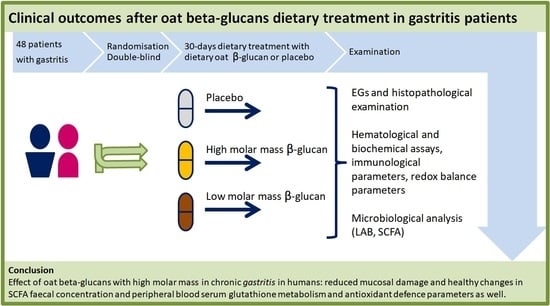
Clinical Outcomes after Oat Beta-Glucans Dietary Treatment in Gastritis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Dietary Supplements
2.4. Esophagogastroduodenoscopy and Histopathological Examination
2.5. Blood Sample Collection, Hematological and Biochemical Assays
2.6. Feces Sample Collection, Determination of the Number of Lactic Acid Bacteria (LAB) and Analysis of Short-Chain Fatty Acids (SCFAs)
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Well-Being and Adverse Effects during the Study
3.3. Histological Examination of Biopsy Specimens
3.4. Peripheral Blood Hematology and Biochemical Parameters
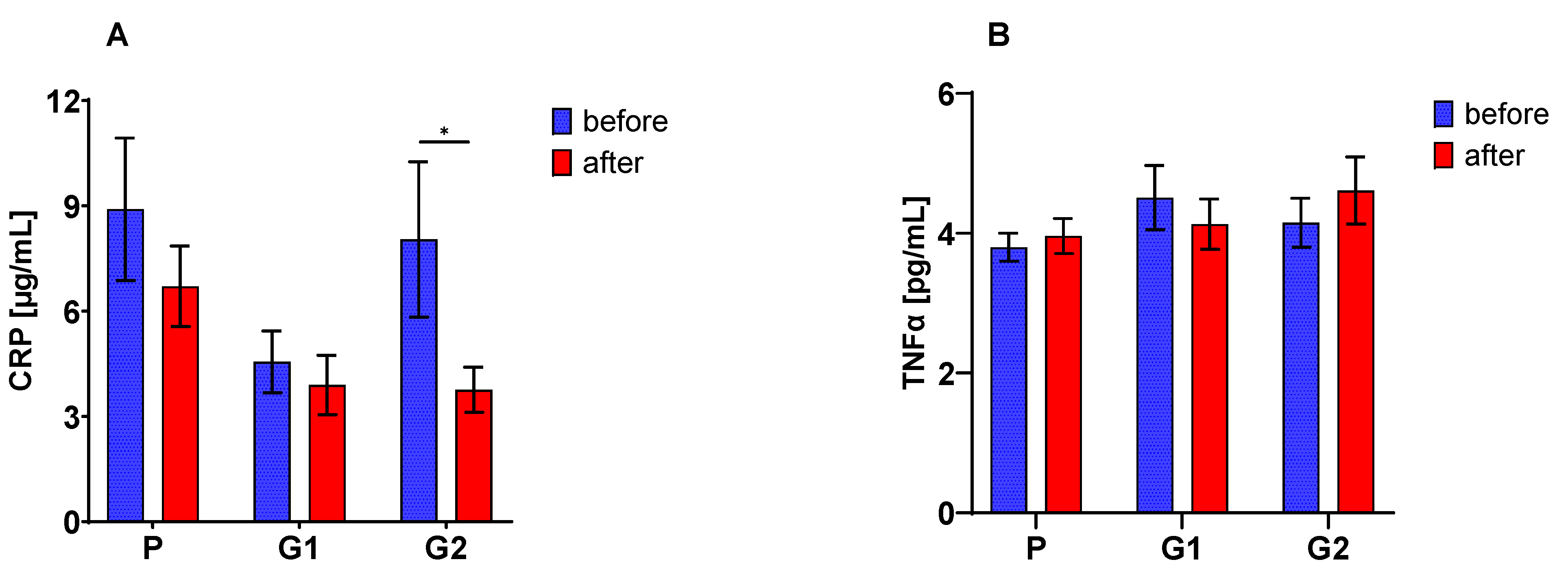
3.5. Blood Serum Immunological Parameters
3.6. Peripheral Blood Redox Balance Parameters
3.7. The Fecal Number of LAB and SCFA Concentration
3.8. Fisher’s Linear Discriminant Analysis (FLD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Bai, Y.; Xie, P.; Fang, J.; Wang, X.; Hou, X.; Tian, D.; Wang, C.; Liu, Y.; Sha, W.; et al. Chronic Gastritis in China: A National Multi-Center Survey. BMC Gastroenterol. 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, M.J.; Atherton, J.C. Helicobacter Pylori Persistence: Biology and Disease. J. Clin. Investig. 2004, 113, 321–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filip, R.; Huk, J.; Jarosz, B.; Skrzydło-Radomańska, B. Endoscopic and histological verification of upper GI tract side effects after long-term therapy with alendronate and strontium ranelate. Gastroenterol. Rev. Przegląd Gastroenterol. 2009, 4, 23–30. [Google Scholar]
- Wolf, E.-M.; Plieschnegger, W.; Geppert, M.; Wigginghaus, B.; Höss, G.M.; Eherer, A.; Schneider, N.I.; Hauer, A.; Rehak, P.; Vieth, M.; et al. Changing Prevalence Patterns in Endoscopic and Histological Diagnosis of Gastritis? Data from a Cross-Sectional Central European Multicentre Study. Dig. Liver Dis. 2014, 46, 412–418. [Google Scholar] [CrossRef]
- Nagata, N.; Niikura, R.; Aoki, T.; Sakurai, T.; Moriyasu, S.; Shimbo, T.; Sekine, K.; Okubo, H.; Watanabe, K.; Yokoi, C.; et al. Effect of Proton-Pump Inhibitors on the Risk of Lower Gastrointestinal Bleeding Associated with NSAIDs, Aspirin, Clopidogrel, and Warfarin. J. Gastroenterol. 2015, 50, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. IJMS 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Tanaka, Y.; Suzuki, T.; Mizushima, T. Protective Effect of β-(1,3 → 1,6)-d-Glucan against Irritant-Induced Gastric Lesions. Br. J. Nutr. 2011, 106, 475–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The Effect of Oat β -Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and ApoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M.S.; Jenkins, A.L.; Prudence, K.; Johnson, J.; Duss, R.; Chu, Y.; Steinert, R.E. Effect of Adding Oat Bran to Instant Oatmeal on Glycaemic Response in Humans—A Study to Establish the Minimum Effective Dose of Oat β-Glucan. Food Funct. 2018, 9, 1692–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetvicka, V.; Vannucci, L.; Sima, P. β-glucan as a New Tool in Vaccine Development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef]
