Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assessment
2.3. Fatty Acid Quantification
2.4. 3-D Spheroid Models
2.5. Measurement of Lipid Peroxidation Potential (BODIPY Assay)
2.6. Quantification of Lipid Peroxidation-Derived Products (MDA Assay)
2.7. Statistics
3. Results
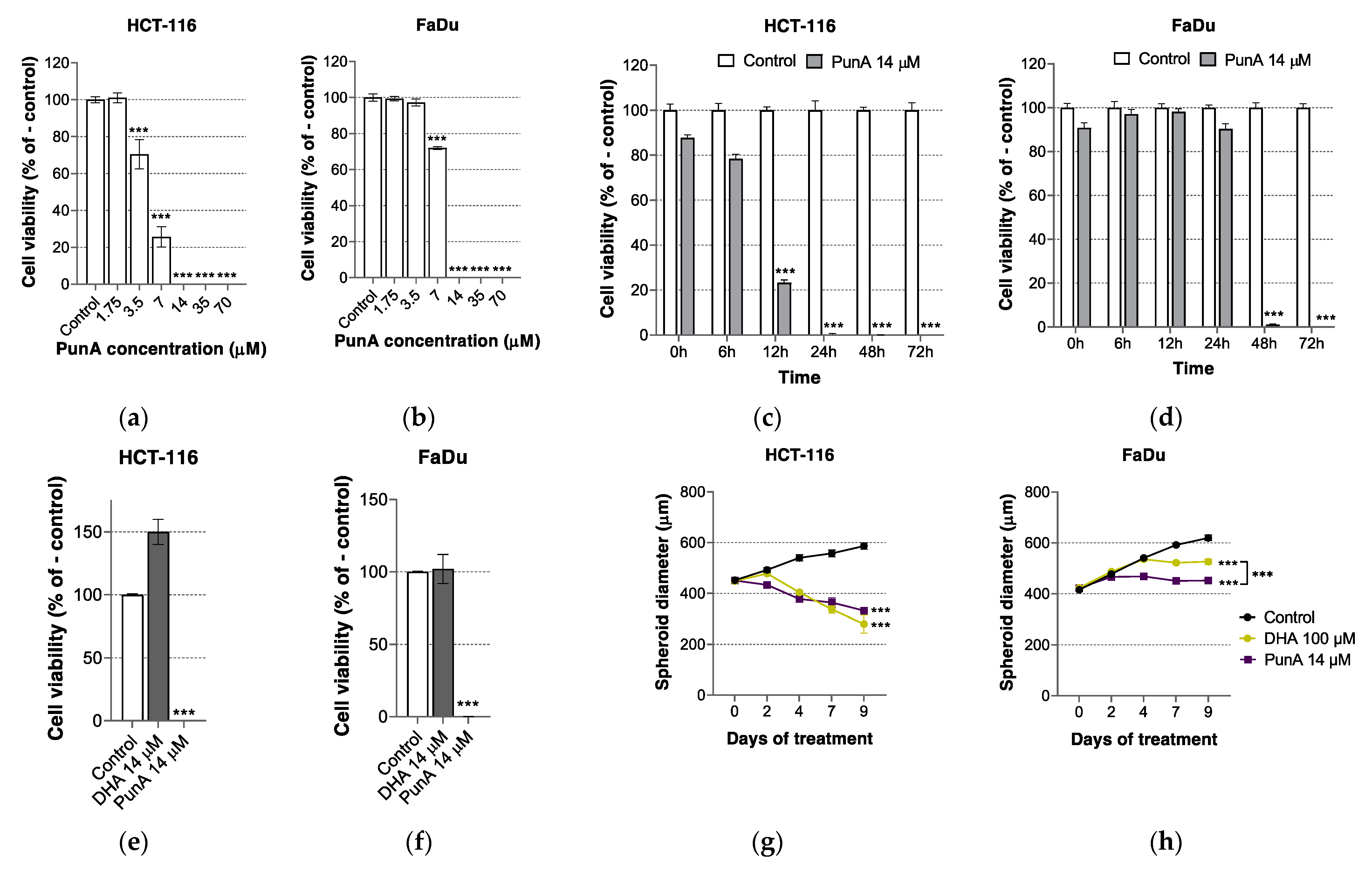
3.1. PunA Is Cytotoxic to Different Carcinoma Cell Lines
3.2. The Effect of PunA on Carcinoma Cells Is Enhanced in the Presence of DHA
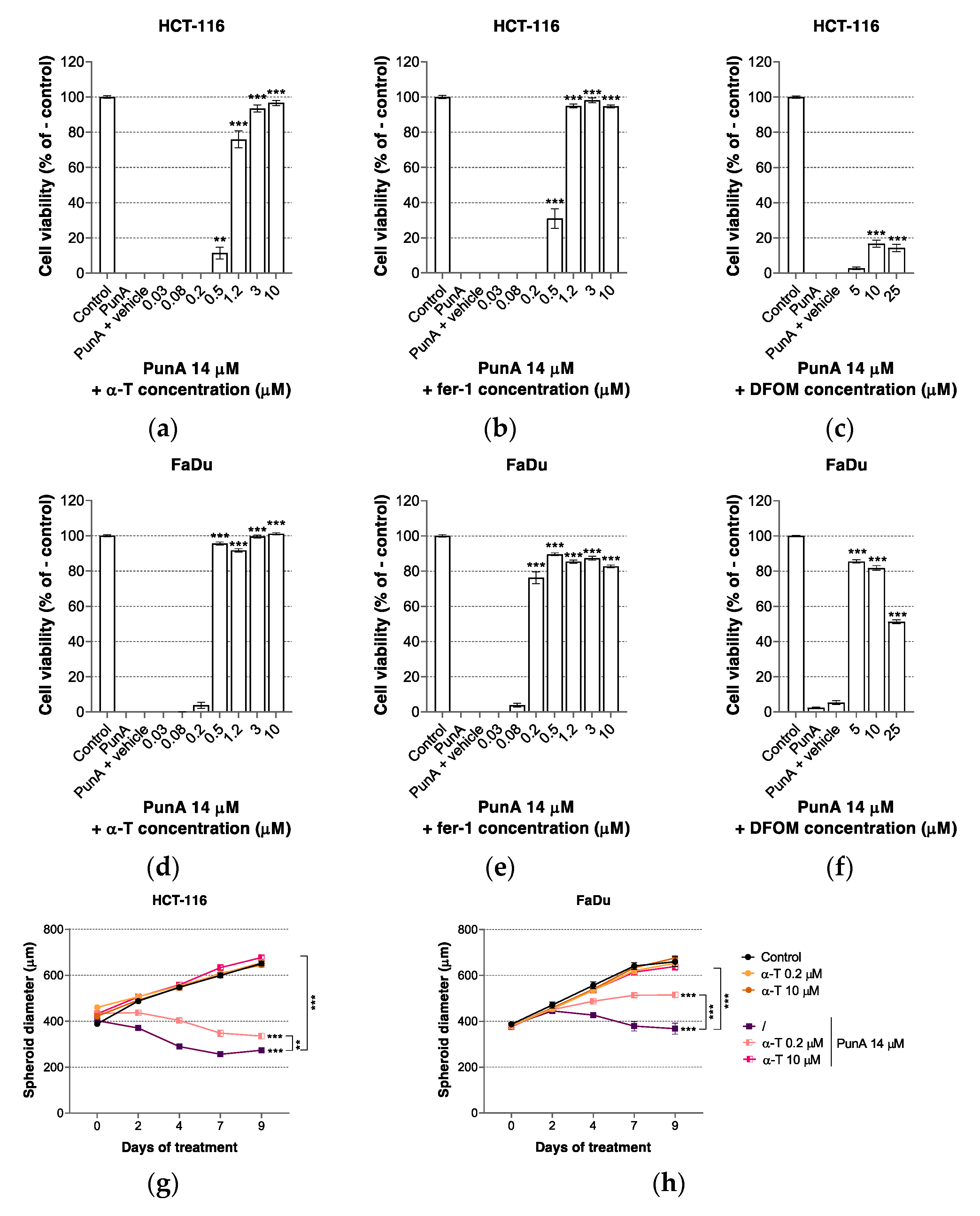
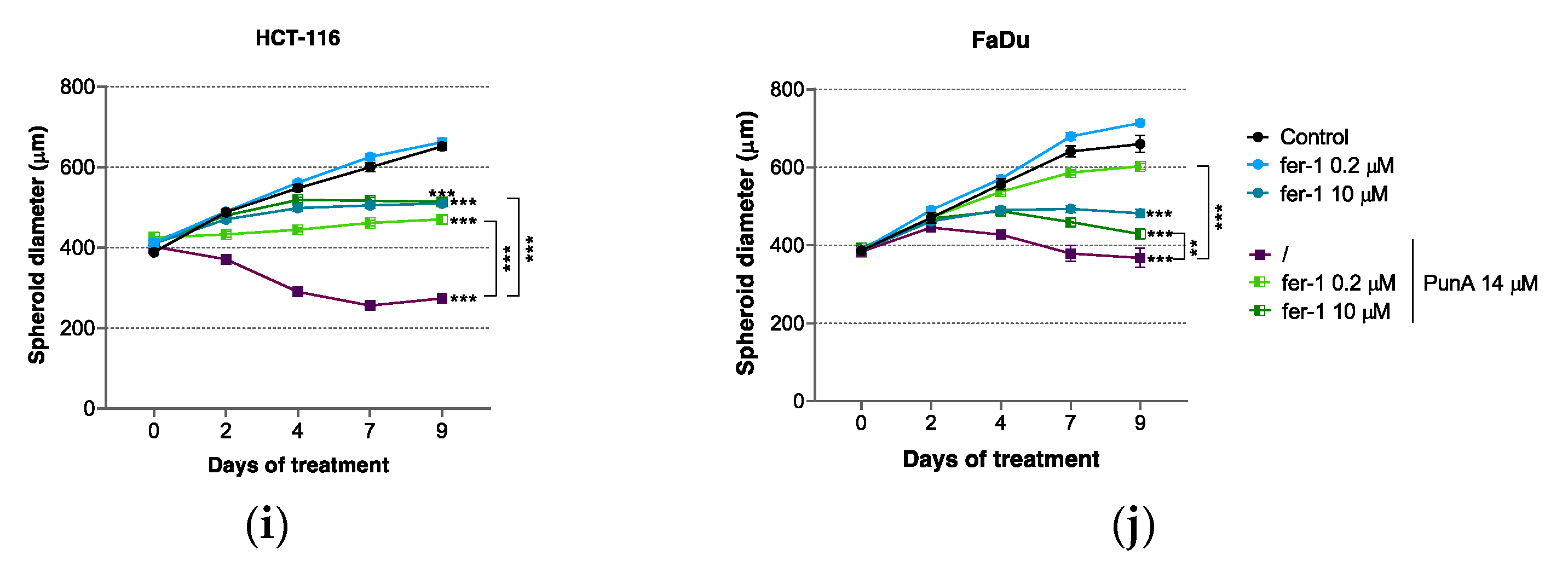
3.3. PunA Effect Is Inhibited by Ferroptosis Inhibitors
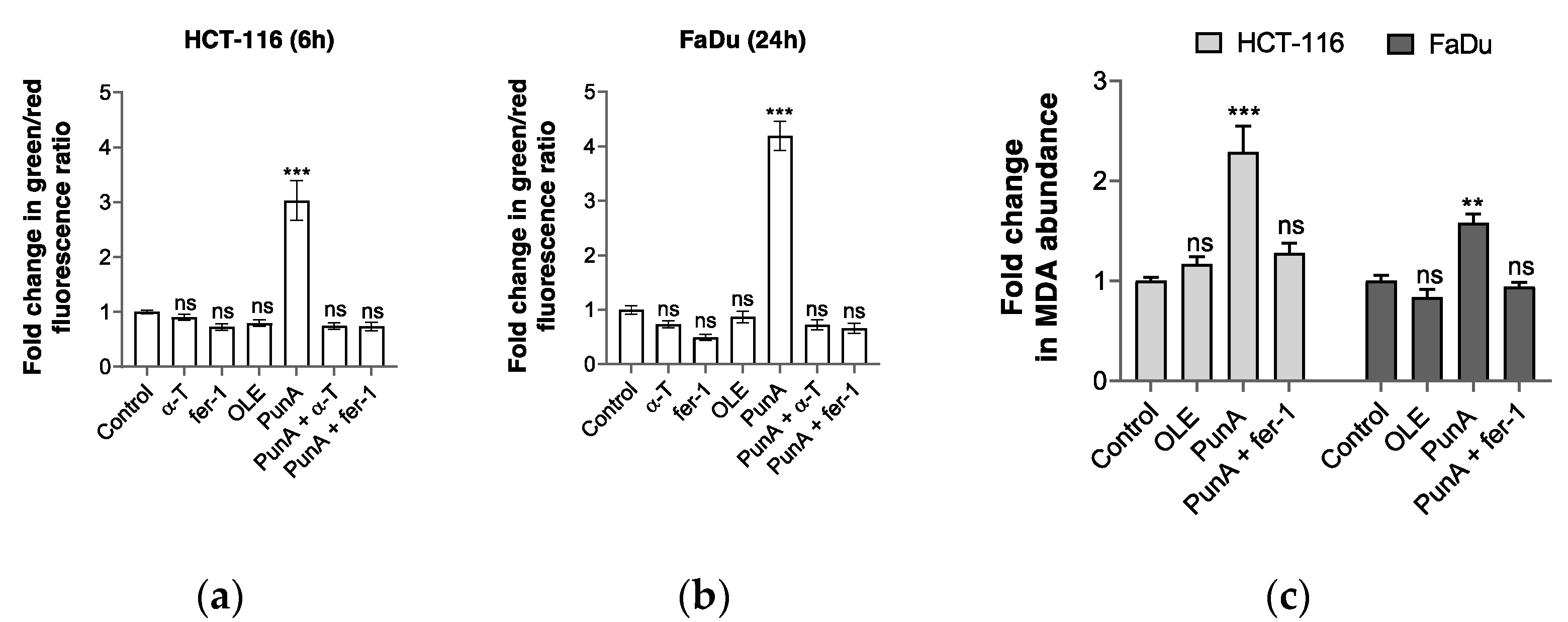
3.4. PunA Treatment Leads to an Increase in Intracellular Lipid Peroxidation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhar Dubey, K.K.; Sharma, G.; Kumar, A. Conjugated Linolenic Acids: Implication in Cancer. J. Agric. Food Chem. 2019, 67, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, J.; Sanderson, J.T. Jacaric Acid and Its Octadecatrienoic Acid Geoisomers Induce Apoptosis Selectively in Cancerous Human Prostate Cells: A Mechanistic and 3-D Structure–Activity Study. Phytomedicine 2013, 20, 734–742. [Google Scholar] [CrossRef][Green Version]
- Dierge, E.; Larondelle, Y.; Feron, O. Cancer Diets for Cancer Patients: Lessons from Mouse Studies and New Insights from the Study of Fatty Acid Metabolism in Tumors. Biochimie 2020, 178, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, A.A.; Ross, P.R.; Fitzgerald, G.F.; Stanton, C. Sources and Bioactive Properties of Conjugated Dietary Fatty Acids. Lipids 2016, 51, 377–397. [Google Scholar] [CrossRef]
- Schneider, A.-C.; Mignolet, E.; Schneider, Y.-J.; Larondelle, Y. Uptake of Conjugated Linolenic Acids and Conversion to Cis-9, Trans-11-or Trans-9, Trans-11-conjugated Linoleic Acids in Caco-2 Cells. Br. J. Nutr. 2013, 109, 57–64. [Google Scholar] [CrossRef]
- Schneider, A.-C.; Beguin, P.; Bourez, S.; Perfield, J.W.; Mignolet, E.; Debier, C.; Schneider, Y.-J.; Larondelle, Y. Conversion of T11t13 CLA into C9t11 CLA in Caco-2 Cells and Inhibition by Sterculic Oil. PLoS ONE 2012, 7, e32824. [Google Scholar] [CrossRef]
