Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes
Abstract
1. Introduction
2. Nutrition Guidelines and Adherence in Children and Adolescents with T1D
3. Fat intake and Glycemic Control
3.1. Food Intake and Postprandial Glycemic Control
3.2. Fat Intake and HbA1c
3.3. Low-Carbohydrate (High-Fat) Diets
4. Fat Intake and Cardiovascular Diseases
4.1. General Population
4.2. Individuals with T1D
5. Fat Intake and Inflammation in Individuals with T1D
5.1. High-Fat Diets and Inflammation
5.2. Inflammation in People with T1D
5.3. The Impact of Diet on Inflammatory Markers in People with T1D
5.4. N-3 PUFA Supplementation in T1D Prevention and Treatment
6. Fat Intake and Microbiota
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| CC | carbohydrate counting |
| CHD | coronary heart disease |
| CVD | cardiovascular diseases |
| DBP | diastolic blood pressure |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| FII | Food Insulin Index |
| HbA1c | glycated hemoglobin A1c |
| HDL | high-density lipoprotein |
| HF/HP | high fat/high protein |
| hsCRP | high-sensitivity C-reactive protein |
| IL-6 | interleukin-6 |
| ISPAD | International Society for Pediatric and Adolescent Diabetes |
| LDL | low-density lipoprotein |
| LF/LP | low fat/low protein; LPS, lipopolysaccharides |
| MUFA | monounsaturated fatty acid |
| Nf-κB | nuclear Factor kappa-light-chain-enhancer-of activated B cells |
| NOD | non-obese diabetic |
| PUFA | polyunsaturated fatty acids |
| SCFA | short-chain fatty acids |
| SFA | saturated fatty acid |
| T1D | type 1 diabetes |
| TLR4 | toll-like receptor 4 |
| TNF-α | Tumor Necrosis Factor-α |
| Treg | regulatory T cells |
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Simmons, K.M. Type 1 Diabetes: A Predictable Disease. World J. Diabetes 2015, 6, 380–390. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic Control Targets and Glucose Monitoring for Children, Adolescents, and Young Adults with Diabetes. Pediatric Diabetes 2018, 19, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional Management in Children and Adolescents with Diabetes. Pediatric Diabetes 2018, 19, 136–154. [Google Scholar] [CrossRef]
- Adolfsson, P.; Riddell, M.C.; Taplin, C.E.; Davis, E.A.; Fournier, P.A.; Annan, F.; Scaramuzza, A.E.; Hasnani, D.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in Children and Adolescents with Diabetes. Pediatric Diabetes 2018, 19, 205–226. [Google Scholar] [CrossRef]
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S180–S199. [Google Scholar] [CrossRef]
- Shi, M.; Tang, R.; Huang, F.; Zhong, T.; Chen, Y.; Li, X.; Zhou, Z. Cardiovascular Disease in Patients with Type 1 Diabetes: Early Evaluation, Risk Factors and Possible Relation with Cardiac Autoimmunity. Diabetes Metab. Res. Rev. 2020, e3423. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Pihoker, C.; Forsander, G.; Fantahun, B.; Virmani, A.; Corathers, S.; Benitez-Aguirre, P.; Fu, J.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: The Delivery of Ambulatory Diabetes Care to Children and Adolescents with Diabetes. Pediatric Diabetes 2018, 19, 84–104. [Google Scholar] [CrossRef]
- Bebu, I.; Braffett, B.H.; Orchard, T.J.; Lorenzi, G.M.; Lachin, J.M. Mediation of the Effect of Glycemia on the Risk of CVD Outcomes in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 2019, 42, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Freese, R.; Mäkimattila, S.; Forsblom, C.; Groop, P.-H. Dietary Patterns Are Associated with Various Vascular Health Markers and Complications in Type 1 Diabetes. J. Diabetes Complicat. 2016, 30, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Mehta, S.; Nansel, T.; Quinn, H.; Lipsky, L.M.; Laffel, L.M.B. Associations of Nutrient Intake with Glycemic Control in Youth with Type 1 Diabetes: Differences by Insulin Regimen. Diabetes Technol. Ther. 2014, 16, 512–518. [Google Scholar] [CrossRef]
