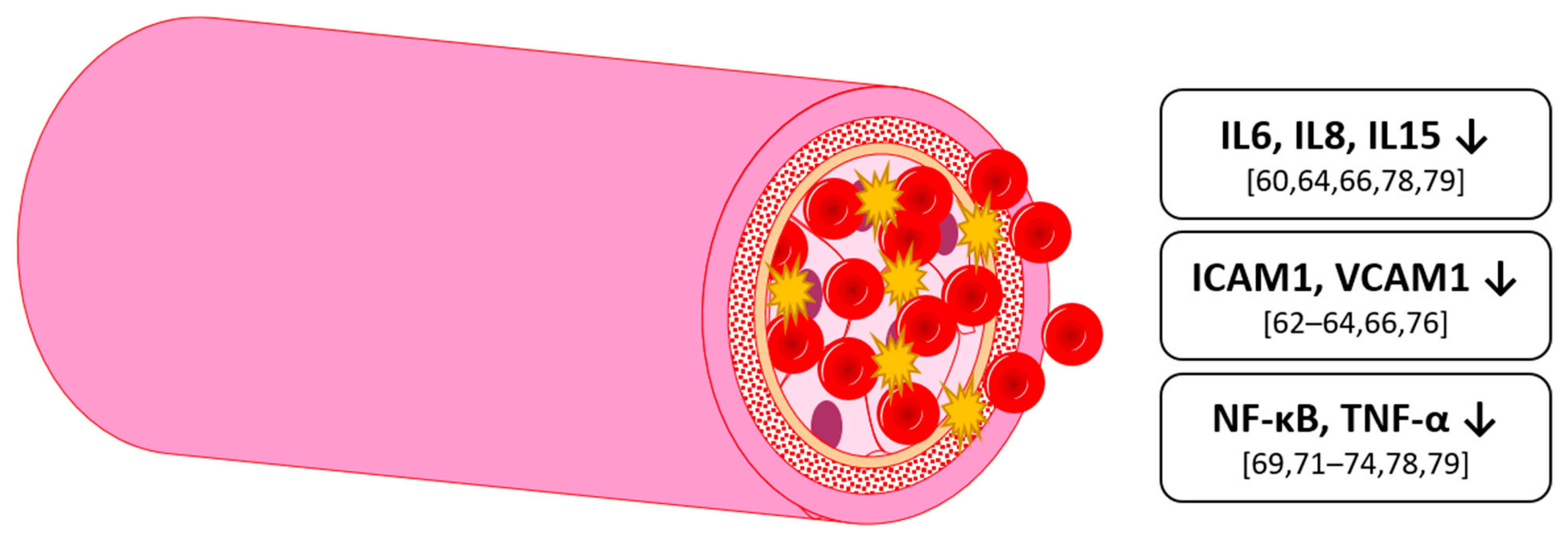
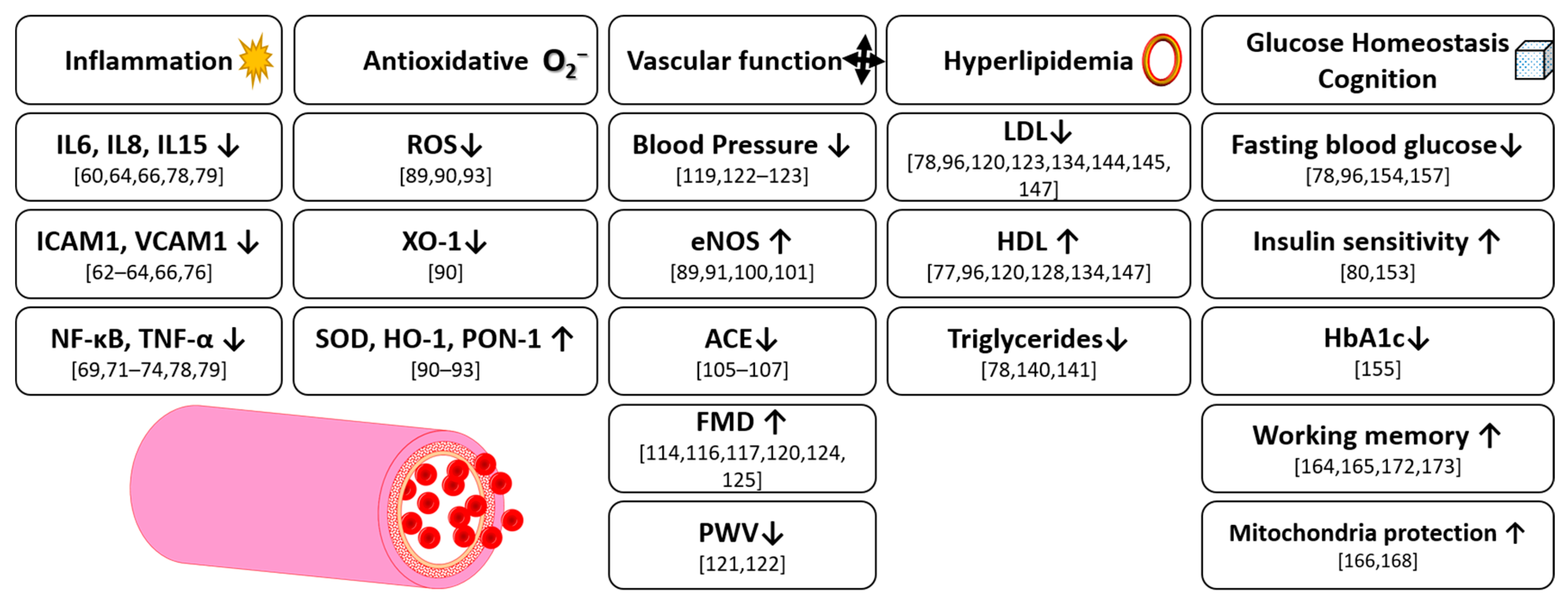
4. Inflammation
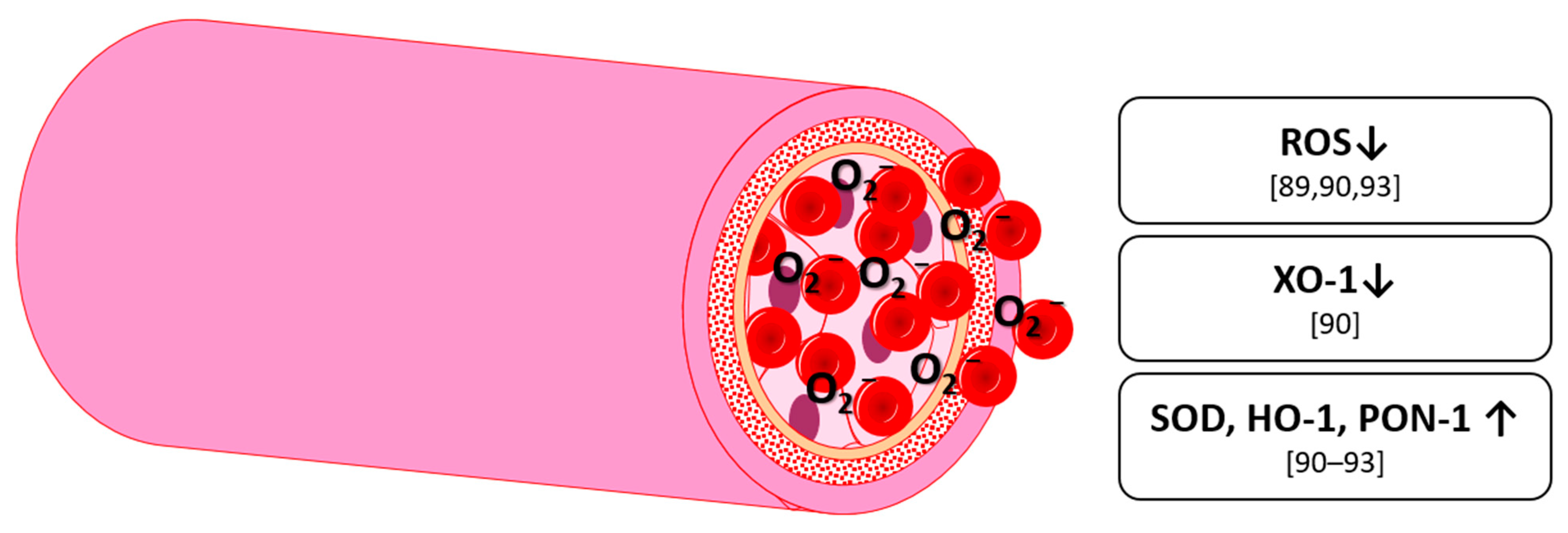
5. Antioxidative Effects
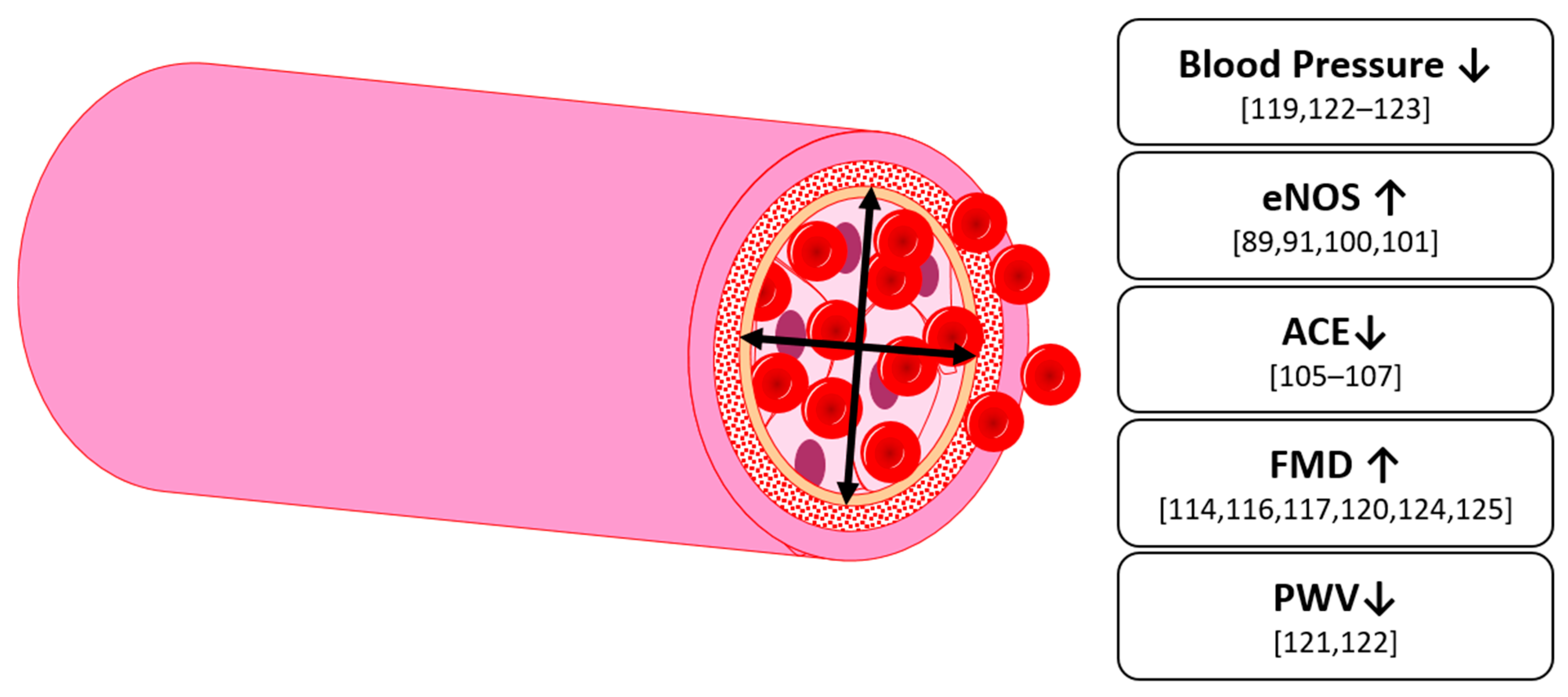
6. Vascular Function
7. Hyperlipidemia
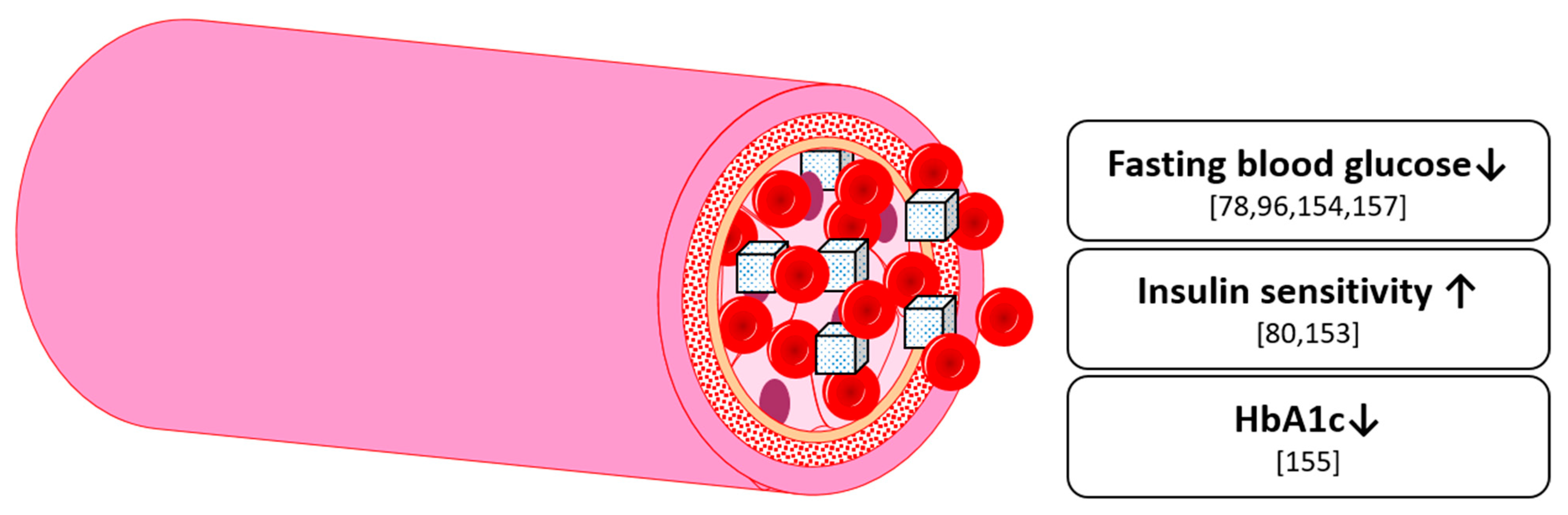
8. Glucose Homeostasis
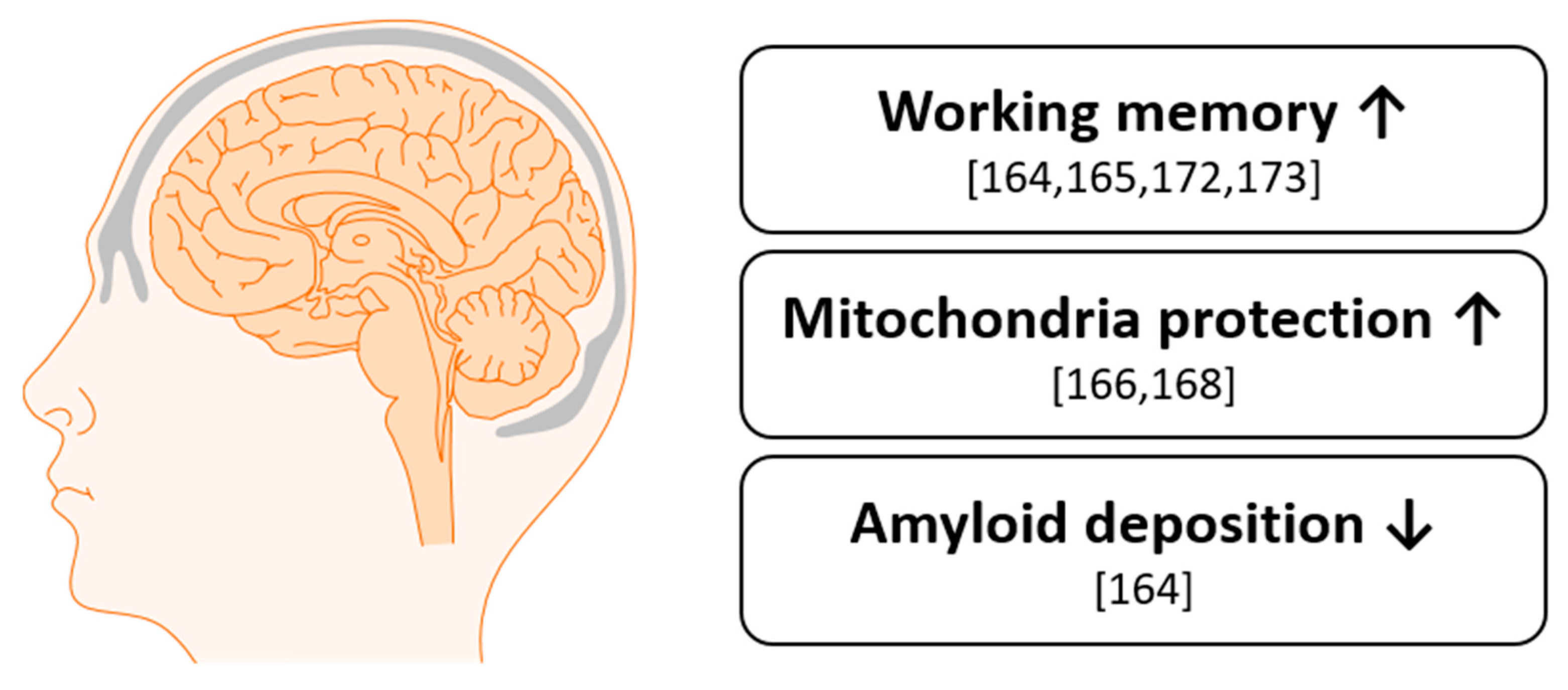
9. Cognition
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Frost & Sullivan. Growth Opportunities in the Gobal Phytonutrient Ingredients Market, Forecast to 2022. Available online: https://ww2.frost.com (accessed on 16 August 2021).
- BCC Research Staff. Nutraceuticals: Global Markets to 2023; BBC: London, UK, 2018. [Google Scholar]
- Peña-Sanhueza, D.; Inostroza-Blancheteau, C.; Ribera-Fonseca, A.; Reyes-Díaz, M. Anthocyanins in berries and their potential use in human health. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; Shiomi, N., Waisundara, V., Eds.; IntechOpen: Temuco, Chile, 2017; pp. 155–172. [Google Scholar]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef]
- Virani Salim, S.; Alonso, A.; Benjamin Emelia, J.; Bittencourt Marcio, S.; Callaway Clifton, W.; Carson April, P.; Chamberlain Alanna, M.; Chang Alexander, R.; Cheng, S.; Delling Francesca, N.; et al. Heart disease and stroke statistics—2020 update: A report from the american heart association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Luan, Y.-Y.; Yao, Y.-M. The clinical significance and potential role of c-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. J. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of fruits and vegetables—Their occurrence, analysis and role in human nutrition. J. Fruit Ornam. Plant Res. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of maqui (Aristotelia chilensis (mol.) stuntz). Phytochem. Anal. PCA 2006, 17, 8–14. [Google Scholar] [CrossRef]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [mol.] stuntz) from different geographical regions in chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Guiné, R.; Gonçalves, F.; Lerat, C.; Idrissi, T.; Rodrigo, E.; Correia, P.; Gonçalves, J.C. Extraction of phenolic compounds with antioxidant activity from beetroot (Beta vulgaris L.). Curr. Nutr. Food Sci. 2018, 14, 350–357. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. Sativus var. Atrorubens alef.) cultivars antonina, beta sweet, deep purple, and purple haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Schantz, M.; Richling, E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2012, 77, C340–C345. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Yoo, K.S.; Bang, H.; Pike, L.; Patil, B.S.; Lee, E.J. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic. Environ. Biotechnol. 2020, 61, 385–393. [Google Scholar] [CrossRef]
- Labbe, M.; Ulloa, P.A.; Lopez, F.; Saenz, C.; Pena, A.; Salazar, F.N. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (’wonderful’, ’chaca’ and ’codpa’) at different maturity stages. Chil. J. Agric. Res. 2016, 76, 479–486. [Google Scholar] [CrossRef][Green Version]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13c-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M. Studien zum Verhalten von Anthocyanen aus Heidelbeeren im Humanstoffwechsel-Stabilisierung und Bindung Durch Proteine. Ph.D. Thesis, University of Wuerzburg, Wuerzburg, Germany, 3 December 2010. [Google Scholar]
