Oral Supplementation with Benzylamine Delays the Onset of Diabetes in Obese and Diabetic db-/- Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Group Composition and Oral Benzylamine Supplementation
2.3. Non-Invasive Explorations
2.4. Tissue Sampling and Determination of Blood Biochemistry
2.5. Adipocyte Preparation, Lipolysis and Glucose Transport Measurements
2.6. RNA Extraction and Quantitative Real-Time RT-PCR
2.7. Assessment of H2O2 Release and of NO Bioavailability
2.8. Reagents
2.9. Statistical Analyses
3. Results
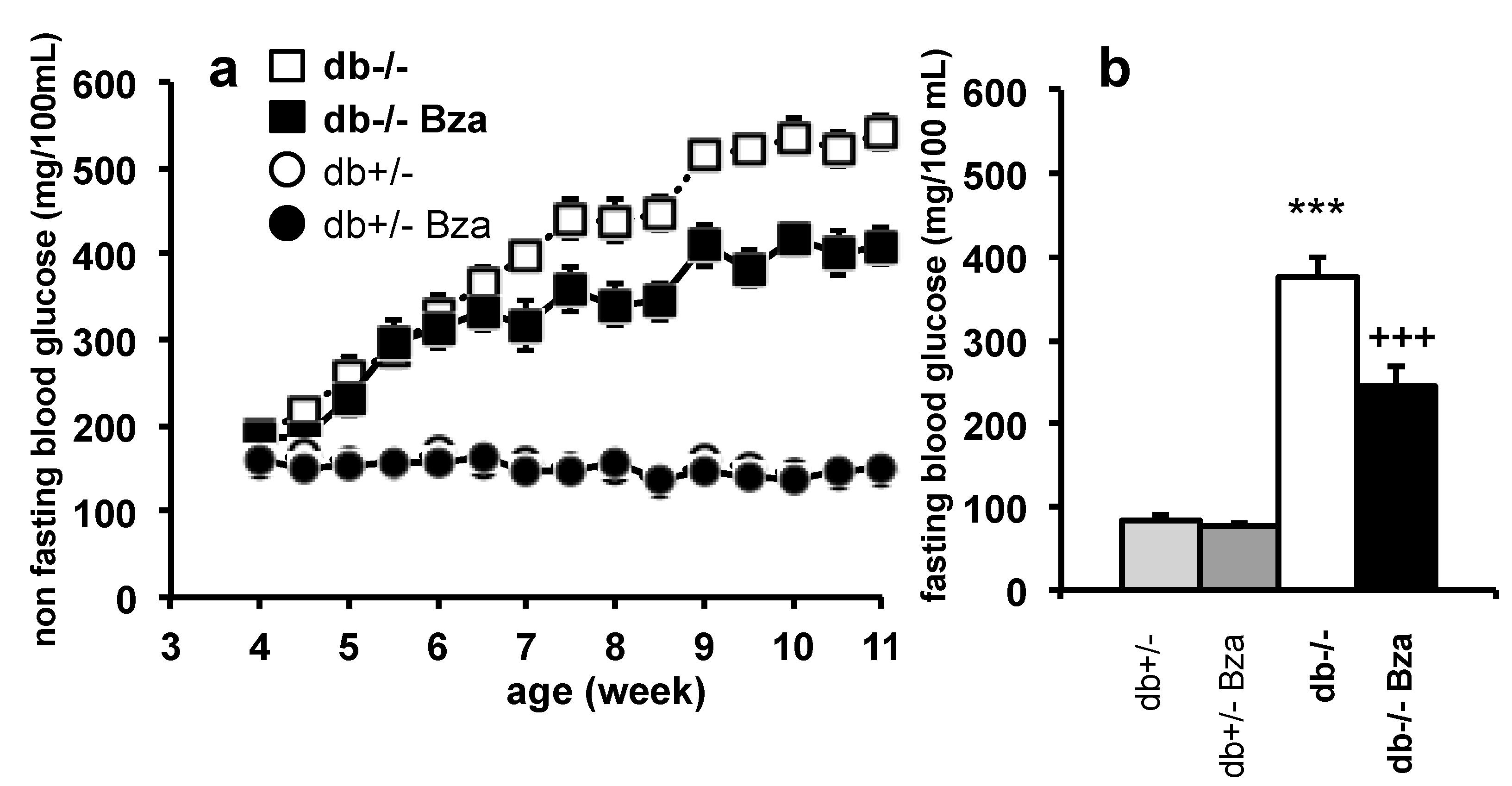
3.1. Oral Supplementation of Benzylamine (Bza-Drinking) Limits the Hyperglycemia of db-/- Mice
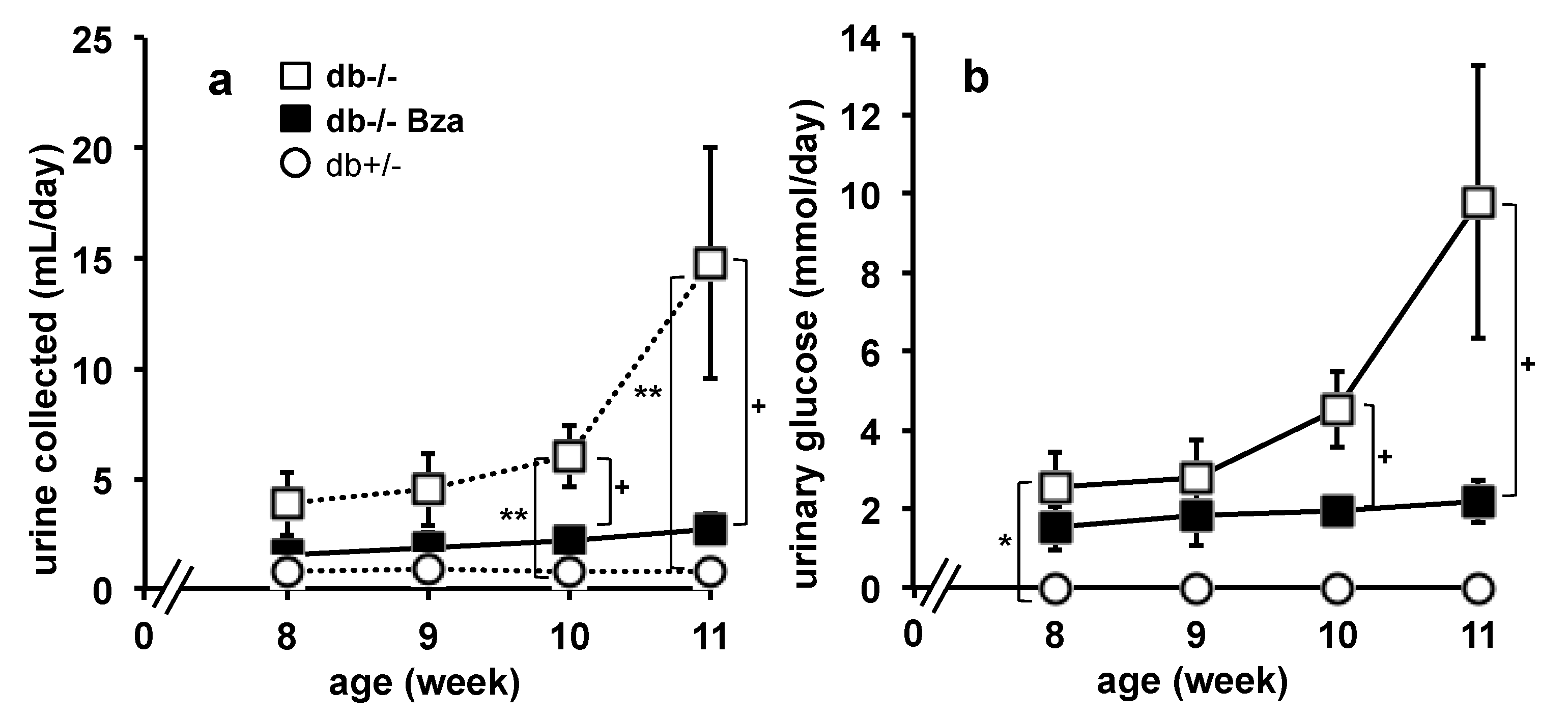
3.2. Daily Urine Production and Glucose Urinary Output
3.3. Water Consumption
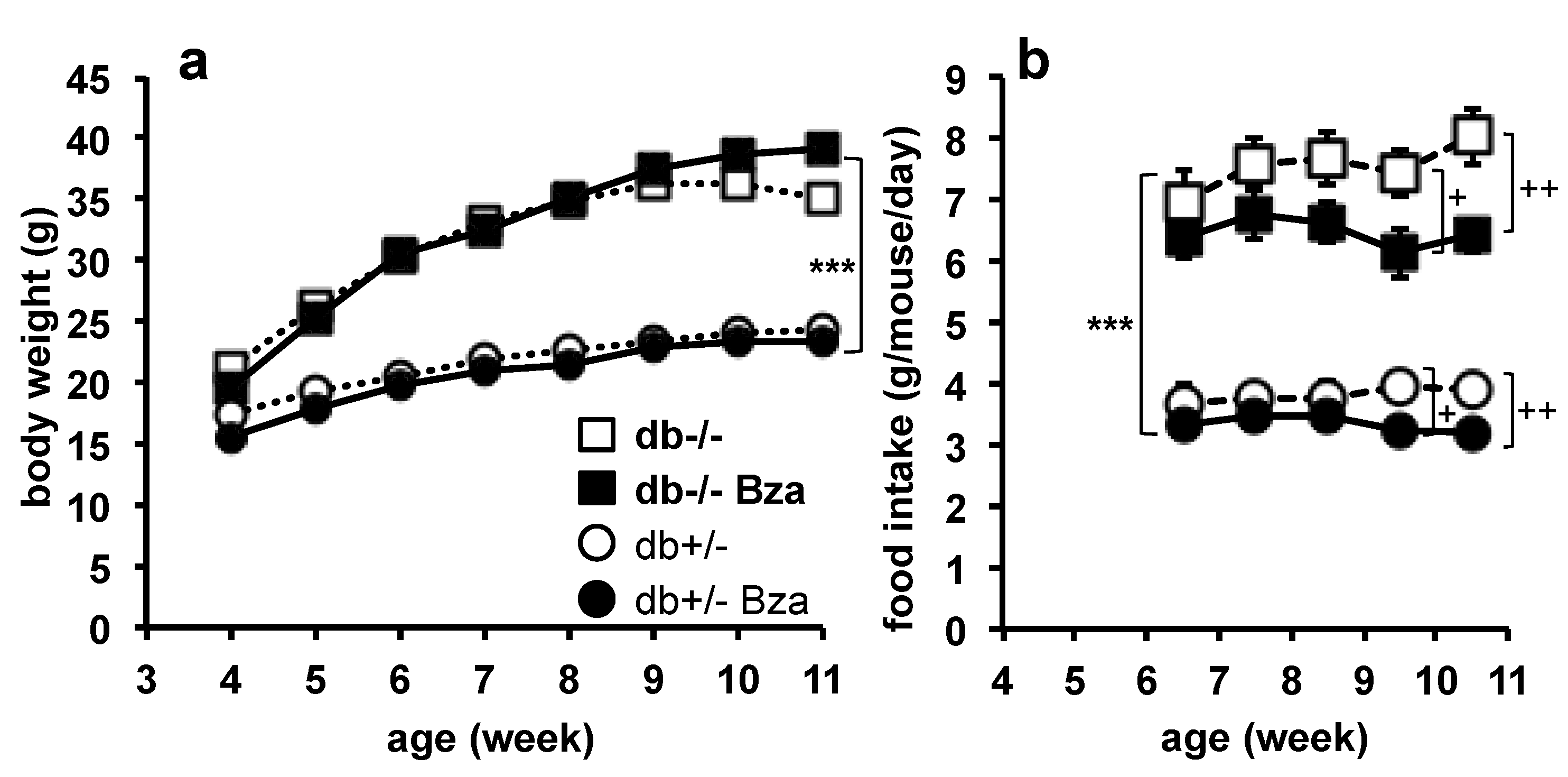
3.4. Body Weight Gain and Food Intake
3.5. Adiposity and Plasma Markers of Metabolic Disturbances
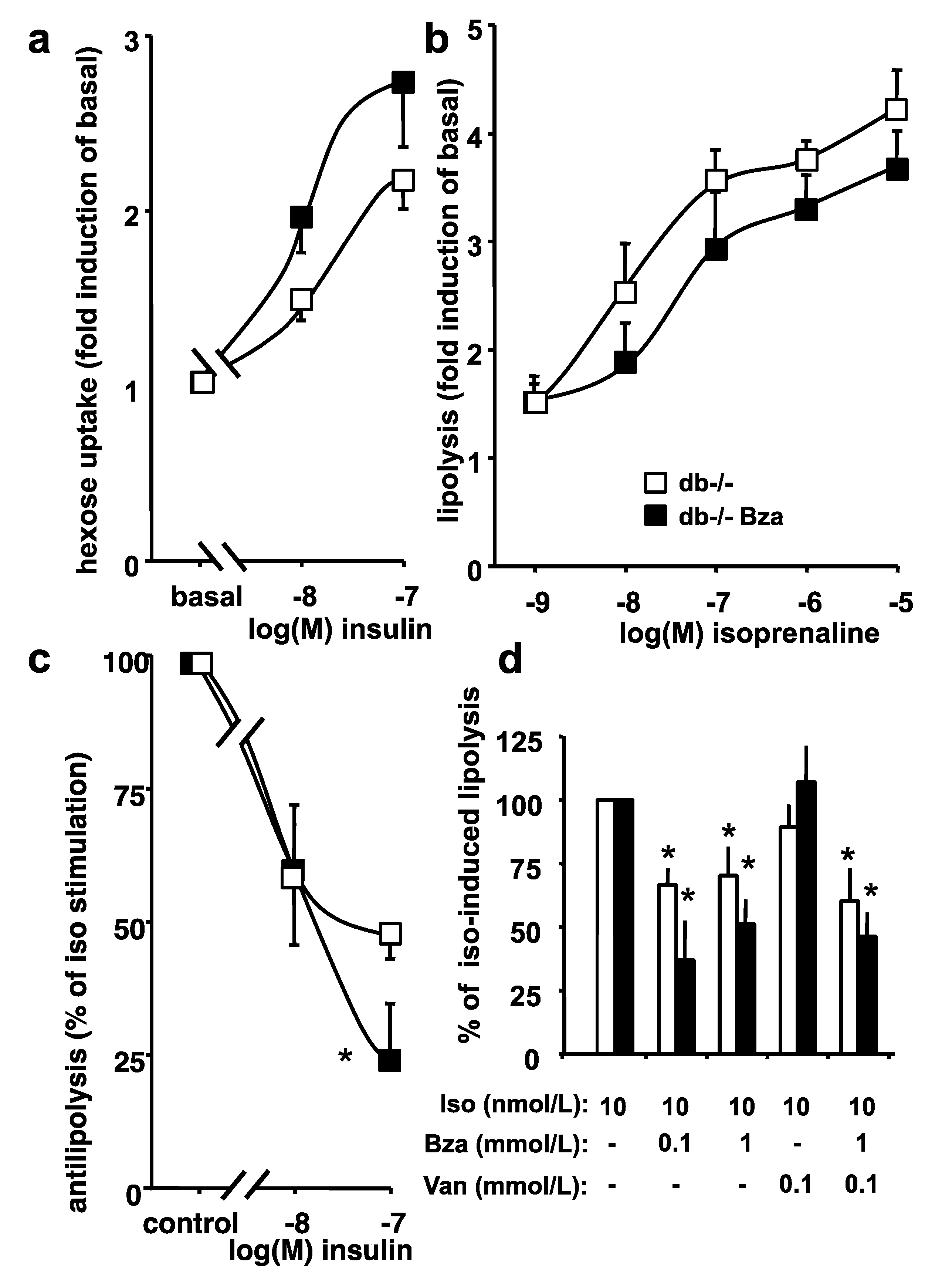
3.6. Glucose Uptake and Lipolytic Activity in Adipocytes from Obese Mice
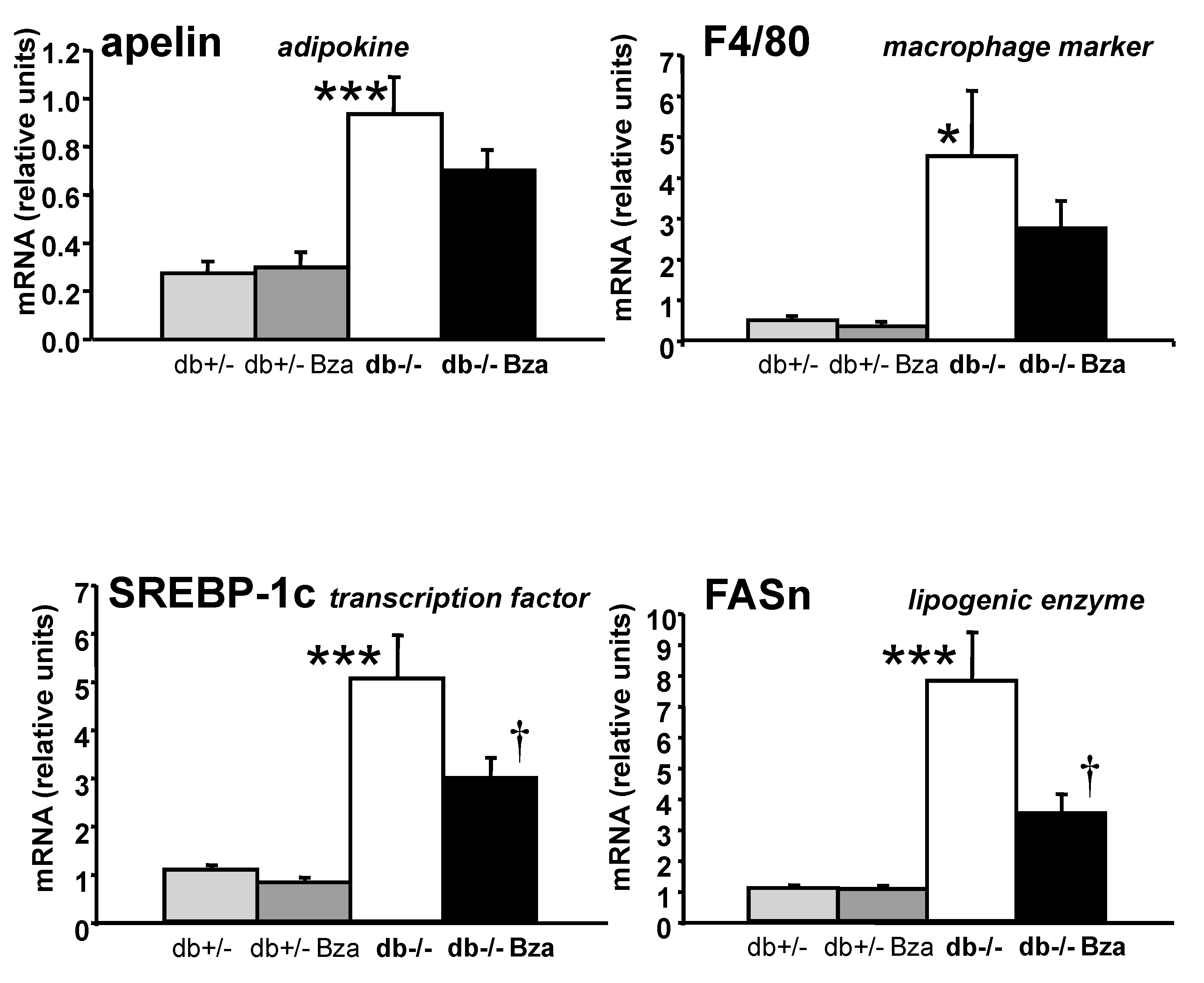
3.7. Metabolism- and Inflammation-Related Genes in Adipose Tissue and Liver
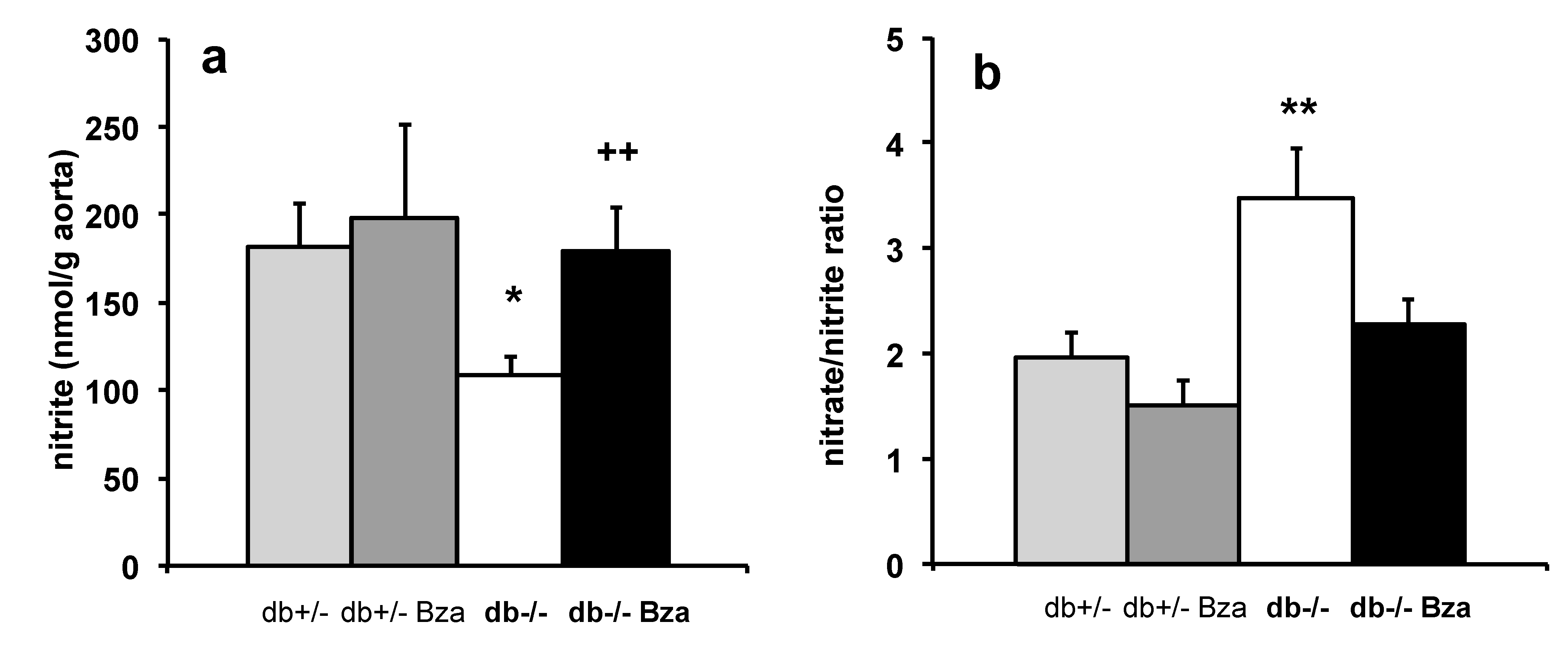
3.8. Oxidative Stress Markers in Adipose Tissue and Aorta NO Bioavailability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Puxty, G.; Conway, W.; Botma, H.; Feron, P.; Maher, D.; Wardhaugh, L. A new CO2 absorbent developed from addressing benzylamine vapour pressure using co-solvents. Energy Procedia 2017, 114, 1956–1965. [Google Scholar] [CrossRef]
- Neurath, G.B.; Dünger, M.; Pein, F.G.; Ambrosius, D.; Schreiber, O. Primary and secondary amines in the human environment. Food Cosmet. Toxicol. 1977, 15, 275–282. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Springer Food chemistry 4th revised and extended edition. Annu. Rev. Biochem. 2009, 79, 655–681. [Google Scholar] [CrossRef]
- Combourieu, B.; Elfoul, L.; Delort, A.M.; Rabot, S. Identification of new derivatives of sinigrin and glucotropaeolin produced by the human digestive microflora using 1H NMR spectroscopy analysis of in vitro incubations. Drug Metab. Dispos. 2001, 29, 1440–1445. [Google Scholar]
- Ignacimuthu, S.; Shanmugam, N. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness. leaves. J. Biosci. 2010, 35, 565–570. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Piscopo, V.; Caputo, R.; Monaco, P. Isolation and structure elucidation of antioxidant polyphenols from quince (Cydonia vulgaris) peels. J. Agric. Food Chem. 2008, 56, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ding, Z.H.; Liu, J.K. The first occurrence in nature of two compounds from hops. Z. für Nat. C 2003, 58, 640–642. [Google Scholar] [CrossRef][Green Version]