- Maffeis, C.; Morandi, A.; Ventura, E.; Sabbion, A.; Contreas, G.; Tomasselli, F.; Tommasi, M.; Fasan, I.; Costantini, S.; Pinelli, L. Diet, Physical, and Biochemical Characteristics of Children and Adolescents with Type 1 Diabetes: Relationship between Dietary Fat and Glucose Control. Pediatric Diabetes 2012, 13, 137–146. [Google Scholar] [CrossRef]
- Delahanty, L.M.; Nathan, D.M.; Lachin, J.M.; Hu, F.B.; Cleary, P.A.; Ziegler, G.K.; Wylie-Rosett, J.; Wexler, D.J. Association of Diet with Glycated Hemoglobin during Intensive Treatment of Type 1 Diabetes in the Diabetes Control and Complications Trial. Am. J. Clin. Nutr. 2009, 89, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ayano-Takahara, S.; Ikeda, K.; Fujimoto, S.; Asai, K.; Oguri, Y.; Harashima, S.I.; Tsuji, H.; Shide, K.; Inagaki, N. Carbohydrate Intake Is Associated with Time Spent in the Euglycemic Range in Patients with Type 1 Diabetes. J. Diabetes Investig. 2015, 6, 678–686. [Google Scholar] [CrossRef]
- Maffeis, C.; Fornari, E.; Morandi, A.; Piona, C.; Tomasselli, F.; Tommasi, M.; Marigliano, M. Glucose-Independent Association of Adiposity and Diet Composition with Cardiovascular Risk in Children and Adolescents with Type 1 Diabetes. Acta Diabetol. 2017, 54, 599–605. [Google Scholar] [CrossRef]
- Mackey, E.R.; Rose, M.; Tully, C.; Monaghan, M.; Hamburger, S.; Herrera, N.; Streisand, R. The Current State of Parent Feeding Behavior, Child Eating Behavior, and Nutrition Intake in Young Children with Type 1 Diabetes. Pediatric Diabetes 2020, 21, 841–845. [Google Scholar] [CrossRef]
- Seckold, R.; Howley, P.; King, B.R.; Bell, K.; Smith, A.; Smart, C.E. Dietary Intake and Eating Patterns of Young Children with Type 1 Diabetes Achieving Glycemic Targets. BMJ Open Diabetes Res. Care 2019, 7, e000663. [Google Scholar] [CrossRef]
- Stechova, K.; Hlubik, J.; Pithova, P.; Cikl, P.; Lhotska, L. Comprehensive Analysis of the Real Lifestyles of T1D Patients for the Purpose of Designing a Personalized Counselor for Prandial Insulin Dosing. Nutrients 2019, 11, 1148. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.; Adams, L.; Anderson, J.; Maftei, O.; Couper, J.; Giles, L.; Peña, A.S. Australian Children with Type 1 Diabetes Consume High Sodium and High Saturated Fat Diets: Comparison with National and International Guidelines. J. Paediatr. Child Health 2019, 55, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Ewers, B.; Trolle, E.; Jacobsen, S.S.; Vististen, D.; Almdal, T.P.; Vilsbøll, T.; Bruun, J.M. Dietary Habits and Adherence to Dietary Recommendations in Patients with Type 1 and Type 2 Diabetes Compared with the General Population in Denmark. Nutrition 2019, 61, 49–55. [Google Scholar] [CrossRef]
- Mackey, E.R.; O’Brecht, L.; Holmes, C.S.; Jacobs, M.; Streisand, R. Teens with Type 1 Diabetes: How Does Their Nutrition Measure Up? J. Diabetes Res. 2018, 2018, 5094569. [Google Scholar] [CrossRef]
- Øverby, N.C.; Flaaten, V.; Veierød, M.B.; Bergstad, I.; Margeirsdottir, H.D.; Dahl-Jørgensen, K.; Andersen, L.F. Children and Adolescents with Type 1 Diabetes Eat a More Atherosclerosis-Prone Diet than Healthy Control Subjects. Diabetologia 2007, 50, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Nichols, M.; Liese, A.D.; Bell, R.A.; Dabelea, D.M.; Johansen, J.M.; Pihoker, C.; Rodriguez, B.L.; Thomas, J.; Williams, D. Dietary Intake among Youth with Diabetes: The SEARCH for Diabetes in Youth Study. J. Am. Diet. Assoc. 2006, 106, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Nansel, T.R.; Haynie, D.L.; Lipsky, L.M.; Laffel, L.M.B.; Mehta, S.N. Multiple Indicators of Poor Diet Quality in Children and Adolescents with Type 1 Diabetes Are Associated with Higher Body Mass Index Percentile but Not Glycemic Control. J. Acad. Nutr. Diet. 2012, 112, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, V.S.; Viccaro, L.; Becker, D.; Escobar, O.; Siminerio, L. Diet of Adolescents With and Without Diabetes: Trading Candy for Potato Chips? Diabetes Care 2006, 29, 982–987. [Google Scholar] [CrossRef]
