Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Assessment of Study Quality
3. Results
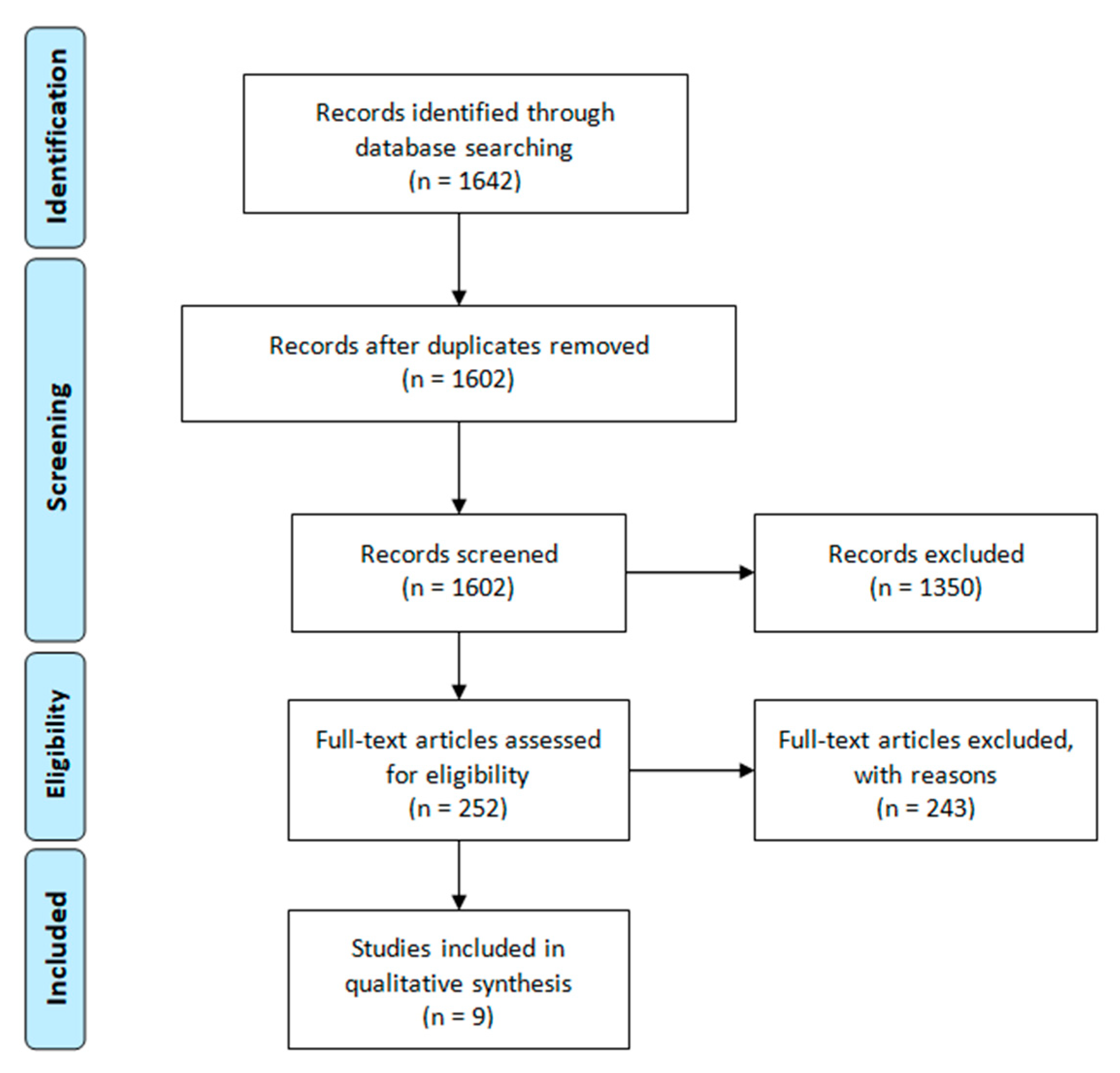
3.1. Identification of Studies
3.2. Study Characteristics
3.3. Methodological Quality
3.4. Prevalence of Metabolic Syndrome
4. Discussion
4.1. Problem of Overweight, Obesity and Metabolic Syndrome in Healthy and Diabetes Population
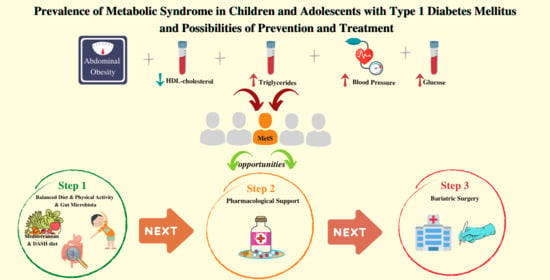
4.2. Strategies for the Prevention and Treatment of Metabolic Syndrome
4.2.1. Diet
4.2.2. Lifestyle and Physical Activity
4.2.3. Combined Approach
4.2.4. Gut Microflora
4.2.5. Pharmacological Support
4.2.6. Bariatric Surgery
4.2.7. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas. 2019. Available online: https://www.diabetesatlas.org (accessed on 5 October 2020).