- Yu, W.; Gao, J.; Hao, R.; Yang, J.; Wei, J. Effects of simulated digestion on black chokeberry (Aronia melanocarpa (michx.) elliot) anthocyanins and intestinal flora. J. Food Sci. Technol. 2021, 58, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef]
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus Microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef]
- Atnip, A.; Giusti, M.M.; Sigurdson, G.T.; Failla, M.L.; Chitchumroonchokchai, C.; Bomser, J.A. The nci-n87 cell line as a gastric epithelial model to study cellular uptake, trans-epithelial transport, and gastric anti-inflammatory properties of anthocyanins. Nutr. Cancer 2020, 72, 686–695. [Google Scholar] [CrossRef]
- Atnip, A.A.; Sigurdson, G.T.; Bomser, J.; Giusti, M.M. Time, concentration, and ph-dependent transport and uptake of anthocyanins in a human gastric epithelial (nci-n87) cell line. Int. J. Mol. Sci. 2017, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Fernandes, I.; Brás, N.F.; Faria, A.; De Freitas, V.; Calhau, C.; Mateus, N. Experimental and theoretical data on the mechanism by which red wine anthocyanins are transported through a human mkn-28 gastric cell model. J. Agric. Food Chem. 2015, 63, 7685–7692. [Google Scholar] [CrossRef]
- Han, F.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; De Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins—Impact of structural complexity and phase ii metabolization. Food Chem. 2020, 317, 126398. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of anthocyanins from bilberries—Effects on bioavailability and intestinal accessibility in humans. Food Chem. 2018, 248, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin absorption and metabolism by human intestinal caco-2 cells—A review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2016, 105, 10–22. [Google Scholar] [CrossRef]
- Kalt, W.; Liu, Y.; McDonald, J.; Vinqvist-Tymchuk, M.; Fillmore, S.A.E. Anthocyanin metabolites are abundant and persistent in human urine. J. Agric. Food Chem. 2014, 62, 3926–3934. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2013, 306, H317–H325. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yambe, M.; Tomiyama, H.; Hirayama, Y.; Gulniza, Z.; Takata, Y.; Koji, Y.; Motobe, K.; Yamashina, A. Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens. Res. 2004, 27, 625–631. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Kjølsrud-Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-κb activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of human umbilical vein endothelial cells (huvec) as a model to study cardiovascular disease: A review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Liu, Y.-M.; Wang, J.; Wang, X.-N.; Li, C.-Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Wang, J.; Liu, Y.-M.; Zheng, Q.-S.; Li, C.-Y. Inhibitory effect of malvidin on tnf-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar] [CrossRef]
