Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade
Abstract
1. Introduction
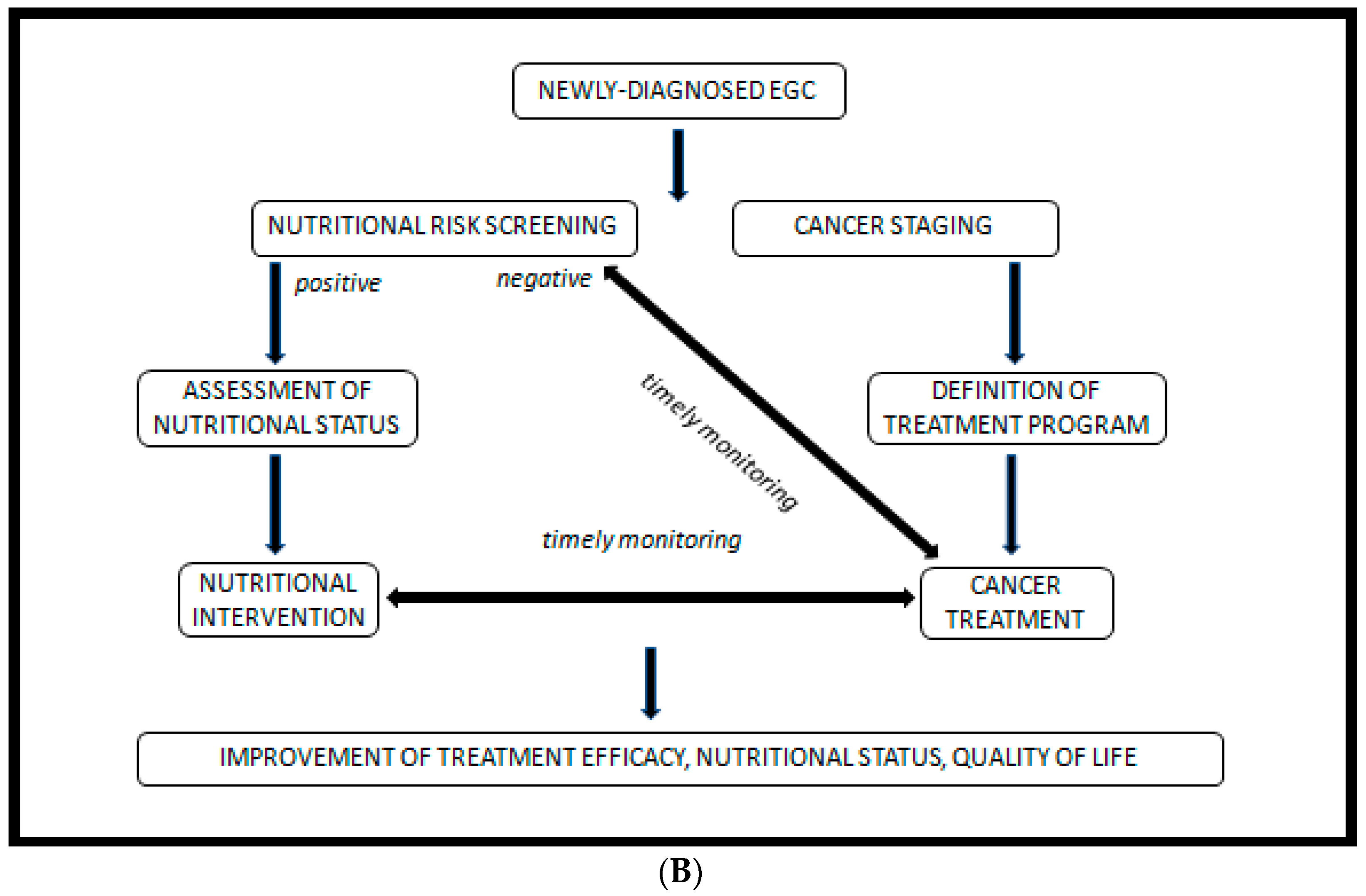
2. Malnutrition: Definition, Screening Methods, Diagnosis, Impact on GC Treatment and Prognosis
- -
- An initial screening, preferably performed by the oncologist at the first visit.
- -
- In case of malnutrition risk, assessment by a nutrition specialist.
- -
- Early and personalized nutritional intervention, if indicated.
- -
- Regular follow-up of to monitor intervention efficacy.
3. Material and Methods
4. Results
5. Nutritional Support Strategies
5.1. Perioperative Setting
5.1.1. The Impact of Malnutrition and Nutritional Interventions in the Perioperative Setting
5.1.2. Dietary Counselling
5.1.3. ONS Intervention
5.1.4. EN Intervention
5.1.5. PN Intervention
5.1.6. Exercise and Nutritional Interventions
5.2. Metastatic Setting
The Impact of Malnutrition and Nutritional Interventions in the Metastatic Setting
5.3. Palliative Setting
5.4. Elderly
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LEGEND | ||
| ADS: Adherence to Dietary Guideline Scale | FOS: Fructo-oligosaccharides | PES: pancreatic enzyme supplementation |
| AEs: Adverse Events | FT: feeding tube | PG: proximal gastrectomy |
| ALB: albumin | FU: follow-up | PGE2: prostaglandin E2 |
| AT: adjuvant therapy | GC: gastric cancer | PI: Prognostic Index |
| BC: body composition | GEC: gastroesophageal cancer | PN: parenteral nutrition |
| BMI: Body Mass Index | GEJC: GE junction cancer | PNI: Prognostic Nutrition Index |
| BWL: Body Weight Loss | GPS: Glasgow Prognostic Score | PO: post-operative |
| CG: control group | H: height | POD: post-operative day |
| CK: cytokines | h: hour | PPDI: patient participation-based dietary intervention |
| CRP: C reactive protein | hEN: home EN | preO: pre-operative |
| CT: chemotherapy | IEN: immune-EN | PPG: pylorus-preserving gastrectomy |
| d: day | IEEN: immune-enhanced EN | PSS: Patient Satisfaction Scale |
| DG: distal gastrectomy | IFN-γ: interferon-γ | QoL: quality of life |
| DHA: docosahexaenoic acid | IG: intervention group | RT: radiotherapy |
| DSS: dietary symptom scale | Ig: immunoglobulin | SDK: Scale of Dietary Knowledge |
| E: esophagus | IL-2, IL-6: Interleukin-2, Interleukin-6 | SEN: standard enteral nutrition |
| EC: esophageal cancer | INA: instant nutritional assessment | SII: Systemic Immune-Inflammation Index |
| ECOG-PS: Eastern Cooperative Oncology Group- Performance Scale | KPS: Karnofsky Performance Status | SIRS: systemic inflammatory response syndrome |
| ED: elemental diet | LBM: lean body mass | SOC: standard of care |
| EEIN: enteral eco-immunonutrition | LOHS: length of hospital stays | sPN: supplemental parenteral nutrition |
| EN: enteral nutrition | m: month | STG: subtotal gastrectomy |
| EON: Early Oral Nutrition | MAC: mid arm circumference | TNF-α: tumor necrosis factor-α |
| EPA: eicosapentanoic acid | MAMC: MA muscle circumference | TG: total gastrectomy |
| FACT-G: Functional Assessment Cancer Therapy- General | MMP-2, MMP-9: matrix metalloproteinase 2, matrix metalloproteinase -9 | tPN: total parenteral nutrition |
| FE: fiber-enriched | NAT: neoadjuvant therapy | TST: triceps skin fold thickness |
| FEP: fiber and probiotic-enriched | NLR: Neutrophil/Lymphocyte Ratio | W: weight |
| FF: fiber-free | NRS: Nutritional Risk Score-2002 | w: week |
| ↓: decrease | OS: overall survival | WL: weight loss |
| ↑: increase | PA: prealbumin | y: year |
| BMI: Body Mass Index | H: height | PYY: gut hormone peptide YY |
| CCK: cholecystokinin | IE: intensive dietary education arm | QoL: quality of life |
| CK: cytokines | IL-1, IL-6: interleukin-1, interleukin-6 | RT: radiotherapy |
| CT: chemotherapy | LOHS: length of hospital stays | SBD: Soft blended diet |
| d: day | m: month | SE: Simplified Dietary Education |
| EC: esophageal cancer | mGPS: modified Glasgow Prognostic Score | SFD: Semifluid diet |
| EN: enteral nutrition | MNA: Mini- Nutritional Assessment | SII: Systemic Immune-Inflammation Index |
| EON: Early Oral Nutrition | NLR: Neutrophil-to-Lymphocyte Ratio | sPN: supplemental parenteral nutrition |
| FU: follow-up | ONS: oral nutritional supplement | STG: subtotal gastrectomy |
| GC: gastric cancer | PI: Prognostic Index | TG: total gastrectomy |
| GEC: gastroesophageal cancer | PNI: Prognostic Nutrition Index | TNF-α:Tumor Necrosis Factor-α |
| GEJC: gastroesophageal junction cancer | PLR: Platelet-to-Lymphocyte Ratio | tPN: total parenteral nutrition |
| GIP: gastric inhibitory polypeptide | PO: post-operative | W: weight |
| GLP-1: glucagon-like peptide-1 | POD: post-operative day | WL: weight loss |
| GPS: Glasgow Prognostic Score, mGPS: modified Glasgow Prognostic Score | preO: pre-operative | Y: year |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Johnston, F.M.; Beckman, M. Updates on management of gastric cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef]
- Rice, T.W.; Ishwaran, H.; Hofstetter, W.L.; Kelsen, D.P.; Apperson-Hansen, C.; Blackstone, E.H.; for the Worldwide Esophageal Cancer Collaboration. Investigators Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis. Esophagus 2016, 29, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Linee Guida AIOM, Neoplasie Dello Stomaco e della Giunzione Esofago-Gastrica. 2020. Available online: https://www.aiom.it/linee-guida-aiom-2020-neoplasie-dello-stomaco-e-della-giunzione-esofago-gastrica/ (accessed on 28 June 2021).
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2018, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aprile, G.; Basile, D.; Giaretta, R.; Schiavo, G.; La Verde, N.; Corradi, E.; Monge, T.; Agustoni, F.; Stragliotto, S. The clinical value of nutritional care before and during active cancer treatment. Nutrients 2021, 13, 1196. [Google Scholar] [CrossRef]
