Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
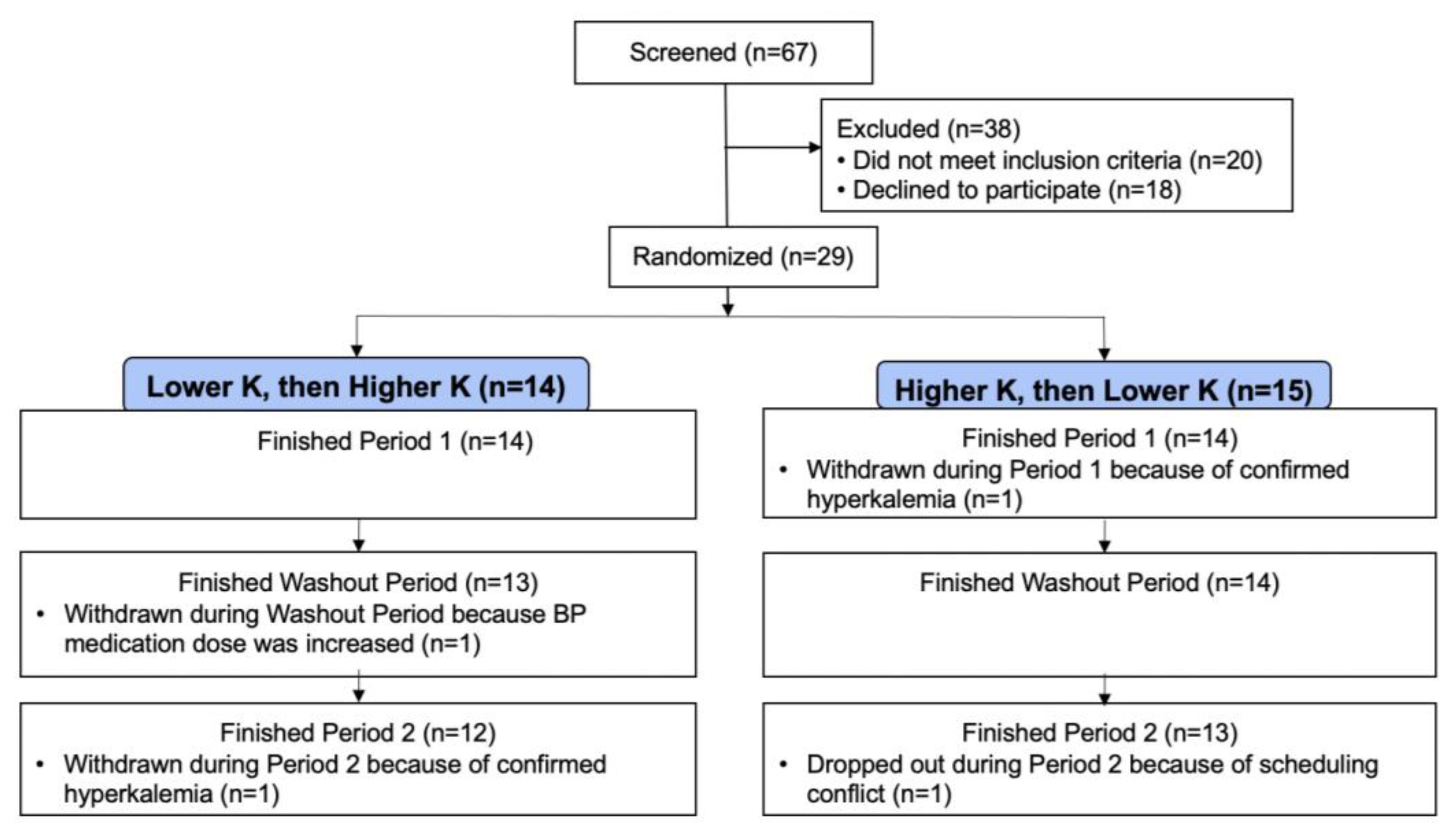
2.1. Participants
2.2. Controlled Feeding
2.3. Measurements
2.4. Analysis
3. Results
3.1. Baseline Characteristics
3.2. BP Results
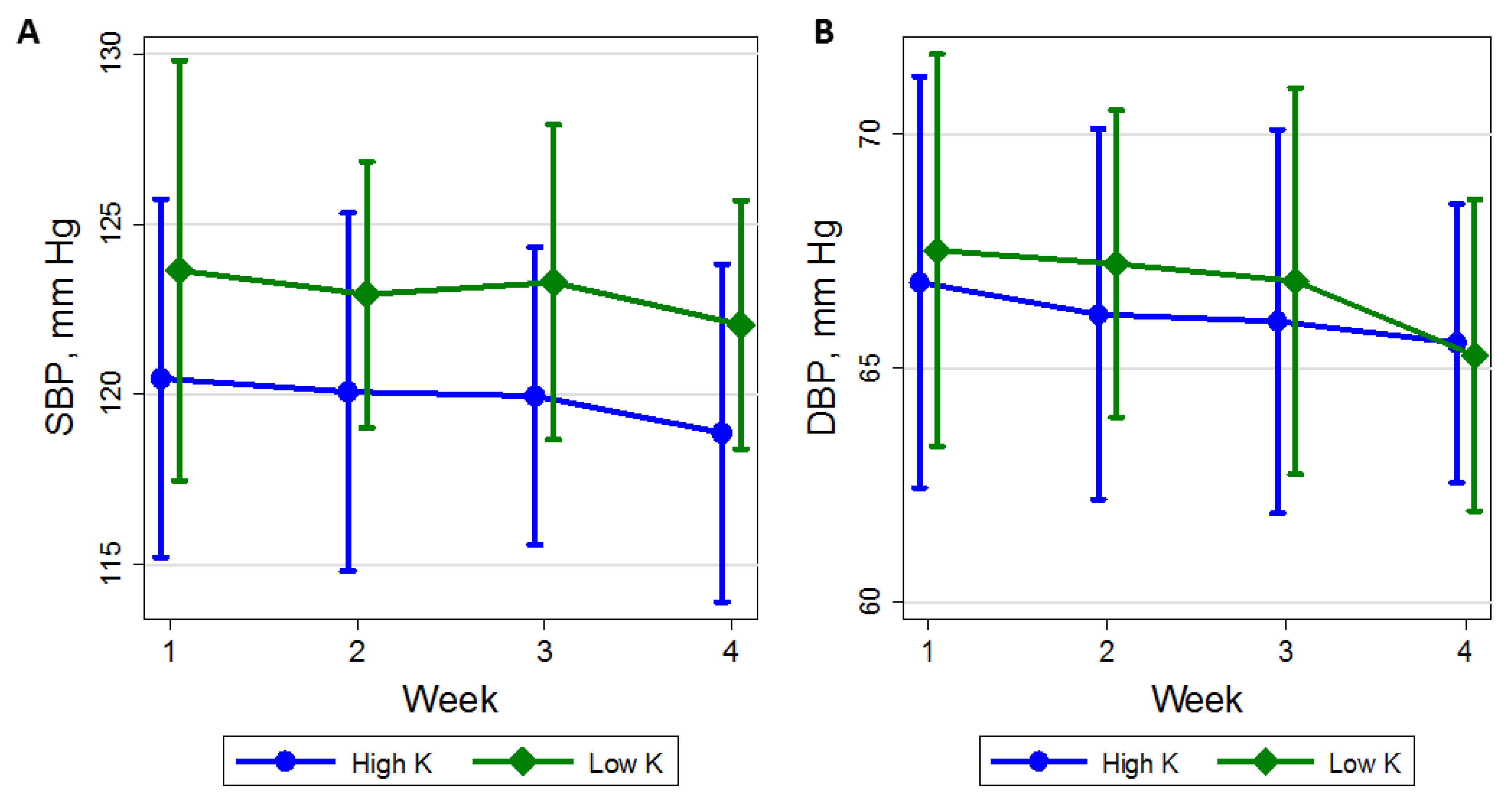
3.3. Time Course of BP
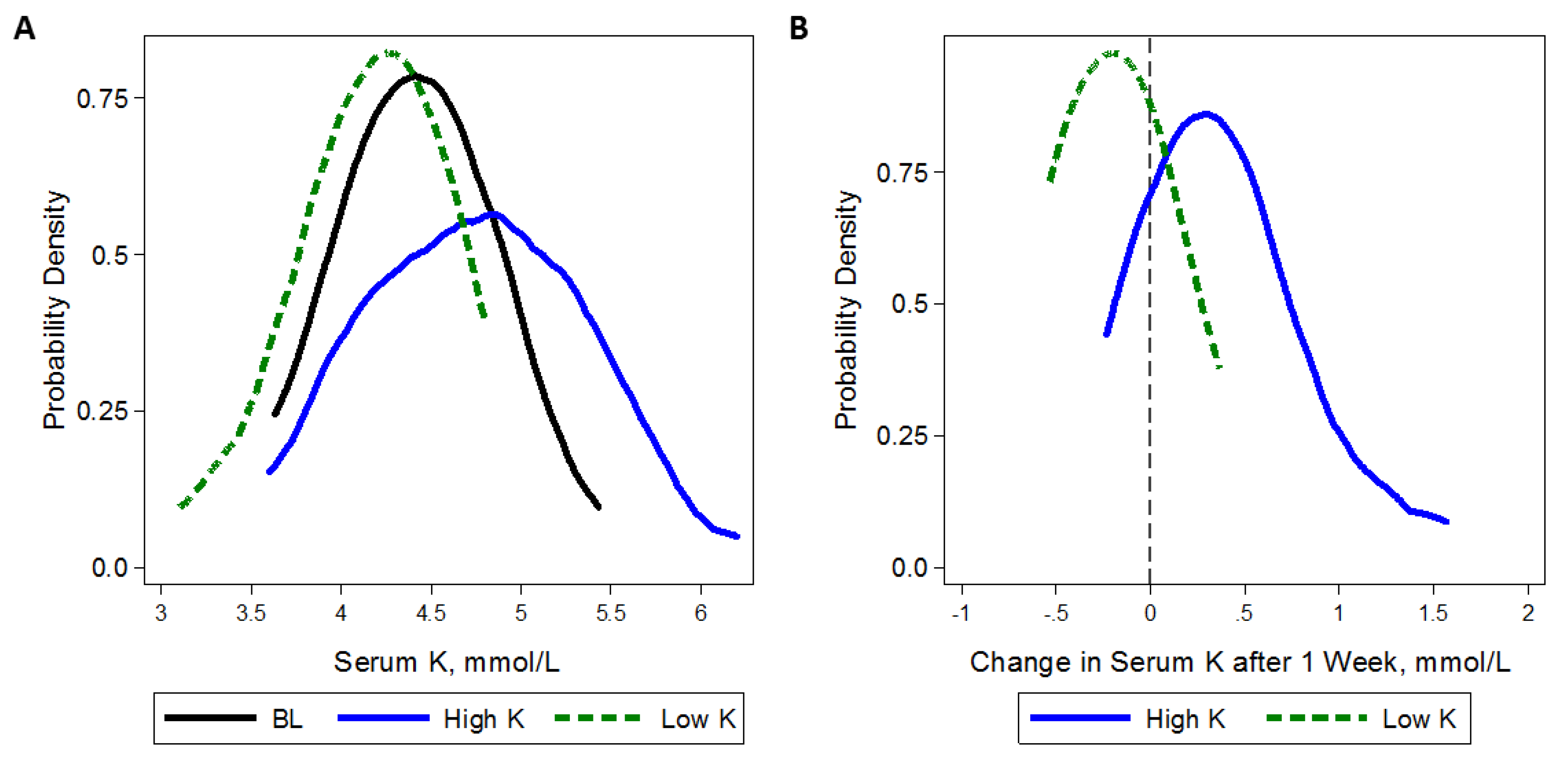
3.4. Adherence Measures and Safety Monitoring
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | Ambulatory Blood Pressure |
| BMI | Body Mass Index |
| K | Potassium |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| CKD | Chronic Kidney Disease |
| CI | Confidence Interval |
References
- National Academies of Sciences, Engineering and Medicine. Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Gritter, M.; Rotmans, J.I.; Hoorn, E.J. Role of Dietary K+ in Natriuresis, Blood Pressure Reduction, Cardiovascular Protection, and Renoprotection. Hypertension 2019, 73, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Banner, B.; Janosky, J.E.; Feig, P.U. Potassium supplementation attenuates experimental hypertensive renal injury. J. Am. Soc. Nephrol. 1992, 2, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Pere, A.K.; Krogerus, L.; Mervaala, E.M.; Karppanen, H.; Ahonen, J.; Lindgren, L. Beneficial effects of dietary magnesium and potassium on cardiac and renal morphologic features in cyclosporin A-induced damage in spontaneously hypertensive rats. Surgery 2000, 128, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pere, A.K.; Lindgren, L.; Tuomainen, P.; Krogerus, L.; Rauhala, P.; Laakso, J.; Karppanen, H.; Vapaatalo, H.; Ahonen, J.; Mervaala, E.M. Dietary potassium and magnesium supplementation in cyclosporine-induced hypertension and nephrotoxicity. Kidney Int. 2000, 58, 2462–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobian, L.; MacNeill, D.; Johnson, M.A.; Ganguli, M.C.; Iwai, J. Potassium protection against lesions of the renal tubules, arteries, and glomeruli and nephron loss in salt-loaded hypertensive Dahl S rats. Hypertension 1984, 6, I170. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, J.T.; Yoo, T.H.; Lee, J.; Chung, W.; Lee, K.B.; Chae, D.W.; Ahn, C.; Kang, S.W.; Choi, K.H.; et al. Urinary potassium excretion and progression of CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-based diets and incident CKD and kidney function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Jhee, J.H.; Kee, Y.K.; Park, J.T.; Chang, T.I.; Kang, E.W.; Yoo, T.H.; Kang, S.W.; Han, S.H. A diet rich in vegetables and fruit and incident CKD: A community-based prospective cohort study. Am. J. Kidney Dis. 2019, 74, 491–500. [Google Scholar] [CrossRef]
- K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am. J. Kidney Dis. 2004, 43, S1–S290.
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005; pp. 186–268. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Fortnightly review: Beneficial effects of potassium. BMJ 2001, 323, 497–501. [Google Scholar] [CrossRef]
- Kelleher, C.; Linas, S. The Patient with Hypokalemia or Hyperkalemia. In Manual of Nephrology; Schrier, R., Ed.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2004; p. 38. [Google Scholar]
- Musso, C.; Liakopoulos, V.; Stefanidis, I.; De Miguel, R.; Imperiali, N.; Algranati, L. Correlation between creatinine clearance and transtubular potassium concentration gradient in old people and chronic renal disease patients. Saudi J. Kidney Dis. Transpl. 2007, 18, 551–555. [Google Scholar]
- Burnier, M. Should we eat more potassium to better control blood pressure in hypertension? Nephrol. Dial. Transpl. 2018, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Picard, K.; Barreto Silva, M.I.; Mager, D.; Richard, C. Dietary potassium intake and risk of chronic kidney disease progression in predialysis patients with chronic kidney disease: A systematic review. Adv. Nutr. 2020, 11, 1002–1015. [Google Scholar] [CrossRef]
- Clase, C.M.; Carrero, J.J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Greene, T.; Kusek, J.W.; Beck, G.J. A simplified equation to predict glomerular filtration rate from serum creatinine. J. Am. Soc. Nephrol. 2000, 11, 155A. [Google Scholar]
- Whelton, P.K.; He, J.; Cutler, J.A.; Brancati, F.L.; Appel, L.J.; Follmann, D.; Klag, M.J. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997, 277, 1624–1632. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Lin, P.; Windhauser, M.; Plaisted, C.; Hoben, K.; McCullough, M.; Obarzanek, E. The Linear Index Model for establishing nutrient goals in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J. Am. Diet. Assoc. 