Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment of Nutritional Intake
2.3. Fecal Collection, DNA Extraction and 16S rRNA Gene Profiles
2.4. Statistical Analysis
3. Results
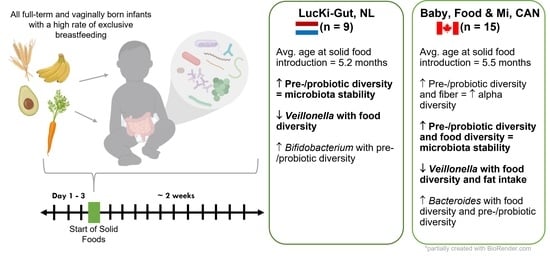
3.1. Study Population
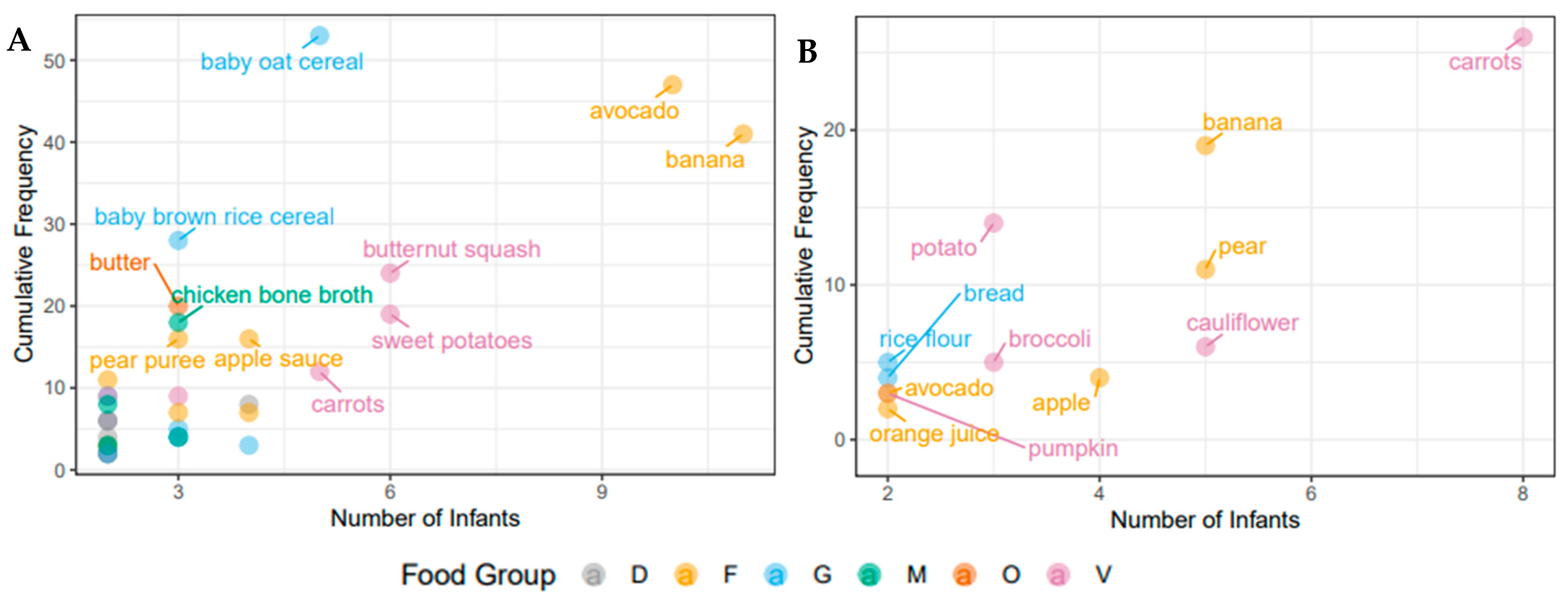
3.2. Nutritional Intake within Each Cohort at the Time of Solid Food Introduction
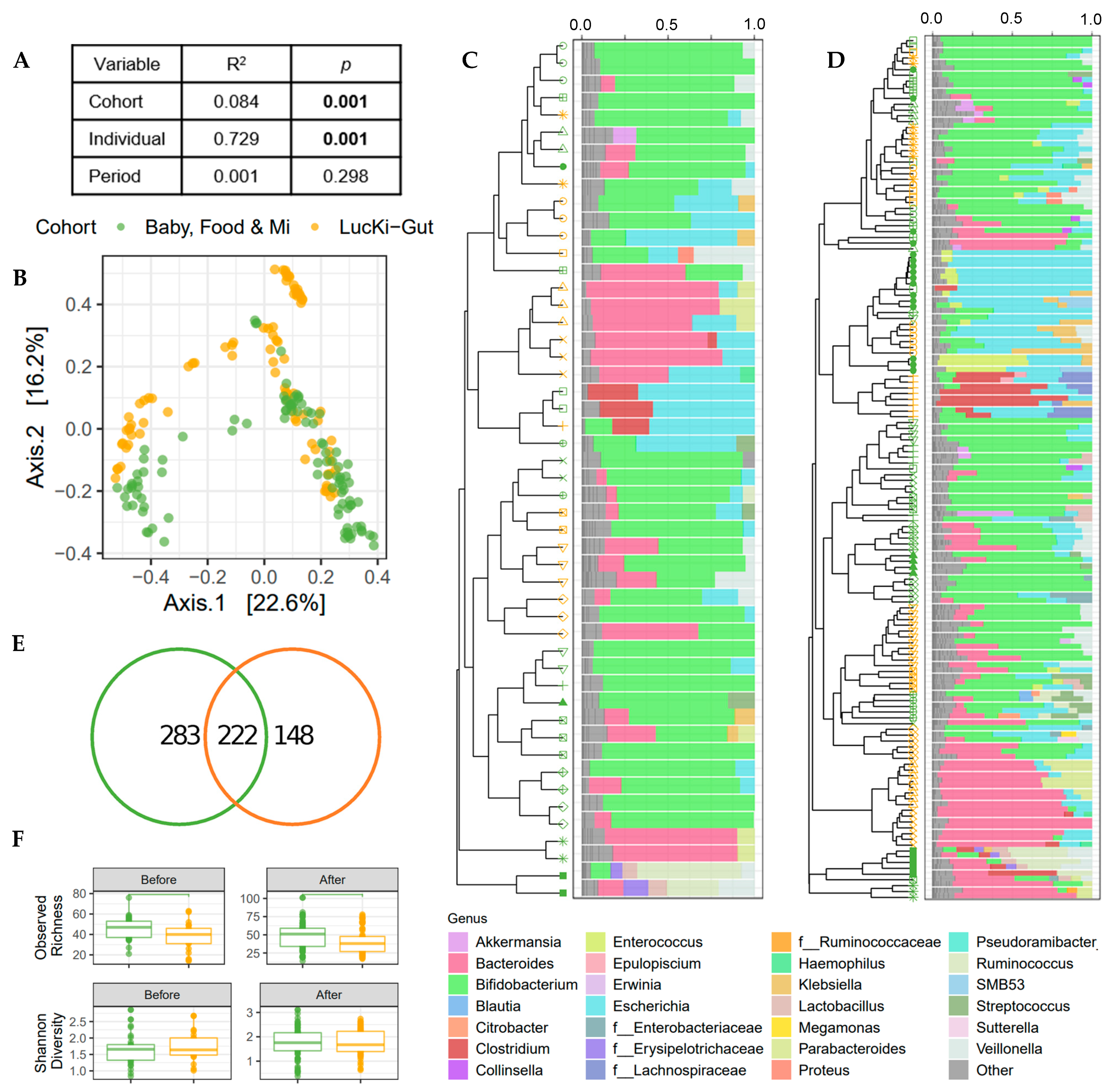
3.3. Microbial Community Structure Was Associated with Individual and Cohort
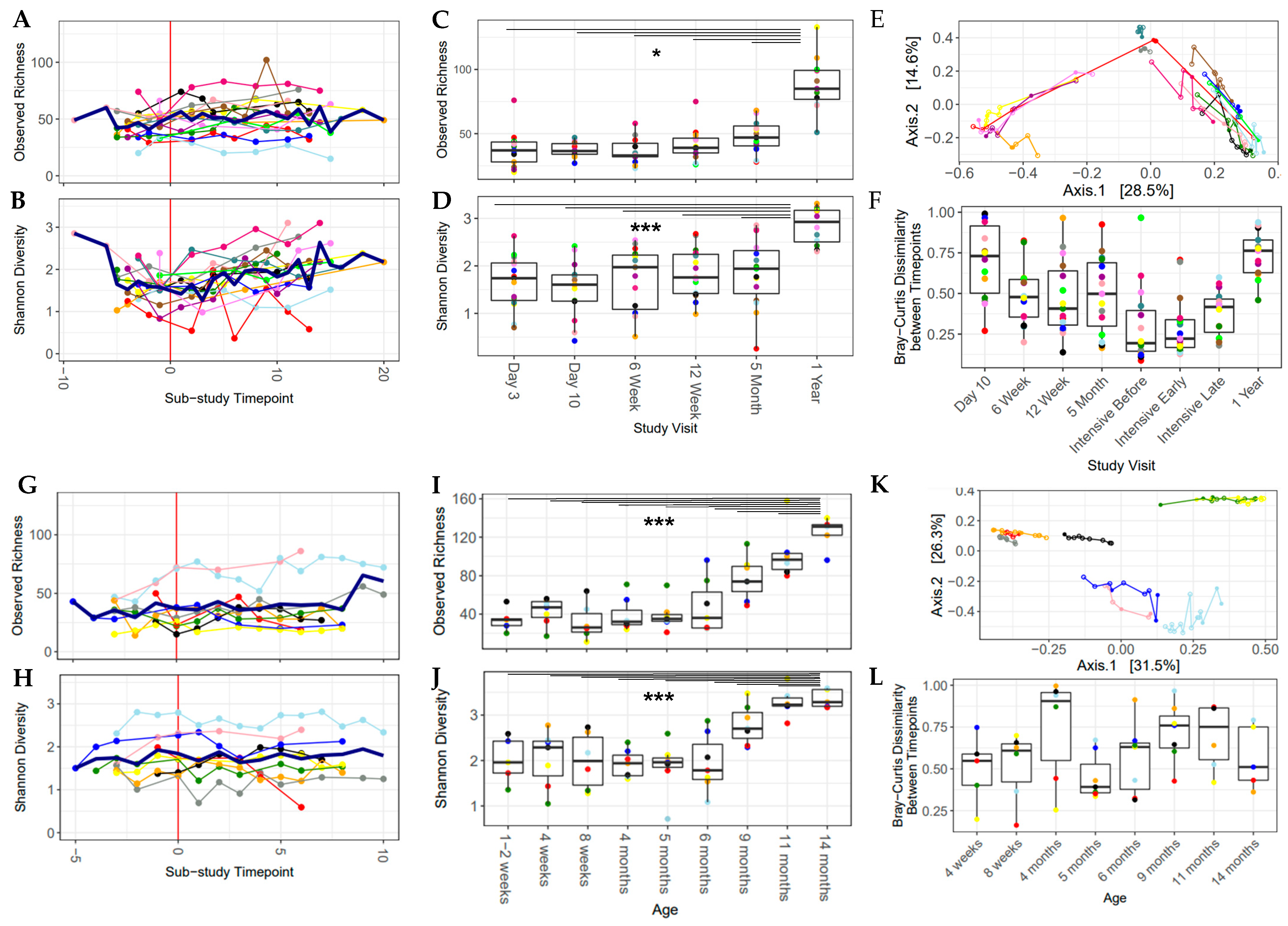
3.4. Alpha Diversity Increased over the Study Period
3.5. Alpha Diversity Was Associated with Dietary Variables at the Time of Solid Food Introduction
3.6. Increased Dietary Diversity Stabilized the Gut Microbiota at the Time of Solid Food Introduction
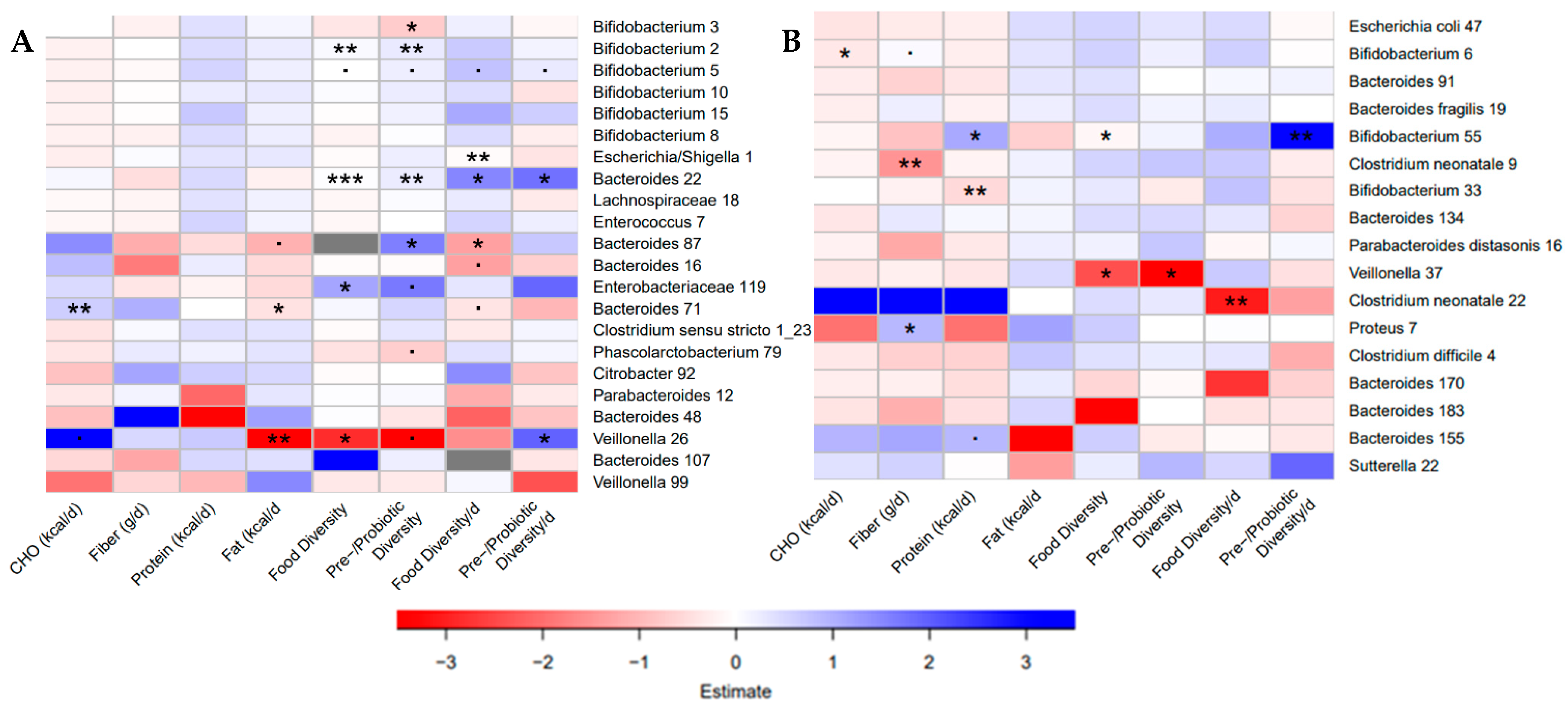
3.7. Bacterial ASVs Associated with Nutritional Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cresci, G.; Bawden, E. The gut microbiome: What we do and don’t know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Jandhyala, S.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Galazzo, G.; van Best, N.; Bervoets, L.; Dapaah, I.; Savelkoul, P.; Hornef, M.; GI-MDH Consortium; Lau, S.; Hamelmann, E.; Penders, J. Development of the microbiota and associations with birth mode, diet and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 2020, 158, 1584–1596. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Gordon, J. The core gut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.; German, J.; Lebrilla, C.; Mills, D. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stearns, J.; Simioni, J.; Gunn, E.; McDonald, H.; Holloway, A.; Thabane, L.; Mousseau, A.; Schertzer, J.; Ratcliffe, E.; Rossi, L.; et al. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Bazanella, M.; Maier, T.; Clavel, T.; Lagkouvardos, I.; Lucio, M.; Maldonado-Gomez, M.; Autran, C.; Walter, J.; Bode, L.; Schmitt-Kopplin, P.; et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am. J. Clin. Nutr. 2017, 106, 1274–1286. [Google Scholar] [CrossRef] [Green Version]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.; Snijders, B.; Kummeling, I.; van den Brandt, P.; Stobberingh, E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernandez, N.; Solis, G.; Hernandez-Barranco, A.; Margolles, A.; de los Reyes-Gavilan, C.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Yatsunenko, T.; Rey, F.; Manary, M.; Trehan, I.; Dominguez-Bello, M.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.; Anokhin, A.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–228. [Google Scholar] [CrossRef]
- Baumann-Dudenhoeffer, A.; D’Souza, A.; Tarr, P.; Warner, B.; Dantas, G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat. Med. 2018, 24, 1822–1829. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.; Norin, E.; Young, D.; et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, J.; Lange, N.; Frick, J.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Versalovic, J. The human microbiome and its potential importance to pediatrics. Pediatrics 2012, 129, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, J.; Spor, A.; Scalfone, N.; Fricker, A.; Stombaugh, J.; Knight, R.; Angenent, L.; Ley, R. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Valles, Y.; Artacho, A.; Pascual-Garcia, A.; Ferrus, M.; Gosalbes, M.; Abellan, J.; Francino, M. Microbial succession in the gut: Directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet. 2014, 10, e1004406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannaraj, P.; Li, F.; Cerini, C.; Bender, J.