- Shinohara, N.; Tsuduki, T.; Ito, J.; Honma, T.; Kijima, R.; Sugawara, S.; Arai, T.; Yamasaki, M.; Ikezaki, A.; Yokoyama, M.; et al. Jacaric Acid, a Linolenic Acid Isomer with a Conjugated Triene System, Has a Strong Antitumor Effect in Vitro and in Vivo. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 980–988. [Google Scholar] [CrossRef]
- Ngo Njembe, M.T.; Dormal, E.; Gardin, C.; Mignolet, E.; Debier, C.; Larondelle, Y. Effect of the Dietary Combination of Flaxseed and Ricinodendron Heudelotii or Punica Granatum Seed Oil on the Fatty Acid Profile of Eggs. Food Chem. 2021, 344, 128668. [Google Scholar] [CrossRef]
- Lee, K.N.; Kritchevsky, D.; Pariza, M.W. Conjugated Linoleic Acid and Atherosclerosis in Rabbits. Atherosclerosis 1994, 108, 19–25. [Google Scholar] [CrossRef]
- Benjamin, S.; Spener, F. Conjugated Linoleic Acids as Functional Food: An Insight into Their Health Benefits. Nutr. Metab. 2009, 6, 36. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Stanton, C.; Ross, R.; Zhang, H.; Chen, Y.; Chen, W. Review of the Roles of Conjugated Linoleic Acid in Health and Disease. J. Funct. Foods 2015, 15, 314–325. [Google Scholar] [CrossRef]
- de Carvalho, E.B.T.; de Melo, I.L.P.; Mancini-Filho, J. Chemical and Physiological Aspects of Isomers of Conjugated Fatty Acids. Food Sci. Technol. 2010, 30, 295–307. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.L.; Simões, C.D.; Gomes, A.M.P.; Rodríguez-Alcalá, L.M. Evidences and Perspectives in the Utilization of CLNA Isomers as Bioactive Compounds in Foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, T.; Raad, H.; Lettéron, P.; Gougerot-Pocidalo, M.-A.; Marie, J.-C.; Driss, F.; El-Benna, J. Punicic Acid a Conjugated Linolenic Acid Inhibits TNFalpha-Induced Neutrophil Hyperactivation and Protects from Experimental Colon Inflammation in Rats. PLoS ONE 2009, 4, e6458. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.; Khan, M.R.; Saeed, M.; Pasha, I.; Khalil, A.A.; Siraj, N. Punicic Acid: A Striking Health Substance to Combat Metabolic Syndromes in Humans. Lipids Health Dis. 2017, 16, 99. [Google Scholar] [CrossRef]