- Pan, W.; Hao, S.; Zheng, M.; Lin, D.; Jiang, P.; Zhao, J.; Shi, H.; Yang, X.; Li, X.; Yu, Y. Oat-Derived β-Glucans Induced Trained Immunity Through Metabolic Reprogramming. Inflammation 2020, 43, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zou, S.; Xu, H.; Liu, Q.; Song, J.; Xu, M.; Xu, X.; Zhang, L. The Linear Structure of β-Glucan from Baker’s Yeast and Its Activation of Macrophage-like RAW264.7 Cells. Carbohydr. Polym. 2016, 148, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C.; Michaud, P. New developments and prospective applications for beta (1,3) glucans. Recent Pat. Biotechnol. 2007, 1, 59–73. [Google Scholar] [CrossRef]
- Harasym, J.; Suchecka, D.; Gromadzka-Ostrowska, J. Effect of Size Reduction by Freeze-Milling on Processing Properties of Beta-Glucan Oat Bran. J. Cereal. Sci. 2015, 61, 119–125. [Google Scholar] [CrossRef]
- Harasym, J.; Brach, J.; Czarnota, J.L.; Stechman, M.; Słabisz, A.; Kowalska, A.; Chorowski, M.; Winkowski, M.; Madera, A. A Kit and a Method of Producing Beta-Glucan, Insoluble Food Fibre as Well as a Preparation of Oat Proteins. European Patent EP2515672B1, 6 July 2016. [Google Scholar]
- Harasym, J.; Gromadzka-Ostrowska, J. PL226915B1 Method for Obtaining Cereal Beta-Glucan with Low Molecular Weight. Method for Obtaining Cereal Beta-Glucan with Low Molecular Weight. PL226915. 2017. Available online: https://grab.uprp.pl (accessed on 25 June 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to Oat Beta Glucan and Lowering Blood Cholesterol and Reduced Risk of (Coronary) Heart Disease Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1885. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis: The Updated Sydney System. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Rebrin, I.; Forster, M.J.; Sohal, R.S. Effects of Age and Caloric Intake on Glutathione Redox State in Different Brain Regions of C57BL/6 and DBA/2 Mice. Brain Res. 2007, 1127, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipponen, P.; Maaroos, H.-I. Chronic Gastritis. Scand. J. Gastroenterol. 2015, 50, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Arend, A.; Loime, L.; Roosaar, P.; Soom, M.; Lõivukene, K.; Sepp, E.; Aunapuu, M.; Zilmer, K.; Selstam, G.; Zilmer, M. Helicobacter pylori substantially increases oxidative stress in indomethacin-exposed rat gastric mucosa. Medicina (Kaunas) 2005, 41, 343–347. [Google Scholar]
- Kwiecien, S.; Jasnos, K.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Brzozowski, B.; Mach, T.; Wojcik, D.; Brzozowski, T. Lipid peroxidation, reactive oxygen species and antioxidative factorsin the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress—Induced gastric injury. J. Physiol. Pharmacol. 2014, 65, 613–622. [Google Scholar]
- Suzuki, R.B. Different Risk Factors Influence Peptic Ulcer Disease Development in a Brazilian Population. WJG 2012, 18, 5404. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Sandhu, V.; Sood, N.; Sood, A.; Malhotra, V. Histopathological Analysis of Chronic Gastritis and Correlation of Pathological Features with Each Other and with Endoscopic Findings. PJP 2012, 3, 172–178. [Google Scholar] [CrossRef]
- Noris, P.; Melazzini, F.; Balduini, C.L. New Roles for Mean Platelet Volume Measurement in the Clinical Practice? Platelets 2016, 27, 607–612. [Google Scholar] [CrossRef]