- Warner, E.F.; Smith, M.J.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Signatures of anthocyanin metabolites identified in humans inhibit biomarkers of vascular inflammation in human endothelial cells. Mol. Nutr. Food Res. 2017, 61, 1700053. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef]
- Bharat, D.; Cavalcanti Rafaela Ramos, M.; Petersen, C.; Begaye, N.; Cutler Brett, R.; Costa Marcella Melo, A.; Ramos Renata Kelly Luna, G.; Ferreira Marina, R.; Li, Y.; Bharath Leena, P.; et al. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2017, 62, 1700601. [Google Scholar] [CrossRef]
- Muscarà, C.; Molonia, M.S.; Speciale, A.; Bashllari, R.; Cimino, F.; Occhiuto, C.; Saija, A.; Cristani, M. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3t3-l1 adipocytes. Phytother. Res. PTR 2019, 33, 1888–1897. [Google Scholar] [CrossRef]
- Krga, I.; Monfoulet, L.-E.; Konic-Ristic, A.; Mercier, S.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to tnfα-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 2016, 599, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-o-glucoside protection against tnf-α-induced endothelial dysfunction: Involvement of nuclear factor-κb signaling. J. Agric. Food Chem. 2010, 58, 12048–12054. [Google Scholar] [CrossRef]
- Luo, X.; Fang, S.; Xiao, Y.; Song, F.; Zou, T.; Wang, M.; Xia, M.; Ling, W. Cyanidin-3-glucoside suppresses tnf-α-induced cell proliferation through the repression of nox activator 1 in mouse vascular smooth muscle cells: Involvement of the stat3 signaling. Mol. Cell. Biochem. 2012, 362, 211–218. [Google Scholar] [CrossRef]
- Yan, X.; Wu, L.; Li, B.; Meng, X.; Dai, H.; Zheng, Y.; Fu, J. Cyanidin-3-o-glucoside induces apoptosis and inhibits migration of tumor necrosis factor-α-treated rat aortic smooth muscle cells. Cardiovasc. Toxicol. 2016, 16, 251–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Lian, F.; Zhu, Y.; Xia, M.; Wang, Q.; Ling, W.; Wang, X.D. Cyanidin-3-o-beta-glucoside inhibits lps-induced expression of inflammatory mediators through decreasing ikappabalpha phosphorylation in thp-1 cells. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2010, 59, 723–730. [Google Scholar]
- Ma, M.M.; Li, Y.; Liu, X.Y.; Zhu, W.W.; Ren, X.; Kong, G.Q.; Huang, X.; Wang, L.P.; Luo, L.Q.; Wang, X.Z. Cyanidin-3-o-glucoside ameliorates lipopolysaccharide-induced injury both in vivo and in vitro suppression of nf-κb and mapk pathways. Inflammation 2015, 38, 1669–1682. [Google Scholar] [CrossRef]
- Paixão, J.; Dinis, T.C.P.; Almeida, L.M. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial no synthase and inhibiting peroxynitrite-induced nf-kb activation. Chem. Biol. Interact. 2012, 199, 192–200. [Google Scholar] [CrossRef]
- Martin, K.R.; Burrell, L.; Bopp, J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: A randomized, crossover pilot study. Food Funct. 2018, 9, 5290–5300. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Hassellund, S.S.; Flaa, A.; Kjeldsen, S.E.; Seljeflot, I.; Karlsen, A.; Erlund, I.; Rostrup, M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2012, 27, 100. [Google Scholar] [CrossRef]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappab activation and increasing ppar-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose–response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Zhang, P.-W.; Chen, F.-X.; Li, D.; Ling, W.-H.; Guo, H.-H. A consort-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef]