- Zheng, Q.; Zhang, Q.-H.; Lu, D.; Leng, L.; Li, P.-Y.; Liu, J.-P. Chemical constituents in Lepidium meyenii cultivated in Jilin. Chin. Trad. Herb. Drugs 2014, 45, 2457–2460. [Google Scholar]
- Esparza, E.; Yi, W.; Limonchi, F.; Cosio, E.G. Glucosinolate catabolism during postharvest drying determines the ratio of bioactive macamides to deaminated benzenoids in Lepidium meyenii (maca) root flour. Phytochemistry 2020, 179, 112502. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Singh, V.K.; Singh, D.K. Characterization of molluscicidal component of Moringa oleifera lead and Momordica charantia fruits and their modes of action in snail Lymnaea acuminata. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 251–259. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, R.; Liu, J.; Huang, Q.; Liu, S.; Jiang, Y. Integrated network pharmacology analysis and experimental validation to reveal the mechanism of anti-insulin resistance effects of Moringa oleifera seeds. Drug Des. Devel. Ther. 2020, 14, 4069–4084. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003, 84, 105–108. [Google Scholar] [CrossRef]
- Jaiswal, D.; Kumar Rai, P.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Mathur, M.; Bajaj, V.K.; Katariya, P.; Yadav, S.; Kamal, R.; Gupta, R.S. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J. Diabetes 2012, 4, 164–171. [Google Scholar] [CrossRef]
- Makedonskaya, M.I.; Veselova, I.A.; Kalmykov, S.N.; Shekhovtsova, T.N. Novel biosensing system for the simultaneous multiplex fluorescent determination of catecholamines and their metabolites in biological liquids. J. Pharm. Biomed. Anal 2018, 156, 133–141. [Google Scholar] [CrossRef]
- Tamim, N.M.; Bennett, L.W.; Shellem, T.A.; Doerr, J.A. High-performance liquid chromatographic determination of biogenic amines in poultry carcasses. J. Agric. Food Chem. 2002, 50, 5012–5015. [Google Scholar] [CrossRef] [PubMed]
- Barrand, M.A.; Fox, S.A. Amine oxidase activities in brown adipose tissue of the rat: Identification of semicarbazide-sensitive (clorgyline-resistant) activity at the fat cell membrane. J. Pharm. Pharmacol. 1984, 36, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Jalkanen, S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 1992, 257, 1407–1409. [Google Scholar] [CrossRef]