- Minges, K.E.; Whittemore, R.; Grey, M. Overweight and obesity in youth with type 1 diabetes. Annu. Rev. Nurs. Res. 2013, 31, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q.; Zinman, B.; Brunzell, J.D. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 2013, 127, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Daniels, S.R.; Eckel, R.H.; Hayman, L.; Lustig, R.H.; McCrindle, B.; Mietus-Snyder, M.L. Progress and Challenges in Metabolic Syndrome in Children and Adolescents. Circulation 2009, 119, 628–647. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.; Bruce, D.G.; Davis, W.A. Prevalence and prognostic implications of the metabolic syndrome in community-based patients with type 1 diabetes: The Fremantle Diabetes Study. Diabetes Res. Clin. Pract. 2007, 78, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the Metabolic Syndrome in Children and Adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of A WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 December 2020).
- Castro-Correia, C.; Santos-Silva, R.; Pinheiro, M.; Costa, C.; Fontoura, M. Metabolic risk factors in adolescent girls with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2018, 31, 631–635. [Google Scholar] [CrossRef]
- Yayıcı Köken, Ö.; Kara, C.; Can Yılmaz, G.; Aydın, H.M. Prevalence of Obesity and Metabolic Syndrome in Children with Type 1 Diabetes: A Comparative Assessment Based on Criteria Established by the International Diabetes Federation, World Health Organisation and National Cholesterol Education Program. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Łuczyński, W.; Szypowska, A.; Głowińska-Olszewska, B.; Bossowski, A. Overweight, obesity and features of metabolic syndrome in children with diabetes treated with insulin pump therapy. Eur. J. Pediatr. 2011, 170, 891–898. [Google Scholar] [CrossRef]
- Saki, F. Prevalence of Metabolic Syndrome in Children with Type 1 Diabetes in South of Iran. J. Compr. Pediatr. 2016, 7, e37703. [Google Scholar] [CrossRef]
- Saki, F.; Setoodehnia, Z.; Javanmardi, H.; Omrani, G. Association between Metabolic Syndrome Criteria and Body-composition Components in Children with Type 1 Diabetes Mellitus. Int. J. Pediatr 2016, 4, 3709–3717. [Google Scholar] [CrossRef]
- Soliman, H.M.; Mosaad, Y.O.; Ibrahim, A. The prevalence and the clinical profile of metabolic syndrome in children and adolescents with Type 1 diabetes. Diabetes Metab. Syndr. 2019, 13, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Szadkowska, A.; Pietrzak, I.; Szlawska, J.; Kozera, A.; Gadzicka, A.; Młynarski, W. Abdominal obesity, metabolic syndrome in type 1 diabetic children and adolescents. Pediatr. Endocrinol. Diabetes Metab. 2009, 15, 233–239. [Google Scholar] [PubMed]
- Van Vliet, M.; Van der Heyden, J.C.; Diamant, M.; Von Rosenstiel, I.A.; Schindhelm, R.K.; Aanstoot, H.J.; Veeze, H.J. Overweight Is Highly Prevalent in Children with Type 1 Diabetes And Associates with Cardiometabolic Risk. J. Pediatr. 2010, 156, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Iafusco, D.; Zucchini, S.; Maffeis, C. Abdominal adiposity and cardiovascular risk factors in adolescents with type 1 diabetes. Diabetes Res. Clin. Pract. 