- Randy, L.H.; Guoying, B. Agonism of peroxisome proliferator receptor-gamma may have therapeutic potential for neuroinflammation and parkinsons disease. Curr. Neuropharmacol. 2007, 5, 35–46. [Google Scholar]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.; Ghaskadbi, S.; Lele, R. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, K.X.; Huang, A.C.; Ho, Y.C.; Wang, C.J. Hibiscus anthocyanins-rich extract inhibited ldl oxidation and oxldl-mediated macrophages apoptosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 1015–1023. [Google Scholar] [CrossRef]
- Yi, L.; Chen, C.Y.; Jin, X.; Mi, M.T.; Yu, B.; Chang, H.; Ling, W.H.; Zhang, T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett. 2010, 584, 583–590. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxid Med. Cell Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef]
- Lazzè Maria, C.; Pizzala, R.; Perucca, P.; Cazzalini, O.; Savio, M.; Forti, L.; Vannini, V.; Bianchi, L. Anthocyanidins decrease endothelin-1 production and increase endothelial nitric oxide synthase in human endothelial cells. Mol. Nutr. Food Res. 2005, 50, 44–51. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable concentrations of delphinidin and its metabolite, gallic acid, induce antioxidant protection associated with increased intracellular glutathione in cultured endothelial cells. Oxid Med. Cell Longev. 2017, 2017, 9260701. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Attias, J.; Mahamid, R.; Aviram, M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010, 1, 99–109. [Google Scholar] [CrossRef]
- Cimino, F.; Speciale, A.; Anwar, S.; Canali, R.; Ricciardi, E.; Virgili, F.; Trombetta, D.; Saija, A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of nrf2 pathway. Genes Nutr. 2013, 8, 391–399. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Van ’t Erve, T.J.; Lih, F.B.; Kadiiska, M.B.; Deterding, L.J.; Eling, T.E.; Mason, R.P. Reinterpreting the best biomarker of oxidative stress: The 8-iso-pgf(2α)/pgf(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic. Biol. Med. 2015, 83, 245–251. [Google Scholar] [CrossRef]
- Nyberg, M.; Gliemann, L.; Hellsten, Y. Vascular function in health, hypertension, and diabetes: Effect of physical activity on skeletal muscle microcirculation. Scand. J. Med. Sci. Sports 2015, 25, 60–73. [Google Scholar] [CrossRef]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the ⋅no/cgmp signaling pathway. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; McCarthy, D.; Burton-Freeman, B.M. Effect of black currant anthocyanins on the activation of endothelial nitric oxide synthase (enos) in vitro in human endothelial cells. J. Agric. Food Chem. 2011, 59, 8616–8624. [Google Scholar] [CrossRef]
- Tulio, A.Z.; Chang, C.; Edirisinghe, I.; White, K.D.; Jablonski, J.E.; Banaszewski, K.; Kangath, A.; Tadapaneni, R.K.; Burton-Freeman, B.; Jackson, L.S. Berry fruits modulated endothelial cell migration and angiogenesis via phosphoinositide-3 kinase/protein kinase b pathway in vitro in endothelial cells. J. Agric. Food Chem. 2012, 60, 5803–5812. [Google Scholar] [CrossRef]