- Buffoni, F.; Dowling, T.G.; Cambi, S. Purification of pig heart benzylamine oxidase. Int. J. Mol. Med. 1998, 2, 187–195. [Google Scholar] [PubMed]
- Olivieri, A.; Rico, D.; Khiari, Z.; Henehan, G.; O’Sullivan, J.; Tipton, K. From caffeine to fish waste: Amine compounds present in food and drugs and their interactions with primary amine oxidase. J. Neural Transm. 2011, 118, 1079–1089. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A cell surface amine oxidase in translation. Antioxid. Redox Signal 2019, 30, 314–332. [Google Scholar] [CrossRef]
- Vakal, S.; Jalkanen, S.; Dahlström, K.M.; Salminen, T.A. Human copper-containing amine oxidases in drug design and development. Molecules 2020, 25, 1293. [Google Scholar] [CrossRef]
- Marti, L.; Abella, A.; Carpéné, C.; Palacin, M.; Testar, X.; Zorzano, A. Combined treatment with benzylamine and low dosages of vanadate enhances glucose tolerance and reduces hyperglycemia in streptozotocin-induced diabetic rats. Diabetes 2001, 50, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Abella, A.; Marti, L.; Camps, M.; Claret, M.; Fernandez-Alvarez, J.; Gomis, R.; Guma, A.; Viguerie, N.; Carpéné, C.; Palacin, M.; et al. Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 activity exerts an antidiabetic action in Goto-Kakizaki rats. Diabetes 2003, 52, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Enrique-Tarancon, G.; Castan, I.; Morin, N.; Marti, L.; Abella, A.; Camps, M.; Casamitjana, R.; Palacin, M.; Testar, X.; Degerman, E.; et al. Substrates of semicarbazide-sensitive amine oxidase co-operate with vanadate to stimulate tyrosine phosphorylation of insulin-receptor-substrate proteins, phosphoinositide 3-kinase activity and GLUT4 translocation in adipose cells. Biochem. J. 2000, 350, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H.; Wang, M.; Fan, H.; Deng, Y.; Gubisne-Haberle, D. Involvement of SSAO-mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E634–E641. [Google Scholar] [CrossRef] [PubMed]
- Zorzano, A.; Abella, A.; Marti, L.; Carpéné, C.; Palacin, M.; Testar, X. Semicarbazide-sensitive amine oxidase activity exerts insulin-like effects on glucose metabolism and insulin-signaling pathways in adipose cells. Biochim. Biophys. Acta 2003, 1647, 3–9. [Google Scholar] [CrossRef]