2012, 97, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Birkebaek, N.H.; Konstantinova, M.; Schwandt, A.; Vazeou, A.; Casteels, K.; Jali, S.; Limbert, C.; Pundziute-Lycka, A.; Toth-Heyn, P.; et al. Prevalence of underweight, overweight, and obesity in children and adolescents with type 1 diabetes: Data from the international SWEET registry. Pediatr. Diabetes 2018, 19, 1211–1220. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- De Keukelaere, M.; Fieuws, S.; Reynaert, N.; Vandoorne, E.; Kerckhove, K.V.; Asscherickx, W.; Casteels, K. Evolution of body mass index in children with type 1 diabetes mellitus. Eur. J. Pediatr. 2018, 177, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; Dewey, D. The association between body mass index and physical activity, and body image, self esteem and social support in adolescents with type 1 diabetes. Can. J. Diabetes 2014, 38, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Bonadonna, R.; Cucinotta, D.; Fedele, D.; Riccardi, G.; Tiengo, A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: Results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care 2006, 29, 2701–2707. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Kułaga, K.; Gurzkowska, B.; Góźdź, M.; Pan, H. Oscillometric blood pressure percentiles for Polish normal-weight school-aged children and adolescents. J. Hypertens. 2012, 30, 1942–1954. [Google Scholar] [CrossRef]
- Ahrens, W.; Moreno, L.A.; Mårild, S.; Molnár, D.; Siani, A.; De Henauw, S.; Böhmann, J.; Günther, K.; Hadjigeorgiou, C.; Iacoviello, L.; et al. Metabolic syndrome in young children: Definitions and results of the IDEFICS study. Int. J. Obes. 2014, 38, S4–S14. [Google Scholar] [CrossRef]
- Ferranti, S.D.d.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the Metabolic Syndrome in American Adolescents. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef]
- Chillarón, J.J.; Flores-Le-Roux, J.A.; Goday, A.; Benaiges, D.; Carrera, M.J.; Puig, J.; Cano-Pérez, J.F.; Pedro-Botet, J. Metabolic syndrome and type-1 diabetes mellitus: Prevalence and associated factors. Rev. Esp. Cardiol. 2010, 63, 423–429. [Google Scholar] [CrossRef]
- Ghosh, S.; Collier, A.; Hair, M.; Malik, I.; Elhadd, T. Metabolic syndrome in type 1 diabetes. Int. J. Diabetes Mellit. 2010, 2, 38–42. [Google Scholar] [CrossRef][Green Version]
- McGill, M.; Molyneaux, L.; Twigg, S.M.; Yue, D.K. The metabolic syndrome in type 1 diabetes: Does it exist and does it matter? J. Diabetes Complicat. 2008, 22, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Vestberg, D.; Rosengren, A.; Olsson, M.; Gudbjörnsdottir, S.; Svensson, A.-M.; Lind, M. Relationship Between Overweight and Obesity with Hospitalization for Heart Failure in 20,985 Patients With Type 1 Diabetes. Diabetes Care 2013, 36, 2857–2861. [Google Scholar] [CrossRef]
- Dietz, W.H. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics 1998, 101, 518–525. [Google Scholar]
- Krishnan, S.; Short, K.R. Prevalence and significance of cardiometabolic risk factors in children with type 1 diabetes. J. Cardiometab. Syndr. 2009, 4, 50–56. [Google Scholar] [CrossRef]
- Pozzilli, P.; Guglielmi, C.; Caprio, S.; Buzzetti, R. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care 2011, 34 (Suppl. 2), S166–S170. [Google Scholar] [CrossRef]