- Studdy, P.R.; Lapworth, R.; Bird, R. Angiotensin-converting enzyme and its clinical significance—A review. J. Clin. Pathol. 1983, 36, 938–947. [Google Scholar] [CrossRef]
- Sica, D.A. Angiotensin-converting enzyme inhibitors’ side effects—Physiologic and non-physiologic considerations. J. Clin. Hypertens. 2005, 7, 17–23. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for potential angiotensin converting enzyme (ace)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef]
- Persson, I.A.L.; Persson, K.; Andersson, R.G.G. Effect of vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J. Agric. Food Chem. 2009, 57, 4626–4629. [Google Scholar] [CrossRef]
- Hidalgo, M.; Martin-Santamaria, S.; Recio, I.; Sanchez-Moreno, C.; de Pascual-Teresa, B.; Rimbach, G.; de Pascual-Teresa, S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ace) activity by the anthocyanins delphinidin- and cyanidin-3-o-sambubiosides from hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef]
- Kim, H.-L.; Kim, S.-H. Pulse wave velocity in atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Clark, K.; Schuschke, D.A.; Klimis-Zacas, D.J. Vascular reactivity is affected by dietary consumption of wild blueberries in the sprague-dawley rat. J. Med. Food 2009, 12, 21–28. [Google Scholar] [CrossRef]
- Côrtes, S.F.; Valadares, Y.M.; de Oliveira, A.B.; Lemos, V.S.; Barbosa, M.P.; Braga, F.C. Mechanism of endothelium-dependent vasodilation induced by a proanthocyanidin-rich fraction from ouratea semiserrata. Planta Med. 2002, 68, 412–415. [Google Scholar] [CrossRef]
- Andriambeloson, E.; Magnier, C.; Haan-Archipoff, G.; Lobstein, A.; Anton, R.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998, 128, 2324–2333. [Google Scholar] [CrossRef]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, J.I.; Yahia, E.M.; Rivera-Pastrana, D.M.; Rojas-Molina, A.; Zavala-Sánchez, M. Nutraceutical value of black cherry prunus serotina ehrh. Fruits: Antioxidant and antihypertensive properties. Molecules 2013, 18, 14597–14612. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E975–E988. [Google Scholar] [CrossRef]
- Shaughnessy, K.S.; Boswall, I.A.; Scanlan, A.P.; Gottschall-Pass, K.T.; Sweeney, M.I. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr. Res. 2009, 29, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J.F. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via no-cgmp activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524. [Google Scholar] [CrossRef]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre-and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating anthocyanin metabolites mediate vascular benefits of blueberries: Insights from randomized controlled trials, metabolomics, and nutrigenomics. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 967–976. [Google Scholar] [CrossRef]