- Dijksterhuis, W.; Latenstein, A.; van Kleef, J.J.; Verhoeven, R.; de Vries, J.; Slingerland, M.; Steenhagen, E.; Heisterkamp, J.; Timmermans, L.M.; de van der Schueren, M.; et al. Cachexia and dietetic inter-ventions in patients with esophagogastric cancer: A multicenter cohort study. J. Natl. Compr. Cancer Netw. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Kuwada, K.; Kuroda, S.; Kikuchi, S.; Yoshida, R.; Nishizaki, M.; Kagawa, S.; Fujiwara, T. Clinical impact of sarcopenia on gastric cancer. Anticancer Res. 2019, 39, 2241–2249. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. 2019, 39, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, E.; Buoro, V.; Cinausero, M.; Caccialanza, R.; Turri, A.; Fanotto, V.; Basile, D.; Vitale, M.G.; Ermacora, P.; Cardellino, G.G.; et al. Sarcopenia in gastric cancer: When the loss costs too much. Gastric Cancer 2017, 20, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, T.; Obama, K.; Sakai, Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl. Gastroenterol. Hepatol. 2020, 5, 22. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2016, 36, 11–48. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Hur, H.; Kim, S.G.; Shim, J.H.; Song, K.Y.; Kim, W.; Park, C.H.; Jeon, H.M. Effect of early oral feeding after gastric cancer surgery: A result of randomized clinical trial. Surgery 2011, 149, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, K.; Tsujinaka, T.; Fujita, J.; Miyashiro, I.; Imamura, H.; Kimura, Y.; Kobayashi, K.; Kurokawa, Y.; Shimokawa, T.; Furukawa, H.; et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br. J. Surg. 2012, 99, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ling, W.; Shen, Z.Y.; Jin, X.; Cao, H. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J. Dig. Dis. 2012, 13, 401–406. [Google Scholar] [CrossRef]
- Marano, L.; Porfidia, R.; Pezzella, M.; Grassia, M.; Petrillo, M.; Esposito, G.; Braccio, B.; Gallo, P.; Boccardi, V.; Cosenza, A.; et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: A prospective randomized study. Ann. Surg. Oncol. 2013, 20, 3912–3918. [Google Scholar] [CrossRef]
- Kim, H.; Suh, E.E.; Lee, H.J.; Yang, H.K. The effects of patient participation-based dietary intervention on nutritional and functional status for patients with gastrectomy: A randomized controlled trial. Cancer Nurs. 2014, 37, E10–E20. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, W.; Chen, J.; Yang, D.; Yan, R.; Cai, Q. A prospective, randomized, controlled study of ω-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumors. Nutr. J. 2014, 24, 13–25. [Google Scholar] [CrossRef]
- Ding, D.; Feng, Y.; Song, B.; Gao, S.; Zhao, J. Effects of preoperative and postoperative enteral nutrition on postoperative nutritional status and immune function of gastric cancer patients. Turk. J. Gastroenterol. 2015, 26, 181–185. [Google Scholar] [CrossRef]
- Wang, F.; Hou, M.X.; Wu, X.L.; Bao, L.D.; Dong, P.D. Impact of enteral nutrition on postoperative immune function and nutritional status. Genet. Mol. Res. 2015, 14, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, H.Y.; Guo, S.H.; Sun, P.; Gong, F.M.; Jia, B.Q. Impact of early enteral and parenteral nutrition on prealbumin and high-sensitivity C-reactive protein after gastric surgery. Genet. Mol. Res. 2015, 14, 7130–7135. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Uitdehaag, M.; Spaander, M.C.W.; Van Steenbergen-Langeveld, S.; De Vos, P.; Berkhout, M.; Lamers, C.H.J.; Rümke, H.; Tilanus, H.W.; Siersema, P.D.; et al. Improved body weight and performance status and reduced serum PGE2levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction. J. Cachex Sarcopenia Muscle 2015, 6, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Bowrey, D.J.; Baker, M.; Halliday, V.; Thomas, A.L.; Pulikottil-Jacob, R.; Smith, K.; Morris, T.; Ring, A. A randomised controlled trial of six weeks of home enteral nutrition versus standard care after oesophagectomy or total gastrectomy for cancer: Report on a pilot and feasibility study. Trials 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Nishikawa, K.; Kishi, K.; Inoue, K.; Matsuyama, J.; Akamaru, Y.; Kimura, Y.; Tamura, S.; Kawabata, R.; Kawada, J.; et al. Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: A randomized controlled clinical trial. Ann. Surg. Oncol. 2016, 23, 2928–2935. [Google Scholar] [CrossRef]
- Ida, S.; Hiki, N.; Cho, H.; Sakamaki, K.; Ito, S.; Fujitani, K.; Takiguchi, N.; Kawashima, Y.; Nishikawa, K.; Sasako, M.; et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. BJS 2017, 104, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Klek, S.; Scislo, L.; Walewska, E.; Choruz, R.; Galas, A. Enriched enteral nutrition may improve short-term survival in stage IV gastric cancer patients: A randomized, controlled trial. Nutrition 2017, 36, 46–53. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Qi, Y. Effect of glutamine-enriched nutritional support on intestinal mucosal barrier function, MMP-2, MMP-9 and immune function in patients with advanced gastric cancer during perioperative chemotherapy. Oncol Lett. 2017, 14, 3606–3610. [Google Scholar] [CrossRef][Green Version]
- Zhao, R.; Wang, Y.; Huang, Y.; Cui, Y.; Xia, L.; Rao, Z.; Zhou, Y.; Wu, X. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: A prospective randomized and controlled trial. Medicine 2017, 96, e8418. [Google Scholar] [CrossRef]
- Baker, M.L.; Halliday, V.; Robinson, P.; Smith, K.; Bowrey, D.J. Nutrient intake and contribution of home enteral nutrition to meeting nutritional requirements after oesophagectomy and total gastrectomy. Eur. J. Clin. Nutr. 2017, 71, 1121–1128. [Google Scholar] [CrossRef]
- Hatao, F.; Chen, K.Y.; Wu, J.M.; Wang, M.Y.; Aikou, S.; Onoyama, H.; Shimizu, N.; Fukatsu, K.; Seto, Y.; Lin, M.T. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbecks Arch. Surg. 2017, 402, 203–211. [Google Scholar] [CrossRef]
- Xie, F.L.; Wang, Y.Q.; Peng, L.F.; Lin, F.Y.; He, Y.L.; Jiang, Z.Q. Beneficial effect of educational and nutritional in-tervention on the nutritional status and compliance of gastric cancer patients undergoing chemotherapy: A randomized trial. Nutr. Cancer 2017, 69, 762–771. [Google Scholar] [CrossRef]