- Pérez, A.; Wägner, A.M.; Carreras, G.; Giménez, G.; Sánchez-Quesada, J.L.; Rigla, M.; Gómez-Gerique, J.A.; Pou, J.M.; de Leiva, A. Prevalence and phenotypic distribution of dyslipidemia in type 1 diabetes mellitus: Effect of glycemic control. Arch. Intern. Med. 2000, 160, 2756–2762. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Rupp, D.; Carnethon, M. Higher levels of HDL cholesterol are associated with a decreased likelihood of albuminuria in patients with long-standing type 1 diabetes. Diabetes Care 2006, 29, 78–82. [Google Scholar] [CrossRef]
- Chillarón, J.J.; Sales, M.P.; Flores-Le-Roux, J.A.; Murillo, J.; Benaiges, D.; Castells, I.; Goday, A.; Cano, J.F.; Pedro-Botet, J. Insulin resistance and hypertension in patients with type 1 diabetes. J. Diabetes Complicat. 2011, 25, 232–236. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Porkka, K.V.K.; Räsänen, L.; Rönnemaa, T.; Viikari, J.S.A. Clustering and six year cluster-tracking of serum total cholesterol, HDL-cholesterol and diastolic blood pressure in children and young adults The cardiovascular risk in young finns study. J. Clin. Epidemiol. 1994, 47, 1085–1093. [Google Scholar] [CrossRef]
- Pandit, D.; Chiplonkar, S.; Khadilkar, A.; Kinare, A.; Khadilkar, V. Efficacy of a continuous metabolic syndrome score in Indian children for detecting subclinical atherosclerotic risk. Int. J. Obes. 2011, 35, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Kamath, C.C.; Vickers, K.S.; Ehrlich, A.; McGovern, L.; Johnson, J.; Singhal, V.; Paulo, R.; Hettinger, A.; Erwin, P.J.; Montori, V.M. Clinical review: Behavioral interventions to prevent childhood obesity: A systematic review and metaanalyses of randomized trials. J. Clin. Endocrinol. Metab. 2008, 93, 4606–4615. [Google Scholar] [CrossRef]
- Overby, N.C.; Flaaten, V.; Veierød, M.B.; Bergstad, I.; Margeirsdottir, H.D.; Dahl-Jørgensen, K.; Andersen, L.F. Children and adolescents with type 1 diabetes eat a more atherosclerosis-prone diet than healthy control subjects. Diabetologia 2007, 50, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Morandi, A.; Ventura, E.; Sabbion, A.; Contreas, G.; Tomasselli, F.; Tommasi, M.; Fasan, I.; Costantini, S.; Pinelli, L. Diet, physical, and biochemical characteristics of children and adolescents with type 1 diabetes: Relationship between dietary fat and glucose control. Pediatr. Diabetes 2012, 13, 137–146. [Google Scholar] [CrossRef]
- Øverby, N.C.; Margeirsdottir, H.D.; Brunborg, C.; Dahl-Jørgensen, K.; Andersen, L.F. Sweets, snacking habits, and skipping meals in children and adolescents on intensive insulin treatment. Pediatr. Diabetes 2008, 9, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.R.; Dolan, L.M.; Powers, S.W. Dietary Adherence and Associated Glycemic Control in Families of Young Children with Type 1 Diabetes. J. Am. Diet. Assoc. 2007, 107, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014, 14, 175. [Google Scholar] [CrossRef]
- Chan, T.F.; Lin, W.T.; Huang, H.L.; Lee, C.Y.; Wu, P.W.; Chiu, Y.W.; Huang, C.C.; Tsai, S.; Lin, C.L.; Lee, C.H. Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents. Nutrients 2014, 6, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Scharf, R.J.; DeBoer, M.D. Sugar-Sweetened Beverages and Children’s Health. Annu. Rev. Public Health 2016, 37, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Yuzbashian, E.; Mirmiran, P.; Hooshmand, F.; Najafi, R.; Azizi, F. Dietary Approaches to Stop Hypertension (DASH) Dietary Pattern Is Associated with Reduced Incidence of Metabolic Syndrome in Children and Adolescents. J. Pediatr. 2016, 174, 178–184.e171. [Google Scholar] [CrossRef] [PubMed]