- Maffeis, C.; Tomasselli, F.; Tommasi, M.; Bresadola, I.; Trandev, T.; Fornari, E.; Marigliano, M.; Morandi, A.; Olivieri, F.; Piona, C. Nutrition Habits of Children and Adolescents with Type 1 Diabetes Changed in a 10 Years Span. Pediatric Diabetes 2020, 21, 960–968. [Google Scholar] [CrossRef]
- Rosi, A.; Paolella, G.; Biasini, B.; Scazzina, F. Dietary Habits of Adolescents Living in North America, Europe or Oceania: A Review on Fruit, Vegetable and Legume Consumption, Sodium Intake, and Adherence to the Mediterranean Diet. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Schutz, Y.; Fornari, E.; Marigliano, M.; Tomasselli, F.; Tommasi, M.; Chini, V.; Morandi, A. Bias in Food Intake Reporting in Children and Adolescents with Type 1 Diabetes: The Role of Body Size, Age and Gender. Pediatric Diabetes 2017, 18, 213–221. [Google Scholar] [CrossRef]
- Rabasa-Lhoret, R.; Garon, J.; Langelier, H.; Poisson, D.; Chiasson, J.L. Effects of Meal Carbohydrate Content on Insulin Requirements in Type 1 Diabetic Patients Treated Intensively with the Basal-Bolus (Ultralente- Regular) Insulin Regimen. Diabetes Care 1999, 22, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T. The Importance of Carbohydrate Counting in the Treatment of Children with Diabetes. Pediatric Diabetes 2007, 8 (Suppl. 6), 57–62. [Google Scholar] [CrossRef] [PubMed]
- Tascini, G.; Berioli, M.G.; Cerquiglini, L.; Santi, E.; Mancini, G.; Rogari, F.; Toni, G.; Esposito, S. Carbohydrate Counting in Children and Adolescents with Type 1 Diabetes. Nutrients 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.E.M.; Evans, M.; O’Connell, S.M.; McElduff, P.; Lopez, P.E.; Jones, T.W.; Davis, E.A.; King, B.R. Both Dietary Protein and Fat Increase Postprandial Glucose Excursions in Children with Type 1 Diabetes, and the Effect Is Additive. Diabetes Care 2013, 36, 3897–3902. [Google Scholar] [CrossRef]
- Paterson, M.A.; Smart, C.E.M.; Lopez, P.E.; Mcelduff, P.; Attia, J.; Morbey, C.; King, B.R. Influence of Dietary Protein on Postprandial Blood Glucose Levels in Individuals with Type 1 Diabetes Mellitus Using Intensive Insulin Therapy. Diabet. Med. 2016, 33, 592–598. [Google Scholar] [CrossRef]
- Abdou, M.; Hafez, M.H.; Anwar, G.M.; Fahmy, W.A.; Al Fattah, M.A.; Salem, R.I.; Arafa, N. Effect of High Protein and Fat Diet on Postprandial Blood Glucose Levels in Children and Adolescents with Type 1 Diabetes in Cairo, Egypt. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Pańkowska, E.; Błazik, M.; Groele, L. Does the Fat-Protein Meal Increase Postprandial Glucose Level in Type 1 Diabetes Patients on Insulin Pump: The Conclusion of a Randomized Study. Diabetes Technol. Ther. 2012, 14, 16–22. [Google Scholar] [CrossRef]
- Paterson, M.A.; Smart, C.E.M.; Lopez, P.E.; Howley, P.; McElduff, P.; Attia, J.; Morbey, C.; King, B.R. Increasing the Protein Quantity in a Meal Results in Dose-Dependent Effects on Postprandial Glucose Levels in Individuals with Type 1 Diabetes Mellitus. Diabet. Med. 2017, 34, 851–854. [Google Scholar] [CrossRef]
- Lodefalk, M.; Åman, J.; Bang, P. Effects of Fat Supplementation on Glycaemic Response and Gastric Emptying in Adolescents with Type 1 Diabetes. Diabet. Med. 2008, 25, 1030–1035. [Google Scholar] [CrossRef]
- Kordonouri, O.; Hartmann, R.; Remus, K.; Bläsig, S.; Sadeghian, E.; Danne, T. Benefit of Supplementary Fat plus Protein Counting as Compared with Conventional Carbohydrate Counting for Insulin Bolus Calculation in Children with Pump Therapy. Pediatric Diabetes 2012, 13, 540–544. [Google Scholar] [CrossRef]