- Catarci, M.; Berlanda, M.; Grassi, G.B.; Masedu, F.; Guadagni, S. Pancreatic enzyme supplementation after gastrectomy for gastric cancer: A randomized controlled trial. Gastric Cancer. 2018, 21, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Scislo, L.; Pach, R.; Nowak, A.; Walewska, E.; Gadek, M.; Brandt, P.; Puto, G.; Szczepanik, A.M.; Kulig, J. The Impact of Postoperative Enteral Immunonutrition on Postoperative Complications and Survival in Gastric Cancer Patients—Randomized Clinical Trial. Nutr. Cancer. 2018, 70, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Lee, H.J.; Na, J.R.; Kim, W.G.; Han, D.S.; Park, S.H.; Hong, H.; Choi, Y.; Ahn, H.S.; Suh, Y.S.; et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: A prospective randomized trial. Surgery 2018, 164, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Oki, E.; Tanizawa, Y.; Suzuki, Y.; Aikou, S.; Kunisaki, C.; Tsuchiya, T.; Fukushima, R.; Doki, Y.; Natsugoe, S.; et al. Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: A Japanese multicenter, randomized controlled trial. Surg. Today 2018, 48, 865–874. [Google Scholar] [CrossRef]
- Jin, Y.; Yong, C.; Ren, K.; Li, D.; Yuan, H. Effects of Post-Surgical Parenteral Nutrition on Patients with Gastric Cancer. Cell. Physiol. Biochem. 2018, 49, 1320–1328. [Google Scholar] [CrossRef]
- Kimura, Y.; Nishikawa, K.; Kishi, K.; Inoue, K.; Matsuyama, J.; Akamaru, Y.; Tamura, S.; Kawada, J.; Kawase, T.; Kawabata, R.; et al. Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann. Gastroenterol. Surg. 2019, 3, 648–656. [Google Scholar] [CrossRef]
- Wang, W.K.; Tu, C.Y.; Shao, C.X.; Chen, W.; Zhou, Q.Y.; Zhu, J.D.; Xu, H.T. Impact of enhanced recovery after surgery on postoperative rehabilitation, inflammation, and immunity in gastric carcinoma patients: A randomized clinical trial. Braz. J. Med. Biol. Res. 2019, 52, e8265. [Google Scholar] [CrossRef]
- Feijó, P.M.; Rodrigues, V.D.; Viana, M.S.; dos Santos, M.P.; Abdelhay, E.; Viola, J.; de Pinho, N.B.; Martucci, R.B. Effects of ω-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: A randomized controlled trial. Nutrition 2018, 61, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Yoshikawa, T.; Ida, S.; Cho, H.; Sakamaki, K.; Ito, Y.; Fujitani, K.; Takiguchi, N.; Kawashima, Y.; Nishikawa, K.; et al. Effects of perioperative Eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J. Cancer 2019, 10, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Li, H.; Li, S. Effects of neoadjuvant chemotherapy combined with enteral nutrition on perioperative immunity, inflammation and intestinal flora in gastric cancer patients. J. BUON 2019, 24, 1113–1119. [Google Scholar] [PubMed]
- Zheng, C.; Chen, T.; Wang, Y.; Gao, Y.; Kong, Y.; Liu, Z.; Deng, X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J. Cancer 2019, 10, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, Y.; Mochiki, E.; Yanai, M.; Suzuki, M.; Yanoma, T.; Kimura, A.; Kogure, N.; Ogata, K.; Kuwano, H. A pro-spective pilot study of an elemental nutritional supplement for prevention of oral mucositis during S-1 adjuvant chemo-therapy for gastric cancer. Surg. Oncol. 2019, 29, 97–101. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, S.; Ding, Z.; Zhao, P. Does early postoperative enteral ecoimmunonutrition enhance intestinal function in gastric cancer. Asia Pac. J. Clin. Nutr. 2020, 29, 469–475. [Google Scholar]
- Wang, H.; Hu, X.; Chen, S.; Xiang, J.; Yang, Z.; Zhou, Z.; Chen, Y.; Lin, Y.; Chen, Y.; Peng, J. Functional jejunal interposition versus Roux-en-Y anastomosis after total gastrectomy for gastric cancer: A prospective randomized clinical trial. Surg. Oncol. 2020, 34, 236–244. [Google Scholar] [CrossRef]
- Meng, Q.; Tan, S.; Jiang, Y.; Han, J.; Xi, Q.; Zhuang, Q.; Wu, G. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin. Nutr. 2021, 40, 40–46. [Google Scholar] [CrossRef]
- Washington, K. 7th edition of the AJCC cancer staging manual: Stomach. Ann. Surg. Oncol. 2010, 17, 3077–3079. [Google Scholar] [CrossRef]
- GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group; Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S.; Ohashi, Y.; Pignon, J.P.; Rougier, P.; Sakamoto, J.; Sargent, D.; et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA 2010, 303, 1729–1737. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Takahari, D.; Hamaguchi, T.; Yoshimura, K.; Katai, H.; Ito, S.; Fuse, N.; Kinoshita, T.; Yasui, H.; Terashima, M.; Goto, M.; et al. Feasibility study of adjuvant chemotherapy with S1 plus cis-platin for gastric cancer. Cancer Chemother. Pharmacol. 2011, 67, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Yoshikawa, T. Adjuvant therapy for locally advanced gastric cancer. Surg. Today 2017, 47, 1295–1302. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, D.H.; Kim, S.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Choi, M.G.; Sohn, T.S.; Noh, J.H.; et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J. Clin. Oncol. 2012, 30, 268–273. [Google Scholar] [CrossRef]
- Segami, K.; Aoyama, T.; Kano, K.; Maezawa, Y.; Nakajima, T.; Ikeda, K.; Sato, T.; Fujikawa, H.; Hayashi, T.; Yamada, T.; et al. Risk factors for severe weight loss at 1 month after gastrectomy for gastric cancer. Asian J. Surg. 2017, 41, 349–355. [Google Scholar] [CrossRef]
- Mirkin, K.A.; Luke, F.E.; Gangi, A.; Pimiento, J.M.; Jeong, D.; Hollenbeak, C.S.; Wong, J. Sarcopenia related to neoad-juvant chemotherapy and perioperative outcomes in resected gastric cancer: A multi-institutional analysis. J. Gastrointest. Oncol. 2017, 8, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Tan, B.H.; Cui, H.; Bhalla, A.; Fearon, K.C.; Parsons, S.L.; Catton, J.A.; Lobo, D.N. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin. Nutr. 2012, 31, 74–77. [Google Scholar] [CrossRef]
- Palmela, C.; Velho, S.; Agostinho, L.; Branco, F.; Santos, M.; Santos, M.P.C.; Oliveira, M.H.; Strecht, J.; Maio, R.; Cravo, M.; et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J. Gastric Cancer 2017, 17, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, N.; Migita, K.; Matsumoto, S.; Wakatsuki, K.; Kunishige, T.; Nakade, H.; Miyao, S.; Sho, M. Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg. Today 2019, 49, 1022–1028. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J.; Zhang, J.; Zou, S.; Luo, R.; Xu, H.; Huang, B. The impact of preoperative underweight status on postoperative complication and survival outcome of gastric cancer patients: A systematic review and meta-analysis. Nutr. Cancer 2019, 70, 1254–1263. [Google Scholar]