1999, 99, S40–S44. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Schulze, M.B.; Kroke, A.; Bergmann, M.M.; Boeing, H. Differences of blood pressure estimates between consecutive measurements on one occasion: Implications for inter-study comparability of epidemiologic studies. Eur. J. Epidemiol. 2000, 16, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.J.; Kroon, A.A.; Kessels, A.G.; Lenders, J.W.; Thien, T.; van Montfrans, G.A.; Smit, A.J.; de Leeuw, P.W. The optimal scheme of self blood pressure measurement as determined from ambulatory blood pressure recordings. J. Hypertens. 2006, 24, 1541–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamler, R. Implications of the INTERSALT study. Hypertension 1991, 17, I16–I20. [Google Scholar] [CrossRef] [Green Version]
- White, H. A Heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980, 48, 817–838. [Google Scholar] [CrossRef]
- Tyson, C.C.; Lin, P.H.; Corsino, L.; Batch, B.C.; Allen, J.; Sapp, S.; Barnhart, H.; Nwankwo, C.; Burroughs, J.; Svetkey, L.P. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: A pilot feeding study. Clin. Kidney J. 2016, 9, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Workman, M.L.; Paller, M.S. Cardiovascular and endocrine effects of potassium in spontaneously hypertensive rats. Am. J. Physiol. 1985, 249, H907–H913. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Kok, F.J.; Grobbee, D.E. Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. J. Hum. Hypertens. 2003, 17, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Ascherio, A.; Rimm, E.B.; Hernán, M.A.; Giovannucci, E.L.; Kawachi, I.; Stampfer, M.J.; Willett, W.C. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 1998, 98, 1198–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguli, M.; Tobian, L.; Sugimoto, T. Deleterious effects of high magnesium diets and beneficial effects of high potassium diets in hypertensive stroke-prone rats. Magnes. Res. 1990, 3, 255–261. [Google Scholar]