- Bour, S.; Prévot, D.; Guigne, C.; Stolen, C.; Jalkanen, S.; Valet, P.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates fail to induce insulin-like effects in fat cells from AOC3 knockout mice. J. Neural Transm. 2007, 114, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Yraola, F.; Garcia-Vicente, S.; Marti, L.; Albericio, F.; Zorzano, A.; Royo, M. Understanding the mechanism of action of the novel SSAO substrate (C7NH10)6(V10O28).2H2O, a prodrug of peroxovanadate insulin mimetics. Chem. Biol. Drug Des. 2007, 69, 423–428. [Google Scholar] [CrossRef]
- Lönnroth, P.; Eriksson, J.W.; Posner, B.I.; Smith, U. Peroxovanadate but not vanadate exerts insulin-like effects in human adipocytes. Diabetologia 1993, 36, 113–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Subra, C.; Fontana, E.; Visentin, V.; Testar, X.; Carpéné, C. Tyramine and benzylamine partially but selectively mimic insulin action on adipose differentiation in 3T3-L1 cells. J. Physiol. Biochem. 2003, 59, 209–216. [Google Scholar] [CrossRef]
- Bour, S.; Visentin, V.; Gres, S.; Saulnier-Blache, J.S.; Wabitsch, M.; Carpéné, C. Tyramine, benzylamine, and to a lesser extent histamine, partially mimic the adipogenic effect of insulin in a human preadipocyte cell strain. Inflamm. Res. 2005, 54, S60–S61. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Daviaud, D.; Boucher, J.; Bour, S.; Visentin, V.; Gres, S.; Duffaut, C.; Fontana, E.; Testar, X.; Saulnier-Blache, J.S.; et al. Short- and long-term insulin-like effects of monoamine oxidases and semicarbazide-sensitive amine oxidase substrates in cultured adipocytes. Metabolism 2006, 55, 1397–1405. [Google Scholar] [CrossRef]
- Iglesias-Osma, M.C.; Garcia-Barrado, M.J.; Visentin, V.; Pastor-Mansilla, M.F.; Bour, S.; Prevot, D.; Valet, P.; Moratinos, J.; Carpéné, C. Benzylamine exhibits insulin-like effects on glucose disposal, glucose transport, and fat cell lipolysis in rabbits and diabetic mice. J. Pharmacol. Exp. Ther. 2004, 309, 1020–1028. [Google Scholar] [CrossRef]
- Iffiu-Soltesz, Z.; Prévot, D.; Grès, S.; Bour, S.; Szoko, E.; Knauf, C.; Burcelin, R.; Fernandez-Quintela, A.; Lomba, A.; Milagro, F.I.; et al. Influence of acute and chronic administration of benzylamine on glucose tolerance in diabetic and obese mice fed on very high-fat diet. J. Physiol. Biochem. 2007, 63, 305–315. [Google Scholar] [CrossRef]
- Morin, N.; Lizcano, J.M.; Fontana, E.; Marti, L.; Smih, F.; Rouet, P.; Prévot, D.; Zorzano, A.; Unzeta, M.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J. Pharmacol. Exp. Ther. 2001, 297, 563–572. [Google Scholar]