- Zamora-López, K.; Noriega, L.G.; Estanes-Hernández, A.; Escalona-Nández, I.; Tobón-Cornejo, S.; Tovar, A.R.; Barbero-Becerra, V.; Pérez-Monter, C. Punica Granatum L.-Derived Omega-5 Nanoemulsion Improves Hepatic Steatosis in Mice Fed a High Fat Diet by Increasing Fatty Acid Utilization in Hepatocytes. Sci. Rep. 2020, 10, 15229. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, G.-C.; Yang, M.-F.; Hsieh, C.-H.; Chuang, S.-H.; Yang, H.-L.; Kuo, Y.-H.; Chyuan, J.-H.; Chao, P.-M. Bitter Melon Seed Oil—Attenuated Body Fat Accumulation in Diet-Induced Obese Mice Is Associated with CAMP-Dependent Protein Kinase Activation and Cell Death in White Adipose Tissue. J. Nutr. 2012, 142, 1197–1204. [Google Scholar] [CrossRef]
- Vroegrijk, I.O.C.M.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Hontecillas, R.; Bassaganya-Riera, J.; Zondag, G.C.M.; Romijn, J.A.; et al. Pomegranate Seed Oil, a Rich Source of Punicic Acid, Prevents Diet-Induced Obesity and Insulin Resistance in Mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1426–1430. [Google Scholar] [CrossRef]
- Anusree, S.S.; Sindhu, G.; Preetha Rani, M.R.; Raghu, K.G. Insulin Resistance in 3T3-L1 Adipocytes by TNF-α Is Improved by Punicic Acid through Upregulation of Insulin Signalling Pathway and Endocrine Function, and Downregulation of Proinflammatory Cytokines. Biochimie 2018, 146, 79–86. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Chen, X.-E.; Li, D. Conjugated Linolenic Acids and Their Bioactivities: A Review. Food Funct. 2014, 5, 1360. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Kawakami, Y. Tumor Angiogenesis Suppression by α-Eleostearic Acid, a Linolenic Acid Isomer with a Conjugated Triene System, via Peroxisome Proliferator-Activated Receptor γ. Carcinogenesis 2008, 29, 797–806. [Google Scholar] [CrossRef][Green Version]
- Yasui, Y.; Hosokawa, M.; Sahara, T.; Suzuki, R.; Ohgiya, S.; Kohno, H.; Tanaka, T.; Miyashita, K. Bitter Gourd Seed Fatty Acid Rich in 9c,11t,13t-Conjugated Linolenic Acid Induces Apoptosis and up-Regulates the GADD45, P53 and PPARγ in Human Colon Cancer Caco-2 Cells. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 113–119. [Google Scholar] [CrossRef]