- Kaya, N.; Kurtoğlu, S.; Gökmen Özel, H. Does Meal-Time Insulin Dosing Based on Fat-Protein Counting Give Positive Results in Postprandial Glycaemic Profile after a High Protein-Fat Meal in Adolescents with Type 1 Diabetes: A Randomised Controlled Trial. J. Hum. Nutr. Diet. 2020, 33, 396–403. [Google Scholar] [CrossRef]
- Bell, K.J.; Gray, R.; Munns, D.; Petocz, P.; Howard, G.; Colagiuri, S.; Brand-Miller, J.C. Estimating Insulin Demand for Protein-Containing Foods Using the Food Insulin Index. Eur. J. Clin. Nutr. 2014, 68, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Gilbertson, H.R.; Gray, A.R.; Munns, D.; Howard, G.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Improving the Estimation of Mealtime Insulin Dose in Adults with Type 1 Diabetes: The Normal Insulin Demand for Dose Adjustment (NIDDA) Study. Diabetes Care 2011, 34, 2146–2151. [Google Scholar] [CrossRef]
- Bell, K.J.; Gray, R.; Munns, D.; Petocz, P.; Steil, G.; Howard, G.; Colagiuri, S.; Brand-Miller, J.C. Clinical Application of the Food Insulin Index for Mealtime Insulin Dosing in Adults with Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Technol. Ther. 2016, 18, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.E.; Evans, M.; King, B.R.; Jones, T.W.; Bell, K.; McElduff, P.; Davis, E.A.; Smart, C.E. A Randomized Comparison of Three Prandial Insulin Dosing Algorithms for Children and Adolescents with Type 1 Diabetes. Diabet. Med. 2018, 35, 1440–1447. [Google Scholar] [CrossRef]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of Fat, Protein, and Glycemic Index on Postprandial Glucose Control in Type 1diabetes: Implications for Intensive Diabetes Management in the Continuous Glucose Monitoring Era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Bell, K.J.; Toschi, E.; Steil, G.M.; Wolpert, H.A. Optimized Mealtime Insulin Dosing for Fat and Protein in Type 1 Diabetes: Application of a Model-Based Approach to Derive Insulin Doses for Open-Loop Diabetes Management. Diabetes Care 2016, 39, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, K.; Dżygało, K.; Szypowska, A. The Additional Dose of Insulin for High-Protein Mixed Meal Provides Better Glycemic Control in Children with Type 1 Diabetes on Insulin Pumps: Randomized Cross-over Study. Pediatric Diabetes 2017, 18, 861–868. [Google Scholar] [CrossRef]
- Lopez, P.E.; Smart, C.E.; McElduff, P.; Foskett, D.C.; Price, D.A.; Paterson, M.A.; King, B.R. Optimizing the Combination Insulin Bolus Split for a High-Fat, High-Protein Meal in Children and Adolescents Using Insulin Pump Therapy. Diabet. Med. 2017, 34, 1380–1384. [Google Scholar] [CrossRef]
- Swedish National Diabetes Registry. Available online: https://www.ndr.nu/#/knappen (accessed on 11 July 2021).
- Balk, S.N.; Schoenaker, D.A.J.M.; Mishra, G.D.; Toeller, M.; Chaturvedi, N.; Fuller, J.H.; Soedamah-Muthu, S.S. Association of Diet and Lifestyle with Glycated Haemoglobin in Type 1 Diabetes Participants in the EURODIAB Prospective Complications Study. Eur. J. Clin. Nutr. 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Lamichhane, A.P.; Crandell, J.L.; Jaacks, L.M.; Couch, S.C.; Lawrence, J.M.; Mayer-Davis, E.J. Longitudinal Associations of Nutritional Factors with Glycated Hemoglobin in Youth with Type 1 Diabetes: The SEARCH Nutrition Ancillary Study. Am. J. Clin. Nutr. 2015, 101, 1278–1285. [Google Scholar] [CrossRef]
- Nansel, T.R.; Lipsky, L.M.; Liu, A. Greater Diet Quality Is Associated with More Optimal Glycemic Control in a Longitudinal Study of Youth with Type 1 Diabetes. Am. J. Clin. Nutr. 2016, 104, 81–87. [Google Scholar] [CrossRef]