- Hassellund, S.S.; Flaa, A.; Sandvik, L.; Kjeldsen, S.E.; Rostrup, M. Effects of anthocyanins on blood pressure and stress reactivity: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2012, 26, 396–404. [Google Scholar] [CrossRef]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Marckmann, P.; Dragsted, L.O.; Finné Nielsen, I.L.; Nielsen, S.E.; Grønbaek, M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005, 59, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Gurrola-Díaz, C.M.; García-López, P.M.; Sánchez-Enríquez, S.; Troyo-Sanromán, R.; Andrade-González, I.; Gómez-Leyva, J.F. Effects of hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (mesy). Phytomedicine 2010, 17, 500–505. [Google Scholar] [CrossRef]
- Nelson, M.R.; Doust, J.A. Primary prevention of cardiovascular disease: New guidelines, technologies and therapies. Med. J. Aust. 2013, 198, 606–610. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2019, 41, 12–85. [Google Scholar] [CrossRef]
- Wouters, K.; Shiri-Sverdlov, R.; van Gorp, P.J.; van Bilsen, M.; Hofker, M.H. Understanding hyperlipidemia and atherosclerosis: Lessons from genetically modified apoe and ldlr mice. Clin. Chem. Lab. Med. 2005, 43, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. Lox-1, oxldl, and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum ldl- and hdl-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef]
- Byfield, F.J.; Tikku, S.; Rothblat, G.H.; Gooch, K.J.; Levitan, I. Oxldl increases endothelial stiffness, force generation, and network formation. J. Lipid Res. 2006, 47, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of il-6 and vcam-1 in cd40l and oxidized ldl challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries and anthocyanins: Promising functional food ingredients with postprandial glycaemia-lowering effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef]
- Jin, X.; Chen, M.; Yi, L.; Chang, H.; Zhang, T.; Wang, L.; Ma, W.; Peng, X.; Zhou, Y.; Mi, M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the ampk/sirt1 signaling pathway. Mol. Nutr. Food Res. 2014, 58, 1941–1951. [Google Scholar] [CrossRef]
- Jin, X.; Yi, L.; Chen, M.L.; Chen, C.Y.; Chang, H.; Zhang, T.; Wang, L.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Delphinidin-3-glucoside protects against oxidized low-density lipoprotein-induced mitochondrial dysfunction in vascular endothelial cells via the sodium-dependent glucose transporter sglt1. PLoS ONE 2013, 8, e68617. [Google Scholar] [CrossRef]
- Xia, X.; Ling, W.; Ma, J.; Xia, M.; Hou, M.; Wang, Q.; Zhu, H.; Tang, Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein e-deficient mice. J. Nutr. 2006, 136, 2220–2225. [Google Scholar] [CrossRef]
- Wu, T.; Qi, X.; Liu, Y.; Guo, J.; Zhu, R.; Chen, W.; Zheng, X.; Yu, T. Dietary supplementation with purified mulberry (morus australis poir) anthocyanins suppresses body weight gain in high-fat diet fed c57bl/6 mice. Food Chem. 2013, 141, 482–487. [Google Scholar] [CrossRef]