- Yang, Y.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Ma, B.; Wang, Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 1176–1182. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Pietrantonio, F.; Rulli, E.; Poli, D.; Berenato, R.; Caporale, M.; Bajetta, E.; Floriani, I.; Catena, L.; Schiavo, M.; et al. Impact on survival of timing and duration of adjuvant chemotherapy in radically resected gastric cancer. Tumori J. 2016, 102, e15–e19. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.S.; Beom, S.-H.; Rha, S.Y.; Chung, H.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.-A.; Heo, S.J.; et al. Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: Single-center study from the CLASSIC trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, W.; Lin, J. Delaying adjuvant chemotherapy in advanced gastric cancer patients: Risk factors and its impact on survival outcome. Curr. Probl. Cancer 2020, 44, 100577. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Fujitani, K.; Tsujinaka, T.; Yamamoto, K.; Hirao, M.; Sekimoto, M. Skeletal muscle loss after total gastrectomy, exacerbated by adjuvant chemotherapy. Gastric Cancer 2014, 18, 382–389. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lu, L.; Fan, K.-H.; Wang, D.-H.; Fu, W.-H. Proximal gastrectomy versus total gastrectomy for adenocarcinoma of the esophagogastric junction: A meta-analysis. J. Comp. Eff. Res. 2019, 8, 753–766. [Google Scholar] [CrossRef]
- Tanioka, T.; Waratchanont, R.; Fukuyo, R.; Saito, T.; Umebayashi, Y.; Kanemoto, E.; Kobayashi, K.; Nakagawa, M.; In-okuchi, M. Surgical and nutritional outcomes of laparoscopic proximal gastrectomy versus total gastrectomy: A me-ta-analysis. Surg. Endosc. 2020, 34, 1061–1069. [Google Scholar] [CrossRef]
- Friess, H.; Tempia-Caliera, A.; Cammerer, G.; Büchler, M. Indication for pancreatic enzyme substitution following gastric resection. Pancreatology 2001, 1, 41–48. [Google Scholar] [CrossRef]
- Davis, J.L.; Ripley, R.T. Postgastrectomy syndromes and nutritional considerations following gastric surgery. Surg. Clin. N. Am. 2017, 97, 277–293. [Google Scholar] [CrossRef]
- Reece, L.; Hogan, S.; Allman-Farinelli, M.; Carey, S. Oral nutrition interventions in patients undergoing gastrointestinal surgery for cancer: A systematic literature review. Support. Care Cancer 2020, 28, 5673–5691. [Google Scholar] [CrossRef]
- Hsu, P.-I.; Chuah, S.-K.; Lin, J.-T.; Huang, S.-W.; Lo, J.-C.; Rau, K.-M.; Chen, I.-S.; Hsu, H.-Y.; Sheu, B.-S.; Chang, W.-K.; et al. Taiwan nutritional consensus on the nutrition management for gastric cancer patients receiving gastrectomy. J. Formos. Med. Assoc. 2019, 120, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, K.; Zhang, X.; Li, K. Meta-analysis of preoperative oral nutritional supplements for patients with gastric cancer: East Asian experience. Eur. J. Clin. Nutr. 2019, 74, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Song, G.M.; Tian, X.; Liang, H.; Yi, L.J.; Zhou, J.G.; Zeng, Z.; Shuai, T.; Ou, Y.X.; Zhang, L.; Wang, Y. Role of enteral immunonutrition in patients undergoing surgery for gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Medicine 2015, 94, e1311. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, J.; Zhang, L.; Wu, J.; Zhan, Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: A systematic review and meta-analysis. BMC Gastroenterol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Mabvuure, N.T.; Roman, A.; Khan, O.A. Enteral immunonutrition versus standard enteral nutrition for patients undergoing oesophagogastric resection for cancer. Int. J. Surg. 2013, 11, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Manfredelli, S.; Delhorme, J.B.; Venkatasamy, A.; Gaiddon, C.; Brigand, C.; Rohr, S.; Romain, B. Could a feeding jeju-nostomy be integrated into a standardized preoperative management of oeso-gastric junction adenocarcinoma? Ann. Surg. Oncol. 2017, 24, 3324–3330. [Google Scholar] [CrossRef]
- Choi, A.H.; O’Leary, M.P.; Merchant, S.J.; Sun, V.; Chao, J.; Raz, D.J.; Kim, J.Y.; Kim, J. Complications of feeding je-junostomy tubes in patients with gastroesophageal cancer. J. Gastrointest. Surg. 2017, 21, 259–265. [Google Scholar] [CrossRef]
- Xin, F.; Mzee, S.A.S.; Botwe, G.; He, H.; Zhiyu, S.; Gong, C.; Said, S.T.; Jixing, C. Short-term evaluation of immune levels and nutritional values of EN versus PN in gastric cancer: A systematic review and a meta-analysis. World J. Surg. Oncol. 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 1997, 273 Pt 1, E122–E129. [Google Scholar] [CrossRef]
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. Metab. 1995, 268, E514–E520. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Nagatsuma, Y.; Fukuda, Y.; Hirao, M.; Nishikawa, K.; Miyamoto, A.; Ikeda, M.; Nakamori, S.; Sekimoto, M.; Fujitani, K.; et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sar-copenic patients with gastric cancer. Gastric Cancer 2017, 20, 913–918. [Google Scholar] [CrossRef]
- Glimelius, B.; Ekström, K.; Hoffman, K.; Graf, W.; Sjödén, P.O.; Haglund, U.; Svensson, C.; Enander, L.K.; Linné, T.; Sellström, H.; et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann. Oncol. 1997, 8, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Grothe, W.; Haerting, J.; Kleber, G.; Grothey, A.; Fleig, W.E. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 2006, 24, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Oki, E.; Makiyama, A.; Miyamoto, Y.; Kotaka, M.; Kawanaka, H.; Miwa, K.; Kabashima, A.; Noguchi, T.; Yuge, K.; Kashiwada, T.; et al. Trifluridine/tipiracil plus bevacizumab as a first-line treatment for elderly patients with metastatic colorectal cancer (KSCC1602): A multicenter phase II trial. Cancer Med. 2020, 10, 454–461. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gas-tro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Ar-beitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R.; et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef]
- Guimbaud, R.; Louvet, C.; Ries, P.; Ychou, M.; Maillard, E.; André, T.; Gornet, J.M.; Aparicio, T.; Nguyen, S.; Azzedine, A.; et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: A French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J. Clin. Oncol. 2014, 32, 3520–3526. [Google Scholar] [PubMed]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Phase III study of docetaxel and cisplatin plus fluorouracil com-pared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Cafferkey, C.; Goode, E.F.; Kouvelakis, K.; Hughes, D.J.; Reguera, P.; Kalaitzaki, E.; Peckitt, C.; Rao, S.; Watkins, D.; et al. Survival in advanced esophagogastric adenocarcinoma improves with use of multiple lines of therapy: Results from an analysis of more than 500 patients. Clin. Color. Cancer 2018, 17, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Thuss-Patience, P.C.; Kretzschmar, A.; Bichev, D.; Deist, T.; Hinke, A.; Breithaupt, K.; Dogan, Y.; Gebauer, B.; Schumacher, G.; Reichardt, P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 2011, 47, 2306–2314. [Google Scholar] [CrossRef]