- Ahola, A.J.; Harjutsalo, V.; Forsblom, C.; Saraheimo, M.; Groop, P.H. Associations of Dietary Macronutrient and Fibre Intake with Glycaemia in Individuals with Type 1 Diabetes. Diabet. Med. 2019, 36, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Crandell, J.L.; Shay, C.M.; Gordon-Larsen, P.; Cole, S.R.; Juhaeri, J.; Kahkoska, A.R.; Maahs, D.M.; Seid, M.; Forlenza, G.P.; et al. Dietary Intake and Risk of Non-Severe Hypoglycemia in Adolescents with Type 1 Diabetes. J. Diabetes Complicat. 2017, 31, 1340–1347. [Google Scholar] [CrossRef]
- Ahola, A.J.; Forsblom, C.; Harjutsalo, V.; Groop, P.H. Dietary Carbohydrate Intake and Cardio-Metabolic Risk Factors in Type 1 Diabetes. Diabetes Res. Clin. Pract. 2019, 155, 107818. [Google Scholar] [CrossRef]
- Meissner, T.; Wolf, J.; Kersting, M.; Fröhlich-Reiterer, E.; Flechtner-Mors, M.; Salgin, B.; Stahl-Pehe, A.; Holl, R.W. Carbohydrate Intake in Relation to BMI, HbA1c and Lipid Profile in Children Andadolescents with Type 1 Diabetes. Clin. Nutr. 2014, 33, 75–78. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-Glycemic Index Diets as an Intervention for Diabetes: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary Carbohydrate Intake and Mortality: A Prospective Cohort Study and Meta-Analysis. Lancet. Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.S.; DeSalvo, D.; Gregory, J.; Hilliard, M.E. Medical and Psychological Considerations for Carbohydrate-Restricted Diets in Youth With Type 1 Diabetes. Curr. Diab. Rep. 2019, 19, 27. [Google Scholar] [CrossRef]
- Scott, S.N.; Anderson, L.; Morton, J.P.; Wagenmakers, A.J.M.; Riddell, M.C. Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients 2019, 11, 1022. [Google Scholar] [CrossRef]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The Ups and Downs of Low-Carbohydrate Diets in the Management of Type 1 Diabetes: A Review of Clinical Outcomes. Diabet. Med. 2019, 36, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy Jr, W.S.; et al. Management of Type 1 Diabetes With a Very Low-Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Laffel, L.M.; Buse, J.B. Management of Type 1 Diabetes with a Very Low–Carbohydrate Diet: A Word of Caution. Pediatrics 2018, 142, e20181536B. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The Relation of Saturated Fatty Acids with Low-Grade Inflammation and Cardiovascular Disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Harcombe, Z.; Baker, J.S.; DiNicolantonio, J.J.; Grace, F.; Davies, B. Evidence from Randomised Controlled Trials Does Not Support Current Dietary Fat Guidelines: A Systematic Review and Meta-Analysis. Open Heart 2016, 3, e000409. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of Fats and Carbohydrate Intake with Cardiovascular Disease and Mortality in 18 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans Fatty Acids and Cardiovascular Disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Available online: https://www.dietaryguidelines.gov/2020-advisory-committee-report (accessed on 4 February 2021).
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-Analysis of Prospective Cohort Studies Evaluating the Association of Saturated Fat with Cardiovascular Disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of Dietary, Circulating, and Supplement Fatty Acids with Coronary Risk: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.; Petersen, K.; Kris-Etherton, P. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major Types of Dietary Fat and Risk of Coronary Heart Disease: A Pooled Analysis of 11 Cohort Studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.O.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 Fats for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 7, CD011094. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Minihane, A.M.; Saleh, R.N.M.; Risérus, U. Intake and Metabolism of Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Nutritional Implications for Cardiometabolic Diseases. Lancet Diabetes Endocrinol. 2020, 8, 915–930. [Google Scholar] [CrossRef]