- Khaw, K.T.; Barrett-Connor, E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N. Engl. J. Med. 1987, 316, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Tobian, L.; Lange, J.; Ulm, K.; Wold, L.; Iwai, J. Potassium reduces cerebral hemorrhage and death rate in hypertensive rats, even when blood pressure is not lowered. Hypertension 1985, 7, I110–I114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobian, L.; Lange, J.M.; Johnson, M.A.; MacNeill, D.A.; Wilke, T.J.; Ulm, K.M.; Wold, L.J. High-K diets reduce brain haemorrhage and infarcts, death rate and mesenteric arteriolar hypertrophy in stroke-prone spontaneously hypertensive rats. J. Hypertens. Suppl. 1986, 4, S205–S207. [Google Scholar] [PubMed]
- McCabe, R.D.; Bakarich, M.A.; Srivastava, K.; Young, D.B. Potassium inhibits free radical formation. Hypertension 1994, 24, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Soltero, L.; Zhang, P.; Huang, X.R.; Lan, H.Y.; Adrogue, H.J. Renal inflammation is modulated by potassium in chronic kidney disease: Possible role of Smad7. Am. J. Physiol. Ren. Physiol. 2007, 293, F1123–F1130. [Google Scholar] [CrossRef]
- McCabe, R.D.; Young, D.B. Potassium inhibits cultured vascular smooth muscle cell proliferation. Am. J. Hypertens 1994, 7, 346–350. [Google Scholar] [CrossRef]
- Sugimoto, T.; Tobian, L.; Ganguli, M.C. High potassium diets protect against dysfunction of endothelial cells in stroke-prone spontaneously hypertensive rats. Hypertension 1988, 11, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Ishimitsu, T.; Tobian, L.; Sugimoto, K.; Lange, J.M. High potassium diets reduce macrophage adherence to the vascular wall in stroke-prone spontaneously hypertensive rats. J. Vasc. Res. 1995, 32, 406–412. [Google Scholar] [CrossRef]
- Kimura, M.; Lu, X.; Skurnick, J.; Awad, G.; Bogden, J.; Kemp, F.; Aviv, A. Potassium chloride supplementation diminishes platelet reactivity in humans. Hypertension 2004, 44, 969–973. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Young, D.B. Interaction between plasma potassium and epinephrine in coronary thrombosis in dogs. Circulation 1994, 89, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Sudhir, K.; Kurtz, T.W.; Yock, P.G.; Connolly, A.J.; Morris, R.C., Jr. Potassium preserves endothelial function and enhances aortic compliance in Dahl rats. Hypertension 1993, 22, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, J.F.; Murakami, S.; Branch, R.A.; Sabra, R. Potassium depletion potentiates amphotericin-B-induced toxicity to renal tubules. Nephron 1995, 70, 235–241. [Google Scholar] [CrossRef]