- Ford, H.E.R.; Marshall, A.; Bridgewater, J.A.; Janowitz, T.; Coxon, F.Y.; Wadsley, J.; Mansoor, W.; Fyfe, D.; Madhusudan, S.; Middleton, G.W.; et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol. 2013, 15, 78–86. [Google Scholar] [CrossRef]
- Hironaka, S.; Ueda, S.; Yasui, H.; Nishina, T.; Tsuda, M.; Tsumura, T.; Sugimoto, N.; Shimodaira, H.; Tokunaga, S.; Moriwaki, T.; et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 Trial. J. Clin. Oncol. 2013, 31, 4438–4444. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastrooesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastrooesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Özgüroglu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- NCCN Guidelines, Gastric Cancer, Version 3.2021. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 28 June 2021).
- Hamamoto, Y.; Piao, Y.; Makiyama, A. Achieving sequential therapy in advanced gastric cancer: The importance of ap-propriate patient management for the elderly and/or those with ascites. Gastric Cancer 2020, 23, 363–372. [Google Scholar] [CrossRef]
- Lu, Z.; Fang, Y.; Liu, C.; Zhang, X.; Xin, X.; He, Y.; Cao, Y.; Jiao, X.; Sun, T.; Pang, Y.; et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: A phase III randomized controlled trial. J. Clin. Oncol. 2021, 39, 748–756. [Google Scholar] [CrossRef]
- Obling, S.R.; Wilson, B.V.; Pfeiffer, P.; Kjeldsen, J. Home parenteral nutrition increases fat free mass in patients with in-curable gastrointestinal cancer. Results of a randomized controlled trial. Clin. Nutr. 2019, 38, 182–190. [Google Scholar] [CrossRef]
- Ma, C.-J.; Huang, C.-W.; Yeh, Y.-S.; Tsai, H.-L.; Su, W.-C.; Chang, T.-K.; Sun, L.-C.; Shih, Y.-L.; Yu, F.-J.; Wu, D.-C.; et al. Supplemental home parenteral nutrition improved nutrition status with comparable quality of life in malnourished unresectable/metastatic gastric cancer receiving salvage chemotherapy. Support. Care Cancer 2020, 29, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef]
- Osman, H.; Shrestha, S.; Temin, S.; Ali, Z.V.; Corvera, R.A.; Ddungu, H.D.; De Lima, L.; Estevez-Diz, M.D.P.; Ferris, F.D.; Gafer, N.; et al. Palliative care in the global setting: ASCO resource-stratified practice guideline. J. Glob. Oncol. 2018, 4, 1–24. [Google Scholar] [CrossRef]
- Zagonel, V.; Torta, R.; Franciosi, V.; Brunello, A.; Biasco, G.; Cattaneo, D.; Cavanna, L.; Corsi, D.; Farina, G.; Fioretto, L.; et al. Early integration of palliative care in oncology practice: Results of the italian association of medical oncology (AIOM) survey. J. Cancer 2016, 7, 1968–1978. [Google Scholar] [CrossRef]
- Cotogni, P.; Stragliotto, S.; Ossola, M.; Collo, A.; Riso, S.; On Behalf of the Intersociety Italian Working Group for Nutritional Support in Cancer. The role of nutritional support for cancer patients in palliative care. Nutrients 2021, 13, 306. [Google Scholar] [CrossRef]
- Min, S.-H.; Son, S.-Y.; Jung, D.-H.; Lee, C.-M.; Ahn, S.-H.; Park, D.J.; Kim, H.-H. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann. Surg. Treat. Res. 2017, 93, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, R.B.; Gerdes, H.; Markowitz, A.J.; DiMaio, C.J.; Schattner, M.A. Carcinomatosis is not a contraindication to enteral stenting in selected patients with malignant gastric outlet obstruction. Gastrointest. Endosc. 2011, 73, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Is there a place for nutrition in palliative care? Support. Care Cancer 2020, 28, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Olguín, G.; Gutiérrez-Salmeán, G.; Posadas-Calleja, J.G.; Padilla-Rubio, M.F.; E Serralde-Zúñiga, A. The effect of enteral stimulation on the immune response of the intestinal mucosa and its application in nutritional support. Eur. J. Clin. Nutr. 2021, 1–7. [Google Scholar] [CrossRef]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hébuterne, X.; Pertkiewicz, M.; et al. ESPEN guidelines on parenteral nutrition: Home parenteral nutrition (HPN) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Mühlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN guideline on home parenteral nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef]
- Cotogni, P.; Monge, T.; Passera, R.; Brossa, L.; De Francesco, A. Clinical characteristics, and predictive factors of sur-vival of 761 cancer patients on home parenteral nutrition: A prospective, cohort study. Cancer Med. 2020, 9, 4686–4698. [Google Scholar] [CrossRef]
- Culine, S.; Chambrier, C.; Tadmouri, A.; Senesse, P.; Seys, P.; Radji, A.; Rotarski, M.; Balian, A.; Dufour, P. Home parenteral nutrition improves quality of life and nutritional status in patients with cancer: A French observational multi-centre study. Support. Care Cancer 2014, 22, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Senesse, P.; Tadmouri, A.; Culine, S.; Dufour, P.R.; Seys, P.; Radji, A.; Rotarski, M.; Balian, A.; Chambrier, C. A pro-spective observational study assessing home parenteral nutrition in patients with gastrointestinal cancer: Benefits for quality of life. J. Pain Symptom Manag. 2015, 49, 183–191.e2. [Google Scholar] [CrossRef] [PubMed]
- Cotogni, P.; De Carli, L.; Passera, R.; Amerio, M.L.; Agnello, E.; Fadda, M.; Ossola, M.; Monge, T.; De Francesco, A.; Bozzetti, F. Longitudinal study of quality of life in advanced cancer patients on home parenteral nutrition. Cancer Med. 2017, 6, 1799–1806. [Google Scholar] [CrossRef]