- Grossmann, M.E.; Mizuno, N.K.; Schuster, T.; Cleary, M.P. Cleary Punicic Acid Is an ω-5 Fatty Acid Capable of Inhibiting Breast Cancer Proliferation. Int. J. Oncol. 2009, 36. [Google Scholar] [CrossRef]
- Suzuki, R.; Noguchi, R.; Ota, T.; Abe, M.; Miyashita, K.; Kawada, T. Cytotoxic Effect of Conjugated Trienoic Fatty Acids on Mouse Tumor and Human Monocytic Leukemia Cells. Lipids 2001, 36, 477–482. [Google Scholar] [CrossRef]
- Gasmi, J.; Sanderson, J.T. Growth Inhibitory, Antiandrogenic, and Pro-Apoptotic Effects of Punicic Acid in LNCaP Human Prostate Cancer Cells. J. Agric. Food Chem. 2010, 58, 12149–12156. [Google Scholar] [CrossRef]
- Kohno, H.; Yasui, Y.; Suzuki, R.; Hosokawa, M.; Miyashita, K.; Tanaka, T. Dietary Seed Oil Rich in Conjugated Linolenic Acid from Bitter Melon Inhibits Azoxymethane-Induced Rat Colon Carcinogenesis through Elevation of Colonic PPAR? Expression and Alteration of Lipid Composition. Int. J. Cancer 2004, 110, 896–901. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Lin, M.; Garcia, M.; Mulholland, D.; Lilly, M.; Martins-Green, M. Luteolin, Ellagic Acid and Punicic Acid Are Natural Products That Inhibit Prostate Cancer Metastasis. Carcinogenesis 2014, 35, 2321–2330. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.; Chen, J.-N.; Chen, Z.-Y. Oxidative Stability of Conjugated Linolenic Acids. J. Agric. Food Chem. 2009, 57, 4212–4217. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D.A. The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Sun, W.-Y.; Tyurin, V.A.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Anthonymuthu, T.S.; Zhai, Y.-J.; Pan, M.-H.; Gong, H.-B.; Lu, D.-H.; Sun, J.; et al. Phospholipase IPLA2β Averts Ferroptosis by Eliminating a Redox Lipid Death Signal. Nat. Chem. Biol. 2021, 17, 465–476. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St. Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, H.; Graham, E.T.; Deik, A.A.; Eaton, J.K.; Wang, W.; Sandoval-Gomez, G.; Clish, C.B.; Doench, J.G.; Schreiber, S.L. Cytochrome P450 Oxidoreductase Contributes to Phospholipid Peroxidation in Ferroptosis. Nat. Chem. Biol. 2020, 16, 302–309. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS–RAF–MEK-Dependent Oxidative Cell Death Involving Voltage-Dependent Anion Channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of Genotype-Selective Antitumor Agents Using Synthetic Lethal Chemical Screening in Engineered Human Tumor Cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef]
- Perez, M.A.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 2020, 54, 447–454.e4. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Beatty, A.; Singh, T.; Tyurina, Y.Y.; Tyurin, V.A.; Samovich, S.; Nicolas, E.; Maslar, K.; Zhou, Y.; Cai, K.Q.; Tan, Y.; et al. Ferroptotic Cell Death Triggered by Conjugated Linolenic Acids Is Mediated by ACSL1. Nat. Commun. 2021, 12, 2244. [Google Scholar] [CrossRef]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of N-3 and n-6 Polyunsaturated Fatty Acids in the Acidic Tumor Environment Leads to Ferroptosis-Mediated Anticancer Effects. Cell Metab. 2021, 33, 1701–1715.e5. [Google Scholar] [CrossRef]
- Edwards, H.M., Jr. Observations on Feeding Tung Oil to Chickens. J. Nutr. 1964, 83, 365–368. [Google Scholar] [CrossRef]
- Spilmont, M.; Léotoing, L.; Davicco, M.-J.; Lebecque, P.; Mercier, S.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate Seed Oil Prevents Bone Loss in a Mice Model of Osteoporosis, through Osteoblastic Stimulation, Osteoclastic Inhibition and Decreased Inflammatory Status. J. Nutr. Biochem. 2013, 24, 1840–1848. [Google Scholar] [CrossRef]
- Smedes, F.; Thomasen, T.K. Evaluation of the Bligh & Dyer Lipid Determination Method. Mar. Pollut. Bull. 1996, 32, 681–688. [Google Scholar] [CrossRef]
- Tanaka, A.; Yamamoto, A.; Murota, K.; Tsujiuchi, T.; Iwamori, M.; Fukushima, N. Polyunsaturated Fatty Acids Induce Ovarian Cancer Cell Death through ROS-Dependent MAP Kinase Activation. Biochem. Biophys. Res. Commun. 2017, 493, 468–473. [Google Scholar] [CrossRef]