- Mannucci, E.; Dicembrini, I.; Lauria, A.; Pozzilli, P. Is Glucose Control Important for Prevention of Cardiovascular Disease in Diabetes? Diabetes Care 2013, 36 (Suppl. 2), S259–S263. [Google Scholar] [CrossRef]
- Sanjeevi, N.; Lipsky, L.M.; Nansel, T.R. Cardiovascular Biomarkers in Association with Dietary Intake in a Longitudinal Study of Youth with Type 1 Diabetes. Nutrients 2018, 10, 1552. [Google Scholar] [CrossRef]
- Tooze, J.A.; The, N.S.; Crandell, J.L.; Couch, S.C.; Mayer-Davis, E.J.; Koebnick, C.; Liese, A.D. An Approach for Examining the Impact of Food Group-Based Sources of Nutrients on Outcomes with Application to PUFAs and LDL in Youth with Type 1 Diabetes. Nutrients 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Toeller, M.; Buyken, A.E.; Heitkamp, G.; Scherbaum, W.A.; Krans, H.M.J.; Fuller, J.H. Associations of Fat and Cholesterol Intake with Serum Lipid Levels and Cardiovascular Disease: The EURODIAB IDDM Complications Study. Exp. Clin. Endocrinol. Diabetes 1999, 107, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis. From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Meessen, E.C.E.; Warmbrunn, M.V.; Nieuwdorp, M.; Soeters, M.R. Human Postprandial Nutrient Metabolism and Low-Grade Inflammation: A Narrative Review. Nutrients 2019, 11, 3000. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A High-Fat Meal Induces Low-Grade Endotoxemia: Evidence of a Novel Mechanism of Postprandial Inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; De Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement from the American Heart Association and American Diabetes Association. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef]
- Snell-Bergeon, J.K.; West, N.A.; Mayer-Davis, E.J.; Liese, A.D.; Marcovina, S.M.; D’Agostino, R.B.; Hamman, R.F.; Dabelea, D. Inflammatory Markers Are Increased in Youth with Type 1 Diabetes: The SEARCH Case-Control Study. J. Clin. Endocrinol. Metab. 2010, 95, 2868–2876. [Google Scholar] [CrossRef]
- De Ferranti, S.; Rifai, N. C-Reactive Protein and Cardiovascular Disease: A Review of Risk Prediction and Interventions. Clin. Chim. Acta 2002, 317, 1–15. [Google Scholar] [CrossRef]
- Mangge, H.; Schauenstein, K.; Stroedter, L.; Griesl, A.; Maerz, W.; Borkenstein, M. Low Grade Inflammation in Juvenile Obesity and Type 1 Diabetes Associated with Early Signs of Atherosclerosis. Exp. Clin. Endocrinol. Diabetes 2004, 112, 378–382. [Google Scholar] [CrossRef]
- van Bussel, B.C.T.; Soedamah-Muthu, S.S.; Henry, R.M.A.; Schalkwijk, C.G.; Ferreira, I.; Chaturvedi, N.; Toeller, M.; Fuller, J.H.; Stehouwer, C.D.A. Unhealthy Dietary Patterns Associated with Inflammation and Endothelial Dysfunction in Type 1 Diabetes: The EURODIAB Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 758–764. [Google Scholar] [CrossRef]
- Ahola, A.J.; Saraheimo, M.; Freese, R.; Forsblom, C.; Mäkimattila, S.; Groop, P.H. Association between Adherence to Dietary Recommendations and High-Sensitivity C-Reactive Protein Level in Type 1 Diabetes. Diabetes Res. Clin. Pract. 2017, 126, 122–128. [Google Scholar] [CrossRef][Green Version]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.H. Dietary Patterns Reflecting Healthy Food Choices Are Associated with Lower Serum LPS Activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef]
- Eccel Prates, R.; Beretta, M.V.; Nascimento, F.V.; Bernaud, F.R.; de Almeira, J.C.; Rodrigues, T.C. Saturated Fatty Acid Intake Decreases Serum Adiponectin Levels in Subjects with Type 1 Diabetes. Diabetes Res. Clin. Pract. 2016, 116, 205–211. [Google Scholar] [CrossRef]