- Vugic, L.; Colson, N.; Nikbakht, E.; Gaiz, A.; Holland, O.J.; Kundur, A.R.; Singh, I. Anthocyanin supplementation inhibits secretion of pro-inflammatory cytokines in overweight and obese individuals. J. Funct. Foods 2020, 64, 103596. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, P.; Liu, Y.; Zha, L.; Ling, W.; Guo, H. A dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: A randomized controlled trial. Nutrition 2020, 74, 110745. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Brewer, H.B., Jr.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl ester transfer protein: A novel target for raising hdl and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves hdl-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Hollands, W.J.; Armah, C.N.; Doleman, J.F.; Perez-Moral, N.; Winterbone, M.S.; Kroon, P.A. 4-week consumption of anthocyanin-rich blood orange juice does not affect ldl-cholesterol or other biomarkers of cvd risk and glycaemia compared with standard orange juice: A randomised controlled trial. Br. J. Nutr. 2018, 119, 415–421. [Google Scholar] [CrossRef]
- Pokimica, B.; García-Conesa, M.-T.; Zec, M.; Debeljak-Martačić, J.; Ranković, S.; Vidović, N.; Petrović-Oggiano, G.; Konić-Ristić, A.; Glibetić, M. Chokeberry juice containing polyphenols does not affect cholesterol or blood pressure but modifies the composition of plasma phospholipids fatty acids in individuals at cardiovascular risk. Nutrients 2019, 11, 850. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H.; et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of amp-activated protein kinase in diabetic mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef]
- Törrönen, R.; Sarkkinen, E.; Tapola, N.; Hautaniemi, E.; Kilpi, K.; Niskanen, L. Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br. J. Nutr. 2010, 103, 1094–1097. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Yang, Y.; Chen, Y.; Tian, Z.; Du, Z.; Chen, J.; Xie, Y.; Liu, Z.; Yang, L. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes—A randomized controlled trial. Nutrients 2017, 9, 1104. [Google Scholar] [CrossRef]
- Bennett, C.M.; Guo, M.; Dharmage, S.C. Hba1c as a screening tool for detection of type 2 diabetes: A systematic review. Diabet. Med. 2007, 24, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Z.; Ling, W.; Wang, L.; Wang, C.; Ma, J.; Peng, X.; Chen, J. Effect of anthocyanins supplementation on serum igfbp-4 fragments and glycemic control in patients with fasting hyperglycemia: A randomized controlled trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3395–3404. [Google Scholar] [CrossRef]
- Fernando, W.; Somaratne, G.; Goozee, K.G.; Williams, S.; Singh, H.; Martins, R.N. Diabetes and alzheimer’s disease: Can tea phytochemicals play a role in prevention? J. Alzheimer’s Dis. 2017, 59, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef]
- Guerra-Araiza, C.; Álvarez-Mejía, A.L.; Sánchez-Torres, S.; Farfan-García, E.; Mondragón-Lozano, R.; Pinto-Almazán, R.; Salgado-Ceballos, H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013, 47, 451–462. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Krause, A.J.; Shearer, S.; Devries, S. The 2015 dietary guidelines advisory committee report concerning dietary cholesterol. Am. J. Cardiol. 2015, 116, 1479–1480. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the app/ps1 mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.C.; Marisco, P.; Agostinho, P.; Rodrigues, M.; Rubin, M.A.; Schmatz, R.; da Silva, C.R.; Cognato, G.D.P. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of alzheimer’s type. Life Sci. 2014, 96, 7–17. [Google Scholar] [CrossRef]