- Huang, Q.; Wen, J.; Chen, G.; Ge, M.; Gao, Y.; Ye, X.; Liu, C.; Cai, C. Omega-3 Polyunsaturated Fatty Acids Inhibited Tumor Growth via Preventing the Decrease of Genomic DNA Methylation in Colorectal Cancer Rats. Nutr. Cancer 2016, 68, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Pizato, N.; Luzete, B.C.; Kiffer, L.F.M.V.; Corrêa, L.H.; de Oliveira Santos, I.; Assumpção, J.A.F.; Ito, M.K.; Magalhães, K.G. Omega-3 Docosahexaenoic Acid Induces Pyroptosis Cell Death in Triple-Negative Breast Cancer Cells. Sci. Rep. 2018, 8, 1952. [Google Scholar] [CrossRef]
- Kim, S.; Jing, K.; Shin, S.; Jeong, S.; Han, S.-H.; Oh, H.; Yoo, Y.-S.; Han, J.; Jeon, Y.-J.; Heo, J.-Y.; et al. Ω3-Polyunsaturated Fatty Acids Induce Cell Death through Apoptosis and Autophagy in Glioblastoma Cells: In Vitro and in Vivo. Oncol. Rep. 2017, 39, 239–246. [Google Scholar] [CrossRef]
- Do, Q.; Lee, D.D.; Dinh, A.N.; Seguin, R.P.; Zhang, R.; Xu, L. Development and Application of a Peroxyl Radical Clock Approach for Measuring Both Hydrogen-Atom Transfer and Peroxyl Radical Addition Rate Constants. J. Org. Chem. 2021, 86, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Luna, P.; de la Fuente, M.A.; Salvador, D.; Márquez-Ruiz, G. Differences in Oxidation Kinetics between Conjugated and Non-Conjugated Methyl Linoleate. Lipids 2007, 42, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Hosokawa, M.; Kohno, H.; Tanaka, T.; Miyashita, K. Growth Inhibition and Apoptosis Induction by All-Trans-Conjugated Linolenic Acids on Human Colon Cancer Cells. Anticancer Res. 2006, 26, 1855–1860. [Google Scholar]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Hu, F.; Feng, H.; Linkermann, A.; Min, W.; Stockwell, B.R. Determination of the Subcellular Localization and Mechanism of Action of Ferrostatins in Suppressing Ferroptosis. ACS Chem. Biol. 2018, 13, 1013–1020. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Magtanong, L.; Ko, P.-J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e9. [Google Scholar] [CrossRef]