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.; Bittinger, K.; et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Simioni, J.; Hutton, E.; Gunn, E.; Holloway, A.; Stearns, J.; McDonald, H.; Mousseau, A.; Schertzer, J.; Ratcliffe, E.; Thabane, L.; et al. A comparison of intestinal microbiota in a population of low-risk infants exposed and not exposed to intrapartum antibiotics: The Baby & microbiota of the intestine cohort study protocol. BMC Pediatr. 2016, 16, 183. [Google Scholar] [CrossRef] [Green Version]
- de Korte-de Boer, D.; Mommers, M.; Creemers, H.; Dompeling, E.; Feron, F.; Gielkens-Sijstermans, C.; Jaminon, M.; Mujakovic, S.; van Schayk, O.; Thijs, C.; et al. LucKi birth cohort study: Rationale and design. BMC Public Health 2015, 15, 934. [Google Scholar] [CrossRef] [Green Version]
- Dizzell, S.; Stearns, J.; Li, J.; Van Best, N.; Bervoets, L.; Mommers, M.; Penders, J.; Morrison, K.; Hutton, E. Investigating colonization patterns of the infant gut microbiome during the introduction of solid food and weaning from breastmilk: A cohort study protocol. PLoS ONE 2021, 16, e0248924. [Google Scholar] [CrossRef]
- Stearns, J.; Davidson, C.; McKeon, S.; Whelan, F.; Fontes, M.; Schryvers, A. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 2015, 9, 1256–1259. [Google Scholar] [CrossRef] [Green Version]
- Bartram, A.; Lynch, M.; Stearns, J.; Moreno-Hagelsieb, G.; Neufeld, J. Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end illumina reads. Appl. Environ. Microbiol. Am. Soc. Microbiol. 2011, 77, 3846–3852. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 1–10. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.; Johnson, A.; Holmes, S. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e061217. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.S.; et al. Vegan: Community Ecology Package. R Package Version 2.5-5. 2020. Available online: https://cran.r-project.org (accessed on 8 January 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Yu, G. Using ggtree to visualize data on tree-like structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef]