- Bock, K.D.; Cremer, W.; Werner, U. Chronic hypokalemic nephropathy: A clinical study. Klin. Wochenschr. 1978, 56 (Suppl. 1), 91–96. [Google Scholar] [CrossRef] [PubMed]
- Elger, M.; Bankir, L.; Kriz, W. Morphometric analysis of kidney hypertrophy in rats after chronic potassium depletion. Am. J. Physiol. 1992, 262, F656–F667. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.E.; Suga, S.; Liu, X.H.; Huang, X.; Johnson, R.J. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int. 2001, 59, 1850–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguro, A.C.; Shimizu, M.H.; Monteiro, J.L.; Rocha, A.S. Effect of potassium depletion on ischemic renal failure. Nephron 1989, 51, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Young, W.F., Jr.; Offord, K.P.; Hattery, R.R. Association of hypokalemia, aldosteronism, and renal cysts. N. Engl. J. Med. 1990, 322, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Scholey, J.W.; Sonnenberg, H. Renal vascular morphology in male Dahl rats on high-salt diet: Effect of potassium. J. Am. Soc. Nephrol. 1996, 7, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.S.; Panese, S.; Virginillo, M.; Gimenez, M.; Litardo, M.; Arrizurieta, E.; Hayslett, J.P. Increased secretion of potassium in the rectum of humans with chronic renal failure. Am. J. Kidney Dis. 1986, 8, 105–110. [Google Scholar] [CrossRef]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient non-equivalence: Does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nicola, L.; Di Lullo, L.; Paoletti, E.; Cupisti, A.; Bianchi, S. Chronic hyperkalemia in non-dialysis CKD: Controversial issues in nephrology practice. J. Nephrol. 2018, 31, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asirvatham, J.R.; Moses, V.; Bjornson, L. Errors in potassium measurement: A laboratory perspective for the clinician. N. Am. J. Med. Sci. 2013, 5, 255–259. [Google Scholar] [CrossRef]
- Dylewski, J.F.; Linas, S. Variability of potassium blood testing: Imprecise nature of blood testing or normal physiologic changes? Mayo Clin. Proc. 2018, 93, 551–554. [Google Scholar] [CrossRef] [Green Version]
- Korgaonkar, S.; Tilea, A.; Gillespie, B.W.; Kiser, M.; Eisele, G.; Finkelstein, F.; Kotanko, P.; Pitt, B.; Saran, R. Serum potassium and outcomes in CKD: Insights from the RRI-CKD cohort study. Clin. J. Am. Soc. Nephrol. 2010, 5, 762–769. [Google Scholar] [CrossRef]