- Tsuzuki, T. Tumor Growth Suppression by -Eleostearic Acid, a Linolenic Acid Isomer with a Conjugated Triene System, via Lipid Peroxidation. Carcinogenesis 2004, 25, 1417–1425. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-Tolerant Persister Cancer Cells Are Vulnerable to GPX4 Inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Kobori, M.; Ohnishi-Kameyama, M.; Akimoto, Y.; Yukizaki, C.; Yoshida, M. α-Eleostearic Acid and Its Dihydroxy Derivative Are Major Apoptosis-Inducing Components of Bitter Gourd. J. Agric. Food Chem. 2008, 56, 10515–10520. [Google Scholar] [CrossRef]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St. Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox Lipid Reprogramming Commands Susceptibility of Macrophages and Microglia to Ferroptotic Death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef]
- Brown, I.; Lee, J.; Sneddon, A.A.; Cascio, M.G.; Pertwee, R.G.; Wahle, K.W.J.; Rotondo, D.; Heys, S.D. Anticancer Effects of N-3 EPA and DHA and Their Endocannabinoid Derivatives on Breast Cancer Cell Growth and Invasion. Prostaglandins Leukot. Essent. Fat. Acids 2019, 156, 102024. [Google Scholar] [CrossRef]
- Kim, N.; Jeong, S.; Jing, K.; Shin, S.; Kim, S.; Heo, J.-Y.; Kweon, G.-R.; Park, S.-K.; Wu, T.; Park, J.-I.; et al. Docosahexaenoic Acid Induces Cell Death in Human Non-Small Cell Lung Cancer Cells by Repressing MTOR via AMPK Activation and PI3K/Akt Inhibition. BioMed Res. Int. 2015, 2015, 239764. [Google Scholar] [CrossRef]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of Fatty Acids on Melanogenesis and Tumor Cell Growth in Melanoma Cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef]
- Camargo, C.Q.; Brunetta, H.S.; Nunes, E.A. Effects of Cotreatment with Omega-3 Polyunsaturated Fatty Acids and Anticancer Agents on Oxidative Stress Parameters: A Systematic Review of in Vitro, Animal, and Human Studies. Nutr. Rev. 2018, 76, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Kostogrys, R.B.; Filipiak-Florkiewicz, A.; Dereń, K.; Drahun, A.; Czyżyńska-Cichoń, I.; Cieślik, E.; Szymczyk, B.; Franczyk-Żarów, M. Effect of Dietary Pomegranate Seed Oil on Laying Hen Performance and Physicochemical Properties of Eggs. Food Chem. 2017, 221, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Franczyk-Żarów, M.; Czyżyńska, I.; Drahun, A.; Maślak, E.; Chłopicki, S.; Kostogrys, R.B. Margarine Supplemented with Conjugated Linolenic Acid (CLnA) Has No Effect on Atherosclerosis but Alleviates the Liver Steatosis and Affects the Expression of Lipid Metabolism Genes in ApoE/LDLR-/- Mice. Eur. J. Lipid Sci. Technol. 2015, 117, 589–600. [Google Scholar] [CrossRef]