- Jatoi, A.; Foster, N.R.; Egner, J.R.; Burch, P.A.; Stella, P.J.; Rubin, J.; Dakhil, S.R.; Sargent, D.J.; Murphy, B.R.; Alberts, S.R. Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and stomach: A pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int. J. Oncol. 2010, 36, 601–606. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Pauligk, C.; Homann, N.; Hartmann, J.T.; Moehler, M.; Probst, S.; Rethwisch, V.; Stoehlma-cher-Williams, J.; Prasnikar, N.; Hollerbach, S.; et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: A randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur. J. Cancer 2013, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.S.; Swinson, D.; Cairns, D.A.; Waters, J.S.; Petty, R.; Allmark, C.; Ruddock, S.; Falk, S.; Wadsley, J.; Roy, R.; et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and cape-citabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: The GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021, 7, 869–877. [Google Scholar] [CrossRef] [PubMed]


| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| Age ≥ 18 years | Age < 18 years |
| Gastric or esophago-gastric cancer | Mixed cancer setting (e.g., Upper GI cancers, GIT cancers) |
| Any oncologic treatment (surgery, chemotherapy, radiotherapy, best supportive care) at any stage | |
| Any nutritional intervention (counseling, oral nutritional support, enteral nutrition, parenteral nutrition) | Not a clear nutritional outcome |
| Primary prevention | |
| Complementary/Alternative Medicine | |
| Randomized clinical trials, prospective observational trials, case-control trials, cross-sectional trials, retrospective analysis, systematic review and metanalysis, narrative review | Case reports, case series, commentary and letters, presentation of protocol, qualitative studies |
| Author, Year DOI | Sample Size, Region | Cancer Site, Therapy | Inclusion Criteria | Nutritional Intervention | Outcome |
|---|---|---|---|---|---|
| Hur, 2010 [17] | 54, Korea | GC, Surgery: TG, DG | Any nutritional status | Early feeding (liquid diet since 1st POD) vs. CG 6 d, 28 d FU | No differences in PO morbidity, hospitalization cost, PO pain ↓ time of gas passage ↓ readmission rate ↓ LOHS ↓ fatigue at discharge Positive effects in LOHS and aspects of QoL (secondary outcomes) |
| Fujitani, 2012 [18] | 244, Japan | GC, Surgery: TG | Well-nourished patients (WL of 10% or less within 6 m before surgery) | 5 d of Immunonutrition PreO vs. CG | No differences in PO complications (Infections, morbidity) Negative study |
| Liu, 2012 [19] | 78, China | GC, Surgery: TG | Not defined (“WL recorded in all patients”) | IEEN vs. SEN vs. CG 7 d, starting 48 h PO | ↑ Biochemical Nutritional parameters in IEEN and SEN vs. CG ↑ Immunological parameters in IEEN vs. SEN and CG ↓ LOHS (IEEN and EN vs. CG) No differences in PO complications Positive effect of EN on nutritional parameters; immunological effect of immune-enriched product |
| Marano, 2013 [20] | 109, Italy | GC, Surgery: TG | Any nutritional status (according to ESPEN Guidelines) | IEN (arginine, RNA, ω3) vs. EN (isonitrogenic, isoenergetic) trough jejunostomy Starting 6 h PO, to POD7 | ↓ duration of SIRS ↓ anastomotic leak and infectious complications (late PO) ↓ LOHS PO ↓ proinflammatory mediators) No differences in mortality and in nutritional parameters Partially positive |
| Kim, 2014 [21] | 56, Korea | GC, Surgery: TG | Any nutritional status | PPDI vs. CG 12 w, starting one d before discharge | ↓ DSS ↑ KPS ↑FACT-G ↑Dietary intake ↑ ADS ↑ SDK ↑ PSS Positive effect of PPDI on nutritional outcome |
| Wei, 2014 [22] | 48, China | GC, Surgery: TG | BMI > 18 kg/m2 and <30 kg/m2 Severely malnourished excluded | ω3-enriched PN vs. isocaloric and isonitrogenous PN >6 consecutive d | No differences in nutritional index No PO ↑ of WBC, IL-6 and TNF-α in intervention group ↓ PO infectious complications Positive effect in flogistic parameters and PO complications |
| Ding, 2015 [23] | 106, China | GC, Surgery: not specified (only abstract available) | Any nutritional status | PreO 1 w EN vs. Early PO EN until 9th POD 1 w before surgery | BW, WBC, ALB, CRP, IgA, CD4, CD8, CD4/CD8, TNF-α ↑ IgG, PA in IG ↓ IL-6 |
| Wang, 2015 [24] | 200, China | GC Surgery: TG | Any nutritional status | PreO EN (7 d before surgery) and early PO EN vs. Early PO EN 1 w before surgery for PreOP ENEN until POD 9 for both groups | ↑ PA ↑ IgG level ↓ IL-6 level Positive effect of PreO EN Comment: different kinds of EN |
| Li, 2015 [25] | 272, China | GC Surgery: PG, DG, TG | Any nutritional status | PO EN (2nd day PO, FT) vs. isocaloric PN (1st day PO) | No differences in BW and ALB ↑ TF, PA ↓ CRP ↓ LOHS No difference in Incidence of complications Partially positive |
| Faber, 2015 [26] | 64, Netherlands | Esophageal or GEJ CT, RT or Surgery | Any nutritional status, | Counseling + ONS enriched in leucine, fish oil and FOS vs. Placebo oral liquid supplement if WL < 5% or Iso-caloric ONS if WL ≥ 5% 4 w before any oncologic treatment | No difference in IL-2, IFNɤ, IL-6, TNF-α ↓ PGE2 ↑ W ↑ ECOG PS No difference in PA, ALB Partially positive (immune function as primary outcome, sample size not adequate) Comment: Oral intake not recorded |
| Bowrey, 2015 [27] | 54, UK | EC Surgery: Esophagectomy or TG with placement of feeding jejunostomy tube | Any nutritional status | Overnight jejunostomy EN for 6 w after discharge (50% of energy needs supplied) vs. CG (discharge without EN, dietetic counseling. Start hEN if WL > 5% from baseline or ↓oral intake < 33% or ↓functional status) 6 w | Mean value of MAC, MAMC, TST, Handgrip > than in CG CG lost 3.9 kg (mean) more than IG Positive, the intervention is feasible, safe, acceptable Comment: 33% of patients in the CG required home EN because of clinical needs (WL, ↓ functional status) |
| Imamura, 2016 [28] | 112, (Japan) | GC, Surgery: DG or TG | Any nutritional status | ED group (SD + elemental diet 300 mL die) vs. CG 6–8 w (before starting AT) | ↓ %BWL in TG (p = 0.012) not in DG (p = 0.059) Positive in TG |
| Ida, 2017 [29] | 126, Japan | GC, Surgery: TG | Any nutritional status | SD + eicosapentaenoic acid enriched ONS vs. SD From 7 to 1 d before surgery and for 21 d PO (when oral intake restarted) | No significant difference in BWL, PO complications, nutritional parameters (ALB, CRP) Negative Comment: 54% compliance PO; not evaluated total intake |
| Klek, 2017 [30] | 145, Poland | GC, Surgery: TG | Any nutritional status | EEN (arginine, glutammine, omega3) vs. SD 7 d, starting 12 h PO | No differences in 5-y OS ↓ risk of dying in the early period (6 m) PO in stage IV cancer Negative |