- Wright, E. DECIPHER: Harnessing local sequence context to improve protein multiple sequence alignment. BMC Bioinform. 2015, 16, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schliep, K. Phangorn: Phylogenetic analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 406–425. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 526–528. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Software, I. GraphPad QuickCalcs. Available online: https://www.graphpad.com/quickcalcs/ttest1/ (accessed on 21 April 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Delignette-Muller, M.; Dutang, C. fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.; Kristensen, K.; van Benthem, K.; Magnusson, A.; Berg, C.; Nielsen, A.; Skaug, H.; Maechler, M.; Bolker, B. glmmTMB Balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Rapley, G. Baby-Led Weaning; Quay Books: London, UK, 2005. [Google Scholar]
- Brown, A.; Jones, S.; Rowan, H. Baby-led weaning: The evidence to date. Curr. Nutr. Rep. 2017, 6, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.; Smits, S.; Tikhonov, M.; Higginbottom, S.; Wingreen, N.; Sonnenburg, J. Diet-induced extinction in the gut microbiota compounds over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, K.; Mokkala, K. Overall dietary quality relates to gut microbiota diversity and abundance. Int. J. Mol. Sci. 2019, 20, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.; Konya, T.; Maughan, H.; Guttman, D.; Field, C.; Chari, R.; Sears, M.; Becker, A.; Scott, J.; Kozyrskyj, A. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. Cmaj 2013, 185, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Schwab, C.; Gaenzle, M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson, M.; Jeffery, I.; Conde, S.; Power, S.; O’Connor, E.; Cusack, S.; Harris, H.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.; Greenway, F. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef]