- Akar, T. Can Mean Platelet Volume Indicate Helicobacter Positivity and Severity of Gastric Inflammation? An Original Study and Review of the Literature. ACC 2019, 58, 576–582. [Google Scholar] [CrossRef]
- Săsăran, M.O.; Meliţ, L.E.; Mocan, S.; Ghiga, D.V.; Dobru, E.D. Pediatric Gastritis and Its Impact on Hematologic Parameters. Medicine 2020, 99, e21985. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging Regulatory Paradigms in Glutathione Metabolism. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 122, pp. 69–101. [Google Scholar] [CrossRef] [Green Version]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione Peroxidases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free. Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) Supplementation in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Insulin Resistance, Endothelial Dysfunction, Genotoxicity, Muscle Strength, and Cognition: Results of a Pilot Clinical Trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef] [PubMed]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione Levels in Human Tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in Health and Diseases. In Glutathione System and Oxidative Stress in Health and Disease; Dulce Bagatini, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.C. Dysregulation of Glutathione Synthesis in Liver Disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Kopiasz, Ł.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Żyła, E.; Kamola, D.; Oczkowski, M.; Królikowski, T.; Gromadzka-Ostrowska, J. Time-Dependent Indirect Antioxidative Effects of Oat Beta-Glucans on Peripheral Blood Parameters in the Animal Model of Colon Inflammation. Antioxidants 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Suchecka, D.; Błaszczyk, K.; Harasym, J.; Gudej, S.; Wilczak, J.; Gromadzka-Ostrowska, J. Impact of Purified Oat 1-3,1-4-β-d-Glucan of Different Molecular Weight on Alleviation of Inflammation Parameters during Gastritis. J. Funct. Foods 2017, 28, 11–18. [Google Scholar] [CrossRef]
- Cano-Garrido, O.; Seras-Franzoso, J.; Garcia-Fruitós, E. Lactic Acid Bacteria: Reviewing the Potential of a Promising Delivery Live Vector for Biomedical Purposes. Microb. Cell Fact. 2015, 14, 137. [Google Scholar] [CrossRef] [Green Version]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The Food-Gut Axis: Lactic Acid Bacteria and Their Link to Food, the Gut Microbiome and Human Health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The Effect of Low or High Molecular Weight Oat Beta-Glucans on the Inflammatory and Oxidative Stress Status in the Colon of Rats with LPS-Induced Enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in Fecal Short-Chain Fatty Acids in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e14513. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormsby, M.J.; Johnson, S.A.; Carpena, N.; Meikle, L.M.; Goldstone, R.J.; McIntosh, A.; Wessel, H.M.; Hulme, H.E.; McConnachie, C.C.; Connolly, J.P.R.; et al. Propionic Acid Promotes the Virulent Phenotype of Crohn’s Disease-Associated Adherent-Invasive Escherichia Coli. Cell Rep. 2020, 30, 2297–2305.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameters | Group | Mean ± SEM | Min | Max |
|---|---|---|---|---|
| Age (years) | Placebo | 50.75 ± 3.61 | 23.00 | 68.00 |