- Mercader, J.; Iffiu-Soltesz, Z.; Brenachot, X.; Foldi, A.; Dunkel, P.; Balogh, B.; Attané, C.; Valet, P.; Matyus, P.; Carpéné, C. SSAO substrates exhibiting insulin-like effects in adipocytes as a promising treatment option for metabolic disorders. Future Med. Chem. 2010, 2, 1735–1749. [Google Scholar] [CrossRef]
- Iffiu-Soltesz, Z.; Wanecq, E.; Lomba, A.; Portillo, M.P.; Pellati, F.; Szoko, E.; Bour, S.; Woodley, J.; Milagro, F.I.; Alfredo Martinez, J.; et al. Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol. Res. 2010, 61, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.G.; Al-Ani, M.R.; Lawson, A. Hippuric acid excretion after benzylamine ingestion in man. Br. J. Ind. Med. 1978, 35, 230–231. [Google Scholar] [CrossRef]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; Moore, K.J.; Breitbart, R.E.; et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Iffiu-Soltesz, Z.; Mercader, J.; Daviaud, D.; Boucher, J.; Carpéné, C. Increased primary amine oxidase expression and activity in white adipose tissue of obese and diabetic db-/- mice. J. Neural Transm. 2011, 118, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigne, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Dusaulcy, R.; Rancoule, C.; Gres, S.; Wanecq, E.; Colom, A.; Guigne, C.; van Meeteren, L.A.; Moolenaar, W.H.; Valet, P.; Saulnier-Blache, J.S. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 2011, 52, 1247–1255. [Google Scholar] [CrossRef]
- Iffiú-Soltész, Z.; Bour, S.; Tercé, F.; Collet, X.; Szökő, E.; Carpéné, C. Hypercholesterolemia of obese mice with deletion of vascular adhesion protein-1 occurs without other atherosclerosis risk factor. Vessel Plus 2021, 5, 16. [Google Scholar] [CrossRef]
- Minet-Ringuet, J.; Even, P.C.; Valet, P.; Carpéné, C.; Visentin, V.; Prevot, D.; Daviaud, D.; Quignard-Boulange, A.; Tome, D.; de Beaurepaire, R. Alterations of lipid metabolism and gene expression in rat adipocytes during chronic olanzapine treatment. Mol. Psychiatry 2007, 12, 562–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bour, S.; Daviaud, D.; Grès, S.; Lefort, C.; Prévot, D.; Zorzano, A.; Wabitsch, M.; Saulnier-Blache, J.S.; Valet, P.; Carpéné, C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007, 89, 916–925. [Google Scholar] [CrossRef]
- Szökő, E.; Tábi, T.; Halász, A.; Pálfi, M.; Magyar, K. High sensitivity analysis of nitrite and nitrate in biological samples by capillary zone electrophoresis with transient isotachophoretic sample stacking. J. Chromatogr. A 2004, 1051, 177–183. [Google Scholar] [CrossRef]