- Johnson, A.; Vangay, P.; Al-Ghalith, G.; Hillmann, B.; Ward, T.; Shields-Cutler, R.; Kim, A.; Shmagel, A.; Syed, A.; Personalized Microbiome Class Students; et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.; Penders, J.; Smidt, H.; Zoetendal, E. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut Microbes 2020, 70, 595–605. [Google Scholar] [CrossRef]
- Zaneveld, J.; McMinds, R.; Vega Thurber, R. Stress and stability: Applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Matsumaya, M.; Gomez-Arango, L.; Fukuma, N.; Morrison, M.; Davies, P.; Hill, R. Breastfeeding: A key modulator of gut microbiota characteristics in late infancy. J. Dev. Orig. Health Dis. 2018, 10, 206–213. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; Van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Baby, Food & Mi | LucKi-Gut | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Median | Range | Mean (SD) | n (%) | Median | Range | Mean (SD) | Sig. 1 | |
| Age at Introduction (months) ++ | 15 (100) | 5.79 | 3.98, 6.5 | 5.5 (0.66) | 9 (100) | 5.03 | 4.37, 6.12 | 5.2 (0.66) | |
| Gestational Age at Birth 2,+ | |||||||||
| Completed 37 weeks | 0 (0) | - | - | - | 1 (14) | - | - | - | |
| Completed 38 weeks | 3 (20) | - | - | - | 0 (0) | - | - | - | |
| Completed 39 weeks | 4 (27) | - | - | - | 1 (14) | - | - | - | |
| Completed 40 weeks | 6 (40) | - | - | - | 2 (29) | - | - | - | |
| Completed 41 weeks | 2 (13) | - | - | - | 2 (29) | - | - | - | |
| Completed 42 weeks | 0 (0) | 1 (14) | - | - | - | ||||
| Female Sex + | 7 (47) | - | - | - | 4 (44) | - | - | - | |
| Parity 2,+ | |||||||||
| 0 | 6 (40) | - | - | - | 4 (57) | - | - | - | |
| 1 | 4 (27) | - | - | - | 2 (29) | - | - | - | |
| 2 | 5 (33) | - | - | - | 1 (13) | - | - | - | |