| G1 | 46.00 ± 3.82 | 23.00 | 74.00 | |
| G2 | 50.41 ± 3.24 | 27.00 | 68.00 | |
| Height [cm] | Placebo | 170.88 ± 2.19 | 156.00 | 183.00 |
| G1 | 169.93 ± 2.10 | 152.00 | 178.00 | |
| G2 | 171.41 ± 2.19 | 156.00 | 184.00 | |
| Weight [kg] | Placebo | 86.63 ± 2.37 | 73.20 | 109.60 |
| G1 | 79.93 ± 3.95 | 54.00 | 116.50 | |
| G2 | 79.17 ± 3.56 | 64.90 | 113.10 | |
| BMI [kg/m2] | Placebo | 29.82 ± 1.00 | 23.43 | 38.83 |
| G1 | 27.72 ± 1.26 | 17.04 | 36.80 | |
| G2 | 26.88 ± 0.93 | 20.97 | 36.51 |
| Parameters | Group | Before Treatment | After Treatment |
|---|---|---|---|
| WBC × 103/µL | Placebo | 6.22 ± 0.34 | 5.81 ± 0.27 |
| G1 | 6.36 ± 0.45 | 6.25 ± 0.47 | |
| G2 | 5.90 ± 0.20 | 6.20 ± 0.30 | |
| RBC × 106/µL | Placebo | 4.94 ± 0.05 | 4.91 ± 0.05 |
| G1 | 5.03 ± 0.10 | 4.92 ± 0.07 | |
| G2 | 4.86 ± 0.08 | 4.91 ± 0.08 | |
| Hemoglobin [g/dL] | Placebo | 14.51 ± 0.38 | 14.48 ± 0.27 |
| G1 | 15.25 ± 0.29 | 14.97 ± 0.29 | |
| G2 | 14.71 ± 0.27 | 14.86 ± 0.30 | |
| Hematocrit [%] | Placebo | 42.84 ± 0.87 | 42.69 ± 0.52 |
| G1 | 44.18 ± 0.68 | 43.37 ± 0.77 | |
| G2 | 42.60 ± 0.72 | 43.08 ± 0.73 | |
| Lymphocytes × 103/µL | Placebo | 1.97 ± 0.14 | 1.93 ± 0.13 |
| G1 | 1.95 ± 0.12 | 1.93 ± 0.11 | |
| G2 | 1.83 ± 0.10 | 2.08 ± 0.09 | |
| Platelets × 103/µL | Placebo | 204.19 ± 9.5 | 199.94 ± 8.07 |
| G1 | 213.23 ± 16.7 | 227.57 ± 19.0 | |
| G2 | 236.65 ± 13.2 * | 250.24 ± 13.6 * |
| Parameters | Group | Before Treatment | After Treatment |
|---|---|---|---|
| Creatinine [mg/dL] | Placebo | 0.74 ± 0.02 | 0.78 ± 0.03 |
| G1 | 0.81 ± 0.03 | 0.83 ± 0.04 | |
| G2 | 0.76 ± 0.04 | 0.78 ± 0.04 | |
| Bilirubin [mg/dL] | Placebo | 0.64 ± 0.08 | 0.65 ± 0.05 |
| G1 | 0.71 ± 0.12 | 0.64 ± 0.09 | |
| G2 | 0.65 ± 0.09 | 0.80 ± 0.13 | |
| ALT [U/L] | Placebo | 33.30 ± 4.50 | 32.68 ± 6.70 |
| G1 | 24.77 ± 3.00 | 23.24 ± 3.10 | |
| G2 | 26.82 ± 1.90 | 26.59 ± 2.19 | |
| AST [U/L] | Placebo | 25.63 ± 1,48 | 26.37 ± 4.00 |
| G1 | 28.48 ± 4.69 | 26.14 ± 3.90 | |
| G2 | 22.47 ± 1.12 | 22.82 ± 1.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudej, S.; Filip, R.; Harasym, J.; Wilczak, J.; Dziendzikowska, K.; Oczkowski, M.; Jałosińska, M.; Juszczak, M.; Lange, E.; Gromadzka-Ostrowska, J. Clinical Outcomes after Oat Beta-Glucans Dietary Treatment in Gastritis Patients. Nutrients 2021, 13, 2791. https://doi.org/10.3390/nu13082791
Gudej S, Filip R, Harasym J, Wilczak J, Dziendzikowska K, Oczkowski M, Jałosińska M, Juszczak M, Lange E, Gromadzka-Ostrowska J. Clinical Outcomes after Oat Beta-Glucans Dietary Treatment in Gastritis Patients. Nutrients. 2021; 13(8):2791. https://doi.org/10.3390/nu13082791
Chicago/Turabian StyleGudej, Sylwia, Rafał Filip, Joanna Harasym, Jacek Wilczak, Katarzyna Dziendzikowska, Michał Oczkowski, Małgorzata Jałosińska, Małgorzata Juszczak, Ewa Lange, and Joanna Gromadzka-Ostrowska. 2021. "Clinical Outcomes after Oat Beta-Glucans Dietary Treatment in Gastritis Patients" Nutrients 13, no. 8: 2791. https://doi.org/10.3390/nu13082791
APA StyleGudej, S., Filip, R., Harasym, J., Wilczak, J., Dziendzikowska, K., Oczkowski, M., Jałosińska, M., Juszczak, M., Lange, E., & Gromadzka-Ostrowska, J. (2021). Clinical Outcomes after Oat Beta-Glucans Dietary Treatment in Gastritis Patients. Nutrients, 13(8), 2791. https://doi.org/10.3390/nu13082791