- Peairs, A.D.; Shah, A.S.; Summer, S.; Hess, M.; Couch, S.C. Effects of the dietary approaches to stop hypertension (DASH) diet on glucose variability in youth with Type 1 diabetes. Diabetes Manag. 2017, 7, 383–391. [Google Scholar]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Shiva, N.; Azizi, F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr. Metab. 2012, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, Y.; Ford, E.S.; Manson, J.E.; Buring, J.E.; Ridker, P.M. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005, 28, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B. Nutritional and endocrine modulation of intracellular calcium: Implications in obesity, insulin resistance and hypertension. Mol. Cell Biochem. 1998, 188, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Gao, Y.; Zhang, M.; Wang, X.; Liu, W.; Zhang, D.; Huang, G. Dietary nutrient intake and metabolic syndrome risk in Chinese adults: A case-control study. Nutr. J. 2013, 12, 106. [Google Scholar] [CrossRef]
- Hart, C.N.; Hawley, N.L.; Wing, R.R. Development of a Behavioral Sleep Intervention as a Novel Approach for Pediatric Obesity in School-aged Children. Pediatr. Clin. N. Am. 2016, 63, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Donga, E.; van Dijk, M.; van Dijk, J.G.; Biermasz, N.R.; Lammers, G.-J.; van Kralingen, K.; Hoogma, R.P.L.M.; Corssmit, E.P.M.; Romijn, J.A. Partial Sleep Restriction Decreases Insulin Sensitivity in Type 1 Diabetes. Diabetes Care 2010, 33, 1573–1577. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Guinhouya, B.C.; Samouda, H.; Zitouni, D.; Vilhelm, C.; Hubert, H. Evidence of the influence of physical activity on the metabolic syndrome and/or on insulin resistance in pediatric populations: A systematic review. Int. J. Pediatr. Obes. 2011, 6, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Nader, P.R.; Bradley, R.H.; Houts, R.M.; McRitchie, S.L.; O’Brien, M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA 2008, 300, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Quirk, H.; Blake, H.; Tennyson, R.; Randell, T.L.; Glazebrook, C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 2014, 31, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; AboElAsrar, M.A.; Elbarbary, N.S.; ElHilaly, R.A.; Refaat, Y.M. Is exercise a therapeutic tool for improvement of cardiovascular risk factors in adolescents with type 1 diabetes mellitus? A randomised controlled trial. Diabetol. Metab. Syndr. 2010, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Caranti, D.A.; de Mello, M.T.; Prado, W.L.; Tock, L.; Siqueira, K.O.; de Piano, A.; Lofrano, M.C.; Cristofalo, D.M.J.; Lederman, H.; Tufik, S.; et al. Short- and long-term beneficial effects of a multidisciplinary therapy for the control of metabolic syndrome in obese adolescents. Metabolism 2007, 56, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.; Milano, G.; Cieslak, F.; Lopes, W.; Rodacki, A.; Radominski, R. Effects of physical exercise and nutritional guidance on metabolic syndrome in obese adolescents. Braz. J. Phys. Ther. 2009, 13, 73–81. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a Metabolic Syndrome Severity Z Score to Track Risk During Treatment of Prediabetes: An Analysis of the Diabetes Prevention Program. Diabetes Care 2018, 41, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Shorey, S.; Ng, E.; Law, E.C.; Wong, J.C.M.; Loke, K.; Tam, W.W. Physical activity interventions and nutrition-based interventions for children and adolescents with type 1 diabetes mellitus. Cochrane Cochrane Database Syst. Rev. 2021, 1, 1–23. [Google Scholar] [CrossRef]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Soyucen, E.; Gulcan, A.; Aktuglu-Zeybek, A.C.; Onal, H.; Kiykim, E.; Aydin, A. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr. Int. 2014, 56, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients with Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Dabelea, D.; Crandell, J.L.; Crume, T.; D’Agostino, R.B., Jr.; Dolan, L.; King, I.B.; Lawrence, J.M.; Norris, J.M.; Pihoker, C.; et al. Nutritional factors and preservation of C-peptide in youth with recently diagnosed type 1 diabetes: SEARCH Nutrition Ancillary Study. Diabetes Care 2013, 36, 1842–1850. [Google Scholar] [CrossRef]
- Al Khalifah, R.A.; Alnhdi, A.; Alghar, H.; Alanazi, M.; Florez, I.D. The effect of adding metformin to insulin therapy for type 1 diabetes mellitus children: A systematic review and meta-analysis. Pediatr Diabetes 2017, 18, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.; Valdivia, N.; Ruiz, S. Overweight adolescents with type 1 diabetes may decrease body mass index, insulin dose and glucose variability on dapagliflozin, a SGLT2 inhibitor. Horm. Res. Paediatr. 2016, 86, 73. [Google Scholar]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Griffen, S.C.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M.; Proietto, J.; et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 864–876. [Google Scholar] [CrossRef]
- Faucher, P.; Poitou, C.; Carette, C.; Tezenas du Montcel, S.; Barsamian, C.; Touati, E.; Bouillot, J.L.; Torcivia, A.; Czernichow, S.; Oppert, J.M.; et al. Bariatric Surgery in Obese Patients with Type 1 Diabetes: Effects on Weight Loss and Metabolic Control. Obes. Surg. 2016, 26, 2370–2378. [Google Scholar] [CrossRef]
- Inge, T.H.; Courcoulas, A.P.; Jenkins, T.M.; Michalsky, M.P.; Brandt, M.L.; Xanthakos, S.A.; Dixon, J.B.; Harmon, C.M.; Chen, M.K.; Xie, C.; et al. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N. Engl. J. Med. 2019, 380, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
| IDF | ATP | WHO | Weiss et al. | |||
|---|---|---|---|---|---|---|
| Age group (years) | <10 | 10–16 | >16 | - | - | - |
| Criteria | Abdominal obesity + two or more of the four criteria | Any three of the five criteria | GI * + two or more of the other components | Any three of the five criteria | ||
| Abdominal obesity | ≥90th percentile WC | WC: ≥94 cm for Europid men, ≥80 cm for Europid women, with ethnicity specific values for other groups | ≥90th WC percentile | WHR >0.9 in males, >0.85 in females or/and BMI>30 kg/m2 | BMI z-score ≥2 | |
| Triglycerides | ** | ≥1.7 mmol/L (≥150 mg/dL) | ≥95th percentile | |||
| HDL- cholesterol | ** | <1.03 mmol/L (<40 mg/dL) | <1.03 mmol/L (<40 mg/dL) in men <1.29 mmol/L (<50 mg/dL) in women or treatment for lipid abnormalities | <0.91 mmol/L (<35 mg/dL) in men <1.01 mmol/L (<39 mg/dL) in women | ≤5th percentile | |