| Wang, 2017 [31] | 94, China | GC, NAT (capecitabine + gemcitabine, 3 courses of 21 d) | Any nutritional status | Glutamine-enriched PN vs. standard PN (isocaloric) 3 cycles of 21 d | ↓ MMP-2 MMP-9 ↑ CD3+, CD4+, CD8+, CD4+/CD8+ ↑ Ig (G, A, M) ↓ Incidence of AEs Positive Comment: no data about oral intake and grade of AEs withdrawal of CT |
| Zhao, 2017 [32] | 120, China | GC, Surgery: DG | Any nutritional status | FF EN vs. FE EN vs FEP EN Start on POD1, FT for 7 d | ↓ diarrhea and intestinal disorders in FEP group No differences in biochemical nutritional index (lymphocyte count, ALB, PA, TF), LOHS Positive on GI symptoms, Negative in biochemical data and LOHS |
| Baker, 2017 [33] | 41, UK | EC, GC Surgery: Oesophagectomy, eosophagogastrectomy or TG. CT (almost all patients) | Any nutritional status | Planned jejunostomy hEN vs. CG EN PO up to 7 d. HEN in IG (50% of estimated requirements) until clinical improvement (mean of 75 d) vs no intervention in CG (rescue intervention with EN in 26%) | ↑ total nutritional intake ↓ BWL ↓ functional deteriorating (hand grip strength) No differences in dietary intake (not a negative impact of EN in oral intake) Positive |
| Hatao, 2017 [34] | 113, Japan/Taiwan | GC, Surgery: DG or TG | Any nutritional status | ONS + SD vs. PO SD 12 w after discharge | Less %BWL PO (significant only in TG group, p = 0.07) No significant differences in BC, biochemical parameters, QoL Partially positive, only in TG group |
| Xie, 2017 [35] | 144, China | GC, Surgery: TG, DG followed by AT (oxaliplatin and capecitabine, every 21 d) | Any nutritional status | Standard educational intervention during hospitalization vs. intensive individualized intervention during entire CT course entire CT course | ↑ kcal intake ↑ iron intake ↑ Hb level Stabilization of BW ↑ protein and ALB ↓ rate of CT withdrawal due to the AEs (p = 0.004) Positive |
| Catarci, 2018 [36] | 43, Italy | GC, Surgery: TG, DG | Any nutritional status (classified by INA, lymphocyte count and ALB) | PES vs. CG 12 m | No differences in BMI ↑ INA class status ↑ PA after 6 mo ↑ QOL Positive |
| Scislo, 2018 [37] | 115, Poland | GC, Surgery: TG or STG | Normal nutritional status, mild or moderate malnutrition | Immunomodulating EN vs. CG 8–16 h after surgery until POD6 | No differences in PO morbidity ↓ PO pulmonary complications ↓ PO 60-day mortality No differences in 6-m and 1-y survival Partially positive (in surgical outcome, not in OS) |
| Kong, 2018 [38] | 127, Korea | GC, Surgery: DG, TG, PG, PPG | Moderately or severely malnourished (PG-SGA) or BMI < 18.5 kg/m2 | PreO and PO ONS vs. CG 2 w PreO and 4 w PO | ↑ total energy intake ↓ incidence of PO complications in IG (not significant overall, significant in PG-SGA grade C, p = 0.24)) No differences in nutritional biochemical parameters Partially positive |
| Shimizu, 2018 [39] | 243, Japan | GC, Surgery: DG or TG | Any nutritional status | Early oral feeding (POD 1) vs. CG (POD 3–4) From POD 1 to POD 7 | No differences in LOHS in DG patients, ↓ LOHS in TG (but not attained the target sample size) No differences in BW, rehospitalization, SIRS incidence ↑ PO complications in DG ↑ oral energy intake Negative |
| Jin, 2018 [40] | 80, China | GC, Surgery: TG DG | Any nutritional status | PO PN vs. CG Starting POD 1 to POD 4–8 | ↑ levels of PA, ALB, Hb Improved QoL ↓Anxiety and depression ↑ CD3+, CD4+, CD4+/CD8+ Positive Comment: No info about oral intake |
| Kimura, 2019 [41] | 106, Japan | GC, Surgery: TG, DG (31 pts received AT, S-1) | Any nutritional status | ONS (elemental diet) vs. CG 6–8 w PO | ↓ %BWL (1 y PO) only in TG subgroup No difference in nutrition-related blood parameters, except for total lymphocyte count (higher in intervention group, p = 0.019) ED give more benefit in TG Partially positive |
| Wang, 2019 [42] | 60, China | GC, Surgery: TG | Any nutritional status except for “severe malnutrition” | ERAS protocol vs. SOC Since the day of surgery to discharge | ↓ PO hospital stay,↓ hospitalization costs ↓ time to first flatus, time to removal of drainage tube ↓ time to oral intake, ↓ time to mobilization ↑ PA, ALB level on POD7 ↓ CRP, N level ↑ Ig (G,A,M) and T lyn Positive effect of ERAS application |
| Feijò, 2019 [43] | 68, Brazil | GC, CT | Any nutritional status | Counseling + ONS-EPA/DHA (IG) vs. isocaloric ONS (CG) 30 d | ↑ W No difference in CD4 and CD8 Partially positive in weight gain and immunologic profile |
| Aoyama 2019 [44] | 123, Japan | GC, Surgery: TG | Any nutritional status | EPA-ONS since 7 d to 1 d PreO and 21 d PO vs. Standard care | No difference in PO complications, mean reduction of LBM at 1 and 3 months after surgery Negative |
| Zong, 2019 [45] | 96, China | GC, CT neoadiuvant (FOLFOX4, 2 courses) | NRS ≥ 3 “Patients with indications of nutritional support” not better specified | Only CT vs. CT + ω3 oral EN, (7 d during each course) 30 days | No variation of nutritional biochemical index Positive effect in nutritional, inflammatory and intestinal flora after surger; |
| Zheng, 2019 [46] | 100, China | GC, Surgery: PG | Any nutritional status | Diet + probiotic vs. Diet + placebo 3–5 d PO for up to 6–7 d | ↓ leukocyte inflammation index ↑ immunity index ↑ ALB and total protein Improved microflora balance (↓ Firmicutes/Bacteroidetes) Positive |
| Toyomasu, 2019 [47] | 22, Japan | GC, Surgery: TG or DG, followed AT (S-1) | Any nutritional status | SD + ONS (elemental diet glutamine-enriched) vs. SD without any restriction From1 to 28 d every cycle | ↓ oral mucositis ↓ median BWL ↑ cumulative S-1 continuation rates Positive |
| Xu, 2020 [48] | 60, China | GC, Surgery: TG | Any nutritional status | EEIN (probiotics, glutamine) via FT vs. Standard EN 7 d, starting 8 h PO | No difference in nutritional variables (biochemical and anthropometric) and PO complications and LOHS ↓ CRP at day 7 ↑ CD4+ at day 7 ↓ time to first flatus Partially positive |
| Wang, 2020 [49] | 113, China | GC, Surgery: TG | Any nutritional status | Functional jejunal interposition) vs. Roux-en-Y group 60 months | No differences in PO food intake ↓ QoL after 12 months ↑ WL Negative |