- Modaresi, J.; Nasri, M.H.F.; Rashidi, L.; Dayani, O.; Kebreab, E. Short Communication: Effects of Supplementation with Pomegranate Seed Pulp on Concentrations of Conjugated Linoleic Acid and Punicic Acid in Goat Milk. J. Dairy Sci. 2011, 94, 4075–4080. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Dey, T.K.; Mukherjee, S.; Ghosh, M.; Dhar, P. Comparative Prophylactic Effects of α-Eleostearic Acid Rich Nano and Conventional Emulsions in Induced Diabetic Rats. J. Food Sci. Technol. 2014, 13, 1724–1736. [Google Scholar] [CrossRef]

| Cell Treatment | HCT-116 | FaDu |
|---|---|---|
| OLE 50 µM | 8.74 ± 2.14 *** | 9.02 ± 3.37 *** |
| PunA 14 µM | 7.53 ± 1.21 *** | 4.79 ± 1.53 *** |
| PunA 14 µM + fer-1 10 µM | 4.94 ± 0.28 *** | 2.66 ± 0.38 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermonden, P.; Vancoppenolle, M.; Dierge, E.; Mignolet, E.; Cuvelier, G.; Knoops, B.; Page, M.; Debier, C.; Feron, O.; Larondelle, Y. Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells. Nutrients 2021, 13, 2751. https://doi.org/10.3390/nu13082751
Vermonden P, Vancoppenolle M, Dierge E, Mignolet E, Cuvelier G, Knoops B, Page M, Debier C, Feron O, Larondelle Y. Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells. Nutrients. 2021; 13(8):2751. https://doi.org/10.3390/nu13082751
Chicago/Turabian StyleVermonden, Perrine, Matthias Vancoppenolle, Emeline Dierge, Eric Mignolet, Géraldine Cuvelier, Bernard Knoops, Melissa Page, Cathy Debier, Olivier Feron, and Yvan Larondelle. 2021. "Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells" Nutrients 13, no. 8: 2751. https://doi.org/10.3390/nu13082751
APA StyleVermonden, P., Vancoppenolle, M., Dierge, E., Mignolet, E., Cuvelier, G., Knoops, B., Page, M., Debier, C., Feron, O., & Larondelle, Y. (2021). Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells. Nutrients, 13(8), 2751. https://doi.org/10.3390/nu13082751