- Moldes, M.; Fève, B.; Pairault, J. Molecular cloning of a major mRNA species in murine 3T3 adipocyte lineage differentiation-dependent expression, regulation, and identification as semicarbazide-sensitive amine oxidase. J. Biol. Chem. 1999, 274, 9515–9523. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kamata, K. Effect of chronic insulin treatment on NO production and endothelium-dependent relaxation in aortae from established STZ-induced diabetic rats. Atherosclerosis 2001, 155, 313–320. [Google Scholar] [CrossRef]
- Jargaud, V.; Bour, S.; Tercé, F.; Collet, X.; Valet, P.; Bouloumié, A.; Guillemot, J.-C.; Mauriège, P.; Jalkanen, S.; Stolen, C.; et al. Obesity of mice lacking VAP-1/SSAO by Aoc3 gene deletion is reproduced in mice expressing a mutated vascular adhesion protein-1 (VAP-1) devoid of amine oxidase activity. J. Physiol. Biochem. 2021, 77, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Cioni, L.; De Siena, G.; Ghelardini, C.; Sernissi, O.; Alfarano, C.; Pirisino, R.; Raimondi, L. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur. J. Pharmacol. 2006, 529, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Visentin, V.; Prevot, D.; Daviaud, D.; Saulnier-Blache, J.S.; Guigne, C.; Valet, P.; Carpéné, C. Effects of oral administration of benzylamine on glucose tolerance and lipid metabolism in rats. J. Physiol. Biochem. 2005, 61, 371–379. [Google Scholar] [CrossRef]
- Banchelli, G.; Ghelardini, C.; Raimondi, L.; Galeotti, N.; Pirisino, R. Selective inhibition of amine oxidases differently potentiate the hypophagic effect of benzylamine in mice. Eur. J. Pharmacol. 2001, 413, 91–99. [Google Scholar] [CrossRef]
- Pirisino, R.; Ghelardini, C.; Banchelli, G.; Galeotti, N.; Raimondi, L. Methylamine and benzylamine induced hypophagia in mice: Modulation by semicarbazide-sensitive benzylamine oxidase inhibitors and aODN towards Kv1.1 channels. Br. J. Pharmacol. 2001, 134, 880–886. [Google Scholar] [CrossRef]
- Weston, C.J.; Adams, D.H. Hepatic consequences of vascular adhesion protein-1 expression. J. Neural. Transm. 2011, 118, 1055–1064. [Google Scholar] [CrossRef]
- Visentin, V.; Prevot, D.; Marti, L.; Carpéné, C. Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur. J. Pharmacol. 2003, 466, 235–243. [Google Scholar] [CrossRef]