| Meng, 2021 [50] | 353, China | GC, Surgery: TG or DG+/− AT | NRS > 3 | Post-discharge ONS + dietary advice vs. dietary advice only 3 m | ↑ BMI ↓ Sarcopenia ↑ CT tolerance ↓ Readmission rate (not significant) ↑ QoL (fatigue, appetite component) Positive effect of post- discharge ONS with dietary advice |
| Field | Title—ID n. | Study | Sample Size Therapy | Cancer Site Setting | Intervention | Primary Outcome | Region |
|---|---|---|---|---|---|---|---|
| NUTRITION SYSTEMIC THERAPY | Early IntraVenous Administration of Nutritional Support (IVANS) in Metastatic GC Patients at Nutritional Risk Undergoing I-line CT NCT03949907 | RCT Phase II | 192 1-line CT | GC GEC Metastatic | Nutritional counseling alone (+/− ONS) vs. Early sPN + nutritional counseling | Early sPN + nutritional counseling ↑ survival and CT feasibility | Italy Fondazione IRCCS Policlinico San Matteo |
| NUTRITION SYSTEMIC THERAPY ELDERLY | A Nutritional Management Algorithm in Older Patients with Locally Advanced EC NCT02027948 | Feasibility study Interventional Single-Group Assignment | 26, >65 yrs Induction CT + CT-RT + surgery or definitive CT-RT | EC GEJC Local disease | Nutritional & functional assessments
MNA category for intervention
| Feasibility of nutritional management algorithm | US, New York Memorial Sloan Kettering Cancer Center |
| NUTRITION SYSTEMIC THERAPY | The Analysis of Immuno-Nutrition Index in Advanced GC Receiving Preoperative Treatments: Observational Cohort Study NCT03493880 | Observational Cohort Study | Child, Adult, Elderly Neoadjuvant CT or CT-RT | GC EGC Local disease | No intervention | Perioperative treatments Immuno-nutrition Index: NLR, PLR, SII, GPS, mGPS, PI, NRS2002, PNI | China, Beijing Peking University Cancer Hospital |
| NUTRITION SYSTEMIC THERAPY | Anorexia in Cancer Patients: Assessment of the Gut HORmone and Cytokine Profile and Body Composition, and the Impact of Dietetic Support in Patients With Gastrointestinal Cancer NCT04791254 | Observational Cohort Study | 450 1-line CT or immune therapy | GC GEJC Metastatic | No intervention | Differences in patterns of pre-prandial and post-prandial plasma gut hormone and CK levels between stage-standardized anorexic and non-anorexic cancer patients and age-matched healthy controls. (Ghrelin, insulin, GLP-1, PYY, pancreatic polypeptide, GIP, Chromogranin A, CCK, IL-1, IL-6, TNF-alpha) Survival at 1 y | United Kingdom, Manchester The Christie NHS Foundation Trust |
| NUTRITION SURGERY | Prospective Study of the Effect of Perioperative Immunonutrition on the Immune Host Defense and the Phagocytic and Bactericidal Activity of Blood Platelets in GC Patients NCT01704664 | RCT Phase II | 240 Neoadjuvant CT | GC Local disease | Group I: EN (Peptisorb) Group II: EN and PN with glutamine (Dipeptiven, Omegaven) in PO period Group III: oral arginine (Cubitan) Group IV: PN with glutamine preO and PO | Phagocytic and bactericidal activity of blood platelets, lymphocytes and their subpopulations, IL-1B, -6, -23 determined before and after nutritional therapy | Poland Medical University of Bialystok |
| NUTRITION SURGERY | The Effect of Postoperative sPN in GC Patients Who Underwent Gastrectomy: A Multicenter Prospective RCT NCT04607057 | RCT Phase II | 224 Adjuvant therapy | GC Local disease | D0: fasting + crystalloid fluid POD1: water sips + crystalloid fl. POD2: SFD + crystalloid fl. POD3: SFD + sPN POD4-7: SBD + sPN vs. D0: fasting + crystalloid fl. POD1: water sips + crystalloid fl. POD2: SFD + dextrose 5% water POD3: SFD + dextrose 5% water POD4-7: SBD | Total amount of kcal during hospitalization W change for 2 m PO Favorable blood test result CT feasibility ↑ QoL ↓ infection rate ↓ mortality | Korea Seoul National University Hospital |
| NUTRITION SURGERY | Personalized Trimodal Prehabilitation for Gastrectomy NCT04223401 | RCT Phase II | 128 | GC Local disease | Prehab. before elective GC surgery
No Intervention | Postoperative morbidity rate by Clavien-Dindo At 90 POD | Lithuania National Cancer Institute |
| NUTRITION SURGERY | A RCT of Simplified Dietary Education Versus Intensive Dietary Education on Nutritional Status After Gastrectomy NCT04798820 | RCT Phase II | 374 | GC Local disease |
| W change between the two groups after surgery at immediate PO period, at 1st,3rd, 6th, 12th, 18th PO m | Korea Samsung medical center |
| NUTRITION SURGERY | Impact on the Hospital Stay, of an EON Protocol Applied to GC Patients After TG: A Prospective RCT NCT03257280 | RCT Phase II | 84 | GC Local disease | EON with ONS start 48 h after TG vs. classical PO management:
| LOHS PO | Barcelona, Spain L’Hospitalet de Llobregat |
| NUTRITION SURGERY | A Multi-center Pilot RCT Examining the Differences of Nutritional Status of Patients Undergoing Functional Jejunal Interposition Or Roux-en-Y After TG for GC NCT01996059 | RCT Phase III | 500 | GC Local disease | Functional Jejunal Interposition vs. Roux-en-Y | BMI 3 m PO | China, Guangdong 6th Affiliated Hospital, Sun Yat-sen University |
| NUTRITION SURGERY | A Prospective Multi-center RCT to Compare Survival Rates and QoL According to Follow-up Period in Patients Who Underwent Radical Gastrectomy for Advanced GC NCT04408859 | RCT | 886 | GC Local disease | FU every 3 m after gastrectomy (Computed tomography, Chest X-ray, and blood test) vs. FU every 6 m after gastrectomy | Survival rates QoL Nutritional status | Korea National Cancer Center, et al. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulazzani, G.E.G.; Corti, F.; Della Valle, S.; Di Bartolomeo, M. Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade. Nutrients 2021, 13, 2766. https://doi.org/10.3390/nu13082766
Mulazzani GEG, Corti F, Della Valle S, Di Bartolomeo M. Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade. Nutrients. 2021; 13(8):2766. https://doi.org/10.3390/nu13082766
Chicago/Turabian StyleMulazzani, Giulia E.G., Francesca Corti, Serena Della Valle, and Maria Di Bartolomeo. 2021. "Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade" Nutrients 13, no. 8: 2766. https://doi.org/10.3390/nu13082766
APA StyleMulazzani, G. E. G., Corti, F., Della Valle, S., & Di Bartolomeo, M. (2021). Nutritional Support Indications in Gastroesophageal Cancer Patients: From Perioperative to Palliative Systemic Therapy. A Comprehensive Review of the Last Decade. Nutrients, 13(8), 2766. https://doi.org/10.3390/nu13082766