| Blood pressure | ** | Systolic ≥ 130 / diastolic ≥ 85 mmHg or treatment hypertension | ≥90th percentile (age-, sex- and race-specific) | Systolic ≥140 Diastolic ≥90 mmHg | ≥95th percentile | |
| Fasting glucose levels | ** | ≥5.6 mmol/L (100 mg/dL) or known diabetes mellitus | ≥6.1 mmol/L (110 mg/dL), which has been changes to ≥ 5.6 mmol/L (100 mg/dL) * | GI (ADA criteria) | ||
| Microalbuminuria | - | Urinary albumin excretion rate ≥20 µg/min or albumin/creatinine ratio ≥30 mg/g | - | |||
| Author | Country | Sample Size Total (F/M) | Age (Year) | Duration of T1DM (Years) | HbA1c (%) | Over- Weight | Obesity | Diagnosis of MetS | Components of MetS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | F/M | Ab. Obesity | Low HDL | High TG | High BP | ||||||||
| n | Min–Max | ± SD Me (IQR) | ± SD Me (IQR) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Castro-Correia [14] | Portugal | 42 | 14–18 | 9.2±3.6 | 8.6±1 | 18 | n/d | n/d | 17 (40.5%) | 7 (16.7%) | 4 (9.5%) | 7 (16.7%) | |
| (42/-) | (42.9%) | ||||||||||||
| Köken [15] | Turkey | 200 | 8–18 | 4.6 ± 3.3 | 8.4 ± 1.6 | 19 | 17 | 17 (8.5%) WHO | 11 (11.5%) | n/d | n/d | n/d | 24 (12.0%) |
| (96/104) | (9.5%) | (8.5%) | 21 (10.5%) IDF | /10 (9.6%) | |||||||||
| 27 (13.5%) ATP | |||||||||||||
| Łuczyński [16] | Poland | 500 | 4–18 | 4.4 (2.1–7.0) | n/a | 78 (15.6%) | 73 (14.6%) | 16 (3.2%) IDF | n/d | 67 (13.4%) | 32 (6.4%) | 31 (6.2%) | 24 (4.8%) |
| (245/255) | |||||||||||||
| Saki [17] | Iran | 87 | 4–21 | 8.0 ± 3.9 | 8 ± 3 | n/d | n/d | 21 (24.1%) IDF | 11 (23.2%) /10 (24.8%) | 6 (6.0%) | 32 (36.8%) | 32 (36.8%) | 13 (14.9%) |
| (48/39) | |||||||||||||
| Saki [18] | Iran | 87 | 4–21 | 8.0 ± 3.9 | 8 ± 3 | n/d | n/d | 26 (29.9%) ATP | 14 (29.2%) /12 (30.7%) | 1 (1.1%) | 33 (41.7%) | 48 (55.2%) | 13 (14.9%) |
| (48/39) | |||||||||||||
| Soliman [19] | Egypt | 160 | <18 | 5.7 ± 3 | 9 ± 2.2 | n/d | n/d | 21 (13.1%) IDF | 15 (18.1%) /6 (7.8%) | n/d | n/d | n/d | n/d |
| (83/77) | |||||||||||||
| Szadkowska [20] | Poland | 163 | 10–18 | 6.2 ± 4.2 | 8 ± 1.5 | n/d | n/d | 14 (8.6%) IDF | 8 (11.1%) /6 (6.6%) | 32 (19.6%) | 4 (2.5%) | 14 (8.6%) | 33 (20.3%) |
| (72/91) | |||||||||||||
| Van Vliet [21] | The Netherlands | 283 | 3–18 | 5.3 (2.9–8.6) | 8.3 (7.5–9.8) | 83 (29.3%) | 26 (9.2%) | 81 (28.6%) WEISS | n/d | 26 (9.2%) | 60 (21.2%) | 49 (17.3%) | 37 (13.1%) |
| (145/138) | |||||||||||||
| Valerio [22] | Italy | 412 | 16–19 | 8.4 ± 3.9 | 8.9 ± 1.7 | 101 (24.5%) | 16 (3.9%) | 39 (9.5%) IDF | 31 (16.1%) /8 (3.7%) | 83 (20.1%) | 66 (16.0%) | 23 (5.6%) | 73 (17.7%) |
| (193/219) | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabia, M.; Markiewicz-Żukowska, R.; Socha, K. Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review. Nutrients 2021, 13, 1782. https://doi.org/10.3390/nu13061782
Grabia M, Markiewicz-Żukowska R, Socha K. Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review. Nutrients. 2021; 13(6):1782. https://doi.org/10.3390/nu13061782
Chicago/Turabian StyleGrabia, Monika, Renata Markiewicz-Żukowska, and Katarzyna Socha. 2021. "Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review" Nutrients 13, no. 6: 1782. https://doi.org/10.3390/nu13061782
APA StyleGrabia, M., Markiewicz-Żukowska, R., & Socha, K. (2021). Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review. Nutrients, 13(6), 1782. https://doi.org/10.3390/nu13061782