- Carpéné, C.; Garcia-Vicente, S.; Serrano, M.; Marti, L.; Belles, C.; Royo, M.; Galitzky, J.; Zorzano, A.; Testar, X. Insulin-mimetic compound hexaquis (benzylammonium) decavanadate is antilipolytic in human fat cells. World J. Diabetes 2017, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Fontana, E.; Boucher, J.; Marti, L.; Lizcano, J.M.; Testar, X.; Zorzano, A.; Carpene, C. Amine oxidase substrates mimic several of the insulin effects on adipocyte differentiation in 3T3 F442A cells. Biochem. J. 2001, 356, 769–777. [Google Scholar] [CrossRef]
- Mercier, N.; Moldes, M.; El Hadri, K.; Fève, B. Semicarbazide-sensitive amine oxidase activation promotes adipose conversion of 3T3-L1 cells. Biochem. J. 2001, 358, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ralle, M.; Wolfgang, M.J.; Dhawan, N.; Burkhead, J.L.; Rodriguez, S.; Kaplan, J.H.; Wong, G.W.; Haughey, N.; Lutsenko, S. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 2018, 16, e2006519. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Miike, T.; Kunishiro, K.; Kanda, M.; Azukizawa, S.; Kurahashi, K.; Shirahase, H. Impairment of endothelium-dependent ACh-induced relaxation in aorta of diabetic db/db mice--possible dysfunction of receptor and/or receptor-G protein coupling. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 401–410. [Google Scholar] [CrossRef]
- Thomas, S.R.; Chen, K.; Keaney, J.F., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002, 277, 6017–6024. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Davis, M.E.; Kanner, W.; Harrison, D.G.; Dudley, S.C., Jr. Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol. Pharmacol. 2003, 63, 325–331. [Google Scholar] [CrossRef]
- Obata, T. Diabetes and semicarbazide-sensitive amine oxidase (SSAO) activity: A review. Life Sci. 2006, 79, 417–422. [Google Scholar] [CrossRef]
- Akagawa, M.; Sasaki, T.; Suyama, K. Oxidative deamination of benzylamine by glycoxidation. Bioorganic Med. Chem. 2003, 11, 1411–1417. [Google Scholar] [CrossRef]
- Sawada, M.; Matsuo, M.; Seki, J. Inhibition of cholesterol synthesis causes both hypercholesterolemia and hypocholesterolemia in hamsters. Biol. Pharm. Bull. 2002, 25, 1577–1582. [Google Scholar] [CrossRef][Green Version]
- Fukushima, H.; Aono, S.; Nakatani, H. Effect of N-(alpha-Methylbenzyl) linoleamide on lipid levels of plasma and liver in cholesterol-fed rats. J. Nutr. 1968, 96, 15–20. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Suyama, K. Amine oxidase-like activity of polyphenols. Mechanism and properties. Eur. J. Biochem. 2001, 268, 1953–1963. [Google Scholar] [CrossRef] [PubMed]

| Mouse Gene | Oligonucleotide Sense |
|---|---|
| /Antisense | |
| Adiponectin |
TGGAATGACAGGAGCTGAAGG TATAAGCGGCTTCTCCAGGCT |
| Aoc3 |