| Hospital Birth (Y) 3,+ | 9 (60) | - | - | - | 5 (71) | - | - | - | |
| Gestational Diabetes (Y) | 4 (27) | - | - | - | - | - | - | - | |
| Pre-pregnancy BMI (kg/m2) ++ | 15 (100) | 23.5 | 17.7, 24.0 | 24.1 (4.16) | 7 (78) | 19.9 | 18.6, 24.7 | 20.5 (1.97) | * |
| GBS Prophylaxis (Y) + | 3 (20) | - | - | - | 0 (0) | - | - | - | |
| Infant Oral Abx (Y) + | 1 (7) | - | - | - | 0 (0) | - | - | - | |
| Infant Oral Pbx (Y) + | 5 (33) | - | - | - | 0 (0) | - | - | - | |
| Maternal Oral Abx (Y) + | 1 (7) | - | - | - | 0 (0) | - | - | - | |
| Baby, Food & Mi | LucKi-Gut | ||||
|---|---|---|---|---|---|
| Mean (SD) | Range (Min, Max) | Mean (SD) | Range (Min, Max) | Sig. 1 | |
| Estimated total energy from solid food (kcal/d) | 53.9 (64.06) | 468.8 (0.00, 468.8) | 28.9 (24.5) | 109 (0.00, 109.00) | *** |
| Carbohydrates (kcal/d) | 25.7 (32.45) | 167.9 (0.00, 167.9) | 18.5 (19.9) | 94.8 (0.00, 94.8) | * |
| Fat (kcal/d) | 21.2 (34.71) | 246.9 (0.00, 346.9) | 6.6 (11.3) | 70.2 (0.00, 70.2) | *** |
| Protein (kcal/d) | 6.92 (9.07) | 60.7 (0.00, 60.7) | 1.9 (2.1) | 11.2 (0.00, 11.2) | *** |
| Fiber (g/d) | 1.0 (1.62) | 8.98 (0.00, 8.98) | 0.8 (0.8) | 3.90 (0.00, 3.90) | |
| Food Diversity | 6.34 (5.05) | 14.8 (0.800, 15.6) | 2.9 (2.8) | 8.90 (0.40, 9.30) | * |
| Pre-/Probiotic Diversity | 7.8 (5.54) | 16.7 (0.00, 16.7) | 5.9 (2.5) | 10.9 (2.00, 12.9) | |
| Food Diversity (/d) | 8.31 (9.80) | 55.0 (0.00, 55.0) | 4.2 (4.7) | 20.0 (0.00, 20.0) | *** |
| Pre-/Probiotic Diversity (/d) | 25.1 (24.15) | 100.0 (0.00, 100.0) | 15.2 (15.2) | 60.0 (0.00, 60.0) | *** |
| Baby, Food & Mi | LucKi-Gut | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable 1 | Univariable | Multivariable 1 | |||||
| Estimate | Sig. | Estimate | Sig. | Estimate | Sig. | Estimate | Sig. | |
| Age (d) | −0.0010 | 0.80 | - | - | −0.0052 | 0.20 | - | - |