- Parrado-Fernández, C.; Sandebring-Matton, A.; Rodriguez-Rodriguez, P.; Aarsland, D.; Cedazo-Mínguez, A. Anthocyanins protect from complex i inhibition and appswe mutation through modulation of the mitochondrial fission/fusion pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 2110–2118. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Kish, S.J. Mitochondria in alzheimer’s disease. In International Review Neurobiology; Academic Press: Cambridge, MA, USA, 2002; Volume 53, pp. 341–385. [Google Scholar]
- Chou, A.P.; Li, S.; Fitzmaurice, A.G.; Bronstein, J.M. Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicology 2010, 31, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Shurland, D.-L.; Ryazantsev, S.N.; van der Bliek, A.M. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 1998, 143, 351–358. [Google Scholar] [CrossRef]
- Marcus, D.L.; Thomas, C.; Rodriguez, C.; Simberkoff, K.; Tsai, J.S.; Strafaci, J.A.; Freedman, M.L. Increased peroxidation and reduced antioxidant enzyme activity in alzheimer’s disease. Exp. Neurol. 1998, 150, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Redha, A.; AlHasan, R. Anthocyanins potentially contribute to defense against Alzheimer’s disease. Molecules 2019, 24, 4255. [Google Scholar] [CrossRef]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]

| Food Source | Anthocyanin Content [mg/100 g Fresh Weight] | Reference |
|---|---|---|
| Beetroot (Beta vulgaris L.) | 23–77 | [24] |
| Bilberry (Vaccinium myrtillus) | 300–698 | [13,17,25,28] |
| Black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) | 1.5–190 | [27,30] |
| Blackberry (Rubus fruticosus L.) | 83–326 | [13,16] |
| Blackcurrant (Ribes nigrum) | 130–476 | [13,16,17,25] |
| Blueberry (Vaccinium angustifolium/corybosum) | 25–495 | [13,16,25,28] |
| Cranberry (Vaccinium macrocarpon) | 46–200 | [13,16,17,25] |
| Elderberry (Sambucus nigra L.) | 200–1560 | [16,17,29] |
| Maqui berry (Aristotelia chilensis) | 137–1250 | [22,23] |
| Pomegranate (Punica granatum) juice | 9–765 mg/L | [13,21,31] |
| Purple corn (Zea mays indurate) | 68–1642 | [13,26] |
| Red cabbage (Brassica oleracea L. var. capitata L.) | 250–322 | [13,16,17] |
| Red Grape (Vitis vinifera) | 26–750 | [13,16,17,25] |
| Redcurrant (Ribes rubrum) | 12–22 | [13,16,17,25] |
| Strawberry (Fragaria × ananassa) | 12–55 | [13,16,17,25] |
| Tart cherry (Prunus cerasus) | 2–450 | [13,16,17,25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients 2021, 13, 2831. https://doi.org/10.3390/nu13082831
Ockermann P, Headley L, Lizio R, Hansmann J. A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients. 2021; 13(8):2831. https://doi.org/10.3390/nu13082831
Chicago/Turabian StyleOckermann, Philipp, Laura Headley, Rosario Lizio, and Jan Hansmann. 2021. "A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health" Nutrients 13, no. 8: 2831. https://doi.org/10.3390/nu13082831
APA StyleOckermann, P., Headley, L., Lizio, R., & Hansmann, J. (2021). A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients, 13(8), 2831. https://doi.org/10.3390/nu13082831