- Luo, J.; Brunelli, S.M.; Jensen, D.E.; Yang, A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin. J. Am. Soc. Nephrol. 2016, 11, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sang, Y.; Ballew, S.H.; Tin, A.; Chang, A.R.; Matsushita, K.; Coresh, J.; Kalantar-Zadeh, K.; Molnar, M.Z.; Grams, M.E. race, serum potassium, and associations with ESRD and mortality. Am. J. Kidney Dis. 2017, 70, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 Update. Am. J. Kidney Dis 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Miller, E.R., III; Weaver, C.M.; Appel, L.J. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef]
| CKD-K Study Diet (Per 2000 kcal/day) | KDOQI Guidelines for Stage 3–4 CKD [10] | ||
|---|---|---|---|
| Lower-K | Higher-K | ||
| K, mg (mmol) | 1600 mg (40 mmol) | 3900 mg (100 mmol) | 2000–4000 mg (51–102 mmol) |
| Na, mg (mmol) | 3300 mg (143 mmol) | 3300 mg (143 mmol) | <2400 mg (<104 mmol) |
| PO4, mg | 1000 | 1000 | 800–1000 |
| Protein, % kcal (g) | 13% (65 g) | 13% (65 g) | ~10% (0.6–0.8 mg/kg) |
| Carbs, % kcal (g) | 50% (250 g) | 50% (250 g) | 50–60 |
| Sat Fat, % kcal | <10 | <10 | <10 |
| Age, years | 67.2 (11.6) |
| Women, % | 58.6 |
| Black, % | 69.0 |
| Medications for: | |
| Diabetes | 24.1% |
| Hypertension | 93.1% |
| Specific types of medications | |
| ACEI or ARB | 58.6% |
| K-sparing diuretic | 13.8% |
| K-wasting diuretic | 58.6% |
| Tacrolimus | 6.9% |
| Body Mass Index, kg/m2 | 31.4 (4.7) |
| Mean clinic BP, mm Hg | |
| SBP | 128.4 (13.0) |
| DBP | 71.5 (7.7) |
| Serum creatinine, mg/dL | 1.3 (0.2) |
| eGFR *, mL/min/1.73 m2 | 54.5 (11.7) |
| N | Baseline | Lower-K | Higher-K | Difference (95% CI)) * | p-Value | |
|---|---|---|---|---|---|---|
| Systolic Blood Pressure (mmHg) | ||||||
| Clinic | 25 | 121.5 (11.9) | 122.8 (9.2) | 118.6 (12.4) | −4.21 (−8.49, 0.07) | 0.054 |
| ** 24 H Ambulatory | 24 | 127.1 (14.5) | 126.2 (12.2) | 124.1 (11.7) | −2.12 (−5.12, 0.87) | 0.16 |
| Daytime Ambulatory | 24 | 131.6 (14.5) | 129.1 (11.4) | 126.9 (10.5) | −2.26 (−5.44, 0.93) | 0.17 |
| Nighttime Ambulatory | 24 | 118.5 (16.4) | 121.0 (14.9) | 118.4 (16.4) | −2.54 (−6.27, 1.19) | 0.18 |
| Diastolic Blood Pressure (mmHg) | ||||||