| Sex | −0.17 | 0.28 | - | - | −0.16 | 0.31 | - | - |
| Total energy (kcal/d) | −0.0028 | 0.025 | - | - | −0.0001 | 0.97 | - | - |
| Carbohydrates (kcal/d) | −0.0043 | 0.12 | −0.0029 | 0.15 | −0.0004 | 0.91 | −0.0016 | 0.80 |
| Fiber (g/d) | −0.069 | 0.17 | 0.043 | 0.60 | −0.025 | 0.76 | −0.048 | 0.68 |
| Protein (kcal/d) | −0.019 | 0.036 | −0.0077 | 0.68 | −0.045 | 0.12 | −0.11 | 0.01 |
| Fat (kcal/d) | −0.044 | 0.030 | 0.0019 | 0.33 | 0.0028 | 0.58 | 0.0033 | 0.60 |
| Food Diversity (/d) | −0.033 | 0.0003 | −0.026 | 0.0079 | −0.008 | 0.62 | −0.0088 | 0.60 |
| Pre-/Probiotic Diversity (/d) | −0.016 | <0.0001 | −0.014 | 0.00014 | −0.0077 | 0.007 | −0.008 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homann, C.-M.; Rossel, C.A.J.; Dizzell, S.; Bervoets, L.; Simioni, J.; Li, J.; Gunn, E.; Surette, M.G.; de Souza, R.J.; Mommers, M.; et al. Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts. Nutrients 2021, 13, 2639. https://doi.org/10.3390/nu13082639
Homann C-M, Rossel CAJ, Dizzell S, Bervoets L, Simioni J, Li J, Gunn E, Surette MG, de Souza RJ, Mommers M, et al. Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts. Nutrients. 2021; 13(8):2639. https://doi.org/10.3390/nu13082639
Chicago/Turabian StyleHomann, Chiara-Maria, Connor A. J. Rossel, Sara Dizzell, Liene Bervoets, Julia Simioni, Jenifer Li, Elizabeth Gunn, Michael G. Surette, Russell J. de Souza, Monique Mommers, and et al. 2021. "Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts" Nutrients 13, no. 8: 2639. https://doi.org/10.3390/nu13082639
APA StyleHomann, C.-M., Rossel, C. A. J., Dizzell, S., Bervoets, L., Simioni, J., Li, J., Gunn, E., Surette, M. G., de Souza, R. J., Mommers, M., Hutton, E. K., Morrison, K. M., Penders, J., van Best, N., & Stearns, J. C. (2021). Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts. Nutrients, 13(8), 2639. https://doi.org/10.3390/nu13082639