GTGGTCAGATCCGTGTCTACCTT CCTGTGGCGTGGAATTTGA |
| Apelin |
TCTTGGCTCTTCCCTCTTTTCA GTGCTGGAATCCACTGGAGAA |
| FASn |
ATCCTGGAACGAGAACACGATCT AGAGACGTGTCACTCCTGGACTT |
| F4/80 |
TGACAACCAGACGGCTTGTG GCAGGCGAGGAAAAGATAGTGT |
| CD31 |
GTCGTCCATGTCCCGAGAA GCACAGGACTCTCGCAATCC |
| HSL |
GGCTTACTGGGCACAGATACCT CTGAAGGCTCTGAGTTGCTCAA |
| PAI-1 |
TCTCCAATTACTGGGTGAGTCAGA GCAGCCGGAAATGACACAT |
| Tie2 |
CAATCAGGCCTGGAAATACATTG TCCGCGGCTCCAAGTAGTT |
| Parameter | db+/- | Bza-db+/- | db-/- | Bza-db-/- | ||
| body mass (g) | 21.7 ± 0.8 | 21.5 ± 0.7 | 32.9 ± 1.0 | *** | 36.2 ± 1.0 | + |
| body weight gain (g) | 7.6 ± 0.7 | 8. 7 ± 0.6 | 14.6 ± 1.1 | *** | 19.7 ± 1.1 | ++ |
| INWAT mass(g) | 0.45 ± 0.02 | 0.42 ± 0.06 | 1.93 ± 0.10 | *** | 2.24 ± 0.11 | |
| SCWAT mass (g) | 0.35 ± 0.03 | 0.29 ± 0.04 | 2.66 ± 0.18 | *** | 3.31 ± 0.16 | + |
| liver mass (g) | 0.89 ± 0.03 | 0.91 ± 0.04 | 1.71 ± 0.07 | *** | 1.87 ± 0.07 | |
| Plasma Level | db+/- | Bza-db+/- | db-/- | Bza-db-/- | ||
|---|---|---|---|---|---|---|
| glucose (g/L) | 1.10 ± 0.08 | 1.07 ± 0.13 | 5.67 ± 0.49 | *** | 3.30 ± 0.43 | ++ |
| insulin (μg/L) | 0.40 ± 0.05 | 0.38 ± 0.05 | 2.89 ± 0.32 | *** | 2.62 ± 0.51 | |
| relative murine HOMA-IR | 1.02 ± 0.16 | 0.76 ± 0.10 | 37.38 ± 5.48 | *** | 20.69 ± 5.38 | + |
| cholesterol (mmol/L) | 1.94 ± 0.13 | 2.00 ± 0.13 | 2.29 ± 0.22 | * | 2.46 ± 0.19 | |
| LDL cholesterol (mmol/L) | 0.18 ± 0.04 | 0.20 ± 0.04 | 0.28 ± 0.12 | 0.23 ± 0.10 | ||
| HDL cholesterol (mmol/L) | 1.62 ± 0.11 | 1.69 ± 0.11 | 2.01 ± 0.16 | ** | 2.08 ± 0.15 | |
| FFA (mmol/L) | 0.64 ± 0.05 | 0.78 ± 0.07 | 0.90 ± 0.05 | *** | 0.94 ± 0.06 | |
| TG (g/L) | 0.97 ± 0.07 | 1.13 ± 0.09 | 1.27 ± 0.10 | 0.87 ± 0.06 | + | |
| uric acid (µmol/L) | 279.3 ± 18.0 | 272.6 ± 17.9 | 406.7 ± 23.5 | *** | 330.6 ± 24.5 | + |
| fructosamine (µmol/L) | 204.5 ± 4.7 | 189.1 ± 7.7 | 291.8 ± 11.2 | *** | 262.7 ± 14.3 | |
| Function | Product | db+/- | db-/- | Bza-db-/- | |
|---|---|---|---|---|---|
| Adipokine secretion | adiponectin | 207.03 ± 16.36 | 298.20 ± 32.30 | * | 302.33 ± 21.49 |
| apelin | 0.27 ± 0.05 | 0.93 ± 0.15 | *** | 0.70 ± 0.08 | |
| Fatty acid metabolism | FASn | 220.40 ± 43.23 | 77.47 ± 48.93 | * | 41.57 ± 4.88 |
| HSL | 55.39 ± 4.06 | 86.08 ± 8.57 | ** | 88.26 ± 6.16 | |
| Inflammation | PAI-1 | 2.04 ± 0.66 | 20.43 ± 4.14 | *** | 26.59 ± 4.21 |
| F4/80 | 0.49 ± 0.08 | 4.51 ± 1.60 | * | 2.74 ± 0.68 | |
| Endothelial markers | CD 31 | 31.52 ± 3.25 | 43.85 ± 4.78 | * | 41.22 ± 3.09 |
| Tie 2 | 5.37 ± 0.62 | 8.06 