| Clinic | 25 | 67.3 (9.7) | 65.2 (8.8) | 65.1 (7.2) | −0.08 (−2.25, 2.09) | 0.94 |
| 24 H Ambulatory | 24 | 71.6 (9.1) | 71.0 (7.8) | 70.3 (7.0) | −0.70 (−2.46, 1.06) | 0.44 |
| Daytime Ambulatory | 24 | 75.0 (9.4) | 74.2 (7.9) | 73.3 (7.5) | −0.97 (−2.90, 0.97) | 0.33 |
| Nighttime Ambulatory | 24 | 65.0 (10.3) | 65.6 (8.5) | 65.0 (8.4) | −0.62 (−2.67, 1.43) | 0.55 |
| Adherence Measures | ||||||
| Urine K, mmol/day | 24 | 53.2 (23.7) | 39.9 (16.2) | 81.4 (33.6) | 41.52 (28.38,54.66) | <0.001 |
| Urine Na, mmol/day | 24 | 128.8 (66.2) | 131.6 (63.1) | 118.5 (41.3) | −13.13 (−36.26,10.00) | 0.27 |
| Serum K, mmol/L | 25 | 4.5 (0.5) | 4.2 (0.4) | 4.4 (0.4) | 0.21 (0.07, 0.35) | 0.003 |
| Urine Cr, gm/day | 24 | 1.1 (0.4) | 1.2 (0.6) | 1.1 (0.4) | −0.08 (−0.30,0.1) | 0.46 |
| Urine Vol, L/day | 25 | 2.0 (1.0) | 1.7 (0.5) | 1.7 (0.7) | 0.04 (−0.1,0.2) | 0.66 |
| Week 1 | Week 2 | Week 4 | OR (95% CI) * p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | N with Hyperkalemia | % | Total N | N with Hyperkalemia | % | Total N | N with Hyperkalemia | % | ||
| High Potassium | 28 | 3 | 10.7 | 27 | 2 | 7.4 | 26 | 0 | 0.0 | 2.50 (1.04, 6.00) 0.04 |
| Low Potassium | 28 | 0 | 0.0 | 26 | 1 | 3.8 | 26 | 1 | 3.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turban, S.; Juraschek, S.P.; Miller, E.R., III; Anderson, C.A.M.; White, K.; Charleston, J.; Appel, L.J. Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease. Nutrients 2021, 13, 2678. https://doi.org/10.3390/nu13082678
Turban S, Juraschek SP, Miller ER III, Anderson CAM, White K, Charleston J, Appel LJ. Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease. Nutrients. 2021; 13(8):2678. https://doi.org/10.3390/nu13082678
Chicago/Turabian StyleTurban, Sharon, Stephen P. Juraschek, Edgar R. Miller, III, Cheryl A. M. Anderson, Karen White, Jeanne Charleston, and Lawrence J. Appel. 2021. "Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease" Nutrients 13, no. 8: 2678. https://doi.org/10.3390/nu13082678
APA StyleTurban, S., Juraschek, S. P., Miller, E. R., III, Anderson, C. A. M., White, K., Charleston, J., & Appel, L. J. (2021). Randomized Trial on the Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease. Nutrients, 13(8), 2678. https://doi.org/10.3390/nu13082678