- Liese, A.D.; Ma, X.; Ma, X.; Mittleman, M.A.; The, N.S.; Standiford, D.A.; Lawrence, J.M.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. Dietary Quality and Markers of Inflammation: No Association in Youth with Type 1 Diabetes. J. Diabetes Complicat. 2018, 32, 179–184. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wu, H.; Zhu, P.; Mo, X.; Ma, X.; Ying, J. Intake of Polyunsaturated Fatty Acids and Risk of Preclinical and Clinical Type 1 Diabetes in Children—a Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2019, 73, 1–8. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The Microbiota-Gut-Brain Axis: Focus on the Fundamental Communication Pathways. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2020; Volume 176, pp. 43–110. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Putignani, L.; Del Chierico, F.; Petrucca, A.; Vernocchi, P.; Dallapiccola, B. The Human Gut Microbiota: A Dynamic Interplay with the Host from Birth to Senescence Settled during Childhood. Pediatr. Res. 2014, 76, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Yap, Y.A.; Mariño, E. An Insight into the Intestinal Web of Mucosal Immunity, Microbiota, and Diet in Inflammation. Front. Immunol. 2018, 9, 2617. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Valentini, A.; Calabrese, G. Gut Microbiota and Type 1 Diabetes Mellitus: The Effect of Mediterranean Diet. Front. Nutr. 2021, 7, 612773. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut Microbiota in Children with Type 1 Diabetes Differs from That in Healthy Children: A Case-Control Study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Toni, G.; Tascini, G.; Santi, E.; Berioli, M.G.; Principi, N. Environmental Factors Associated with Type 1 Diabetes. Front. Endocrinol. 2019, 10, 592. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Ribeiro, D.A.; Pisani, L.P. High-Fat or High-Sugar Diets as Trigger Inflammation in the Microbiota-Gut-Brain Axis. Crit. Rev. Food Sci. Nutr. 2021, 61, 836–854. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut Microbial Metabolites Limit the Frequency of Autoimmune T Cells and Protect against Type 1 Diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Uusitalo, U.; Liu, X.; Yang, J.; Aronsson, C.A.; Hummel, S.; Butterworth, M.; Lernmark, Å.; Rewers, M.; Hagopian, W.; She, J.X.; et al. Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study. JAMA Pediatr. 2016, 170, 20–28. [Google Scholar] [CrossRef]
- Ahola, A.J.; Harjutsalo, V.; Forsblom, C.; Freese, R.; Makimattila, S.; Groop, P.H. The Self-Reported Use of Probiotics Is Associated with Better Glycaemic Control and Lower Odds of Metabolic Syndrome and Its Components in Type 1 Diabetes. J. Probiotics Health 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Savilahti, E.; Härkönen, T.; Savilahti, E.M.; Kukkonen, K.; Kuitunen, M.; Knip, M. Probiotic Intervention in Infancy Is Not Associated with Development of Beta Cell Autoimmunity and Type 1 Diabetes. Diabetologia 2018, 61, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Population (n *), Design | Key Findings |
|---|---|---|
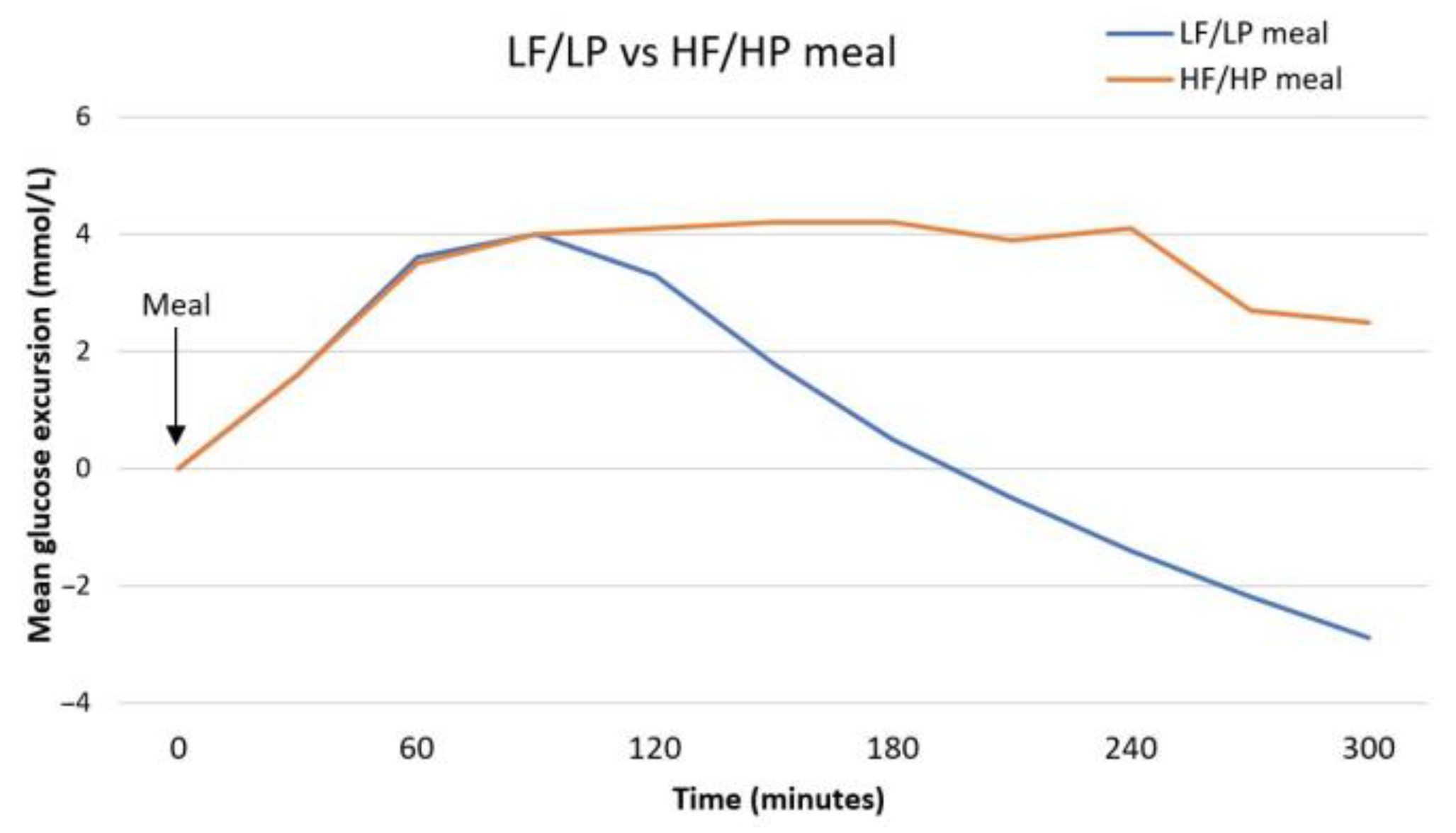
| Smart et al., 2013 [33] | Pediatric (n = 33), four-by-four randomized crossover trial. | Meals with high content of fat or protein increase postprandial glucose excursion, 3–5 h after meal. |
| Balk et al., 2016 [50] | Adult 1 (n = 1659), 7-year prospective cohort analysis. | Total fat, SFA, PUFA and MUFA were not associated with HbA1c. |
| Lamichhane et al., 2015 [51] | Pediatric (n = 908), multicenter observational study. | No significant association between total fat and HbA1c. |
| Katz et al., 2014 [12] | Pediatric (n = 252), cross-sectional study. | Higher risk of suboptimal HbA1c between individuals consuming the highest quartile of fat intake. |
| Maffeis et al., 2012 [13] | Pediatric (n = 114), cross-sectional study. | HbA1c levels positively correlated with lipid intake and SFA and negatively correlated with MUFA intake. |
| Maffeis et al., 2020 [27] | Pediatric (n = 229), retrospective cross-sectional study. | Higher MUFA intake lowered the risk of having HbA1c higher than 7.5%. |
| Delahanty et al., 2009 [14] | Mixed 2 (n = 532), randomized, controlled clinical trial. | Higher fat, SFA and MUFA intake are associated with higher HbA1c levels. |
| Nansel et al., 2016 [52] | Pediatric (n = 136), behavioral nutrition intervention study. | Lower HbA1c was associated with lower unsaturated fat intake, no significant associations found for total fat and SFA. |
| Ahola et al., 2019 [53] | Adults (n = 1000), observational, cross-sectional study. | MUFA intake was associated with higher variability in blood glucose measurements. |
| Sanjeevi et al., 2018 [83] | Pediatric (n = 136), 18-months intervention study. | Positive association between SFA intake and HDL-cholesterol and between PUFA intake and diastolic blood pressure. |
| Maffeis et al., 2017 [16] | Pediatric (n = 180), cross-sectional study. | Non-HDL cholesterol was associated with lipids and lipid-to-carbohydrate intake ratio, not associated with SFA, MUFA, PUFA and cholesterol intake. |
| Snell-Bergeon et al., 2010 [91] | Pediatric 3 (n = 553 + 215 controls), cross-sectional, observational study. | Elevated inflammatory markers in youth with T1D, which were associated with an atherogenic lipid profile. |
| van Bussel et al., 2013 [94] | Adult (n = 491), prospective study. | Lower consumption of PUFA and higher of cholesterol were associated with a greater low-grade inflammation. |
| Ahola et al., 2017 [95] | Adult (n = 677), cross-sectional study. | A diet closer to recommendations was associated to a lower level of low-grade inflammation in men. |
| Liese et al., 2018 [98] | Pediatric 4 (n = 2520), observational, multicenter study. | No association between dietary quality indices and inflammation biomarkers. |
| Murri et al., 2013 [108] | Pediatric (n = 16 + 16 controls), case-control study. | Marked differences in the fecal microbial composition in individuals with T1D. |
| Nutritional Recommendations on Diet Composition for Children and Adolescents with T1D |
|---|
| Individualized assessment of nutrition therapy and the related best distribution of macronutrient, aiming at improving glycemic control and lower cardiovascular risk [4,6]. |
| As a guide: - carbohydrate intake: 45–50% of total daily energy intake, - fat intake no greater than 30–35% (saturated fat < 10%), - protein intake 15–20% [4]. |
| Appropriate energy intake for optimal growth and keeping an ideal body weight [4]. |
| Diet should be assorted with healthy foods, such as fruits, vegetables, dairy, whole grains, legumes and lean meat [4]. |
| Restrictions in one macronutrient are discouraged (risk of growth compromising and nutritional deficiencies) [4]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garonzi, C.; Forsander, G.; Maffeis, C. Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes. Nutrients 2021, 13, 2625. https://doi.org/10.3390/nu13082625
Garonzi C, Forsander G, Maffeis C. Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes. Nutrients. 2021; 13(8):2625. https://doi.org/10.3390/nu13082625
Chicago/Turabian StyleGaronzi, Chiara, Gun Forsander, and Claudio Maffeis. 2021. "Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes" Nutrients 13, no. 8: 2625. https://doi.org/10.3390/nu13082625
APA StyleGaronzi, C., Forsander, G., & Maffeis, C. (2021). Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes. Nutrients, 13(8), 2625. https://doi.org/10.3390/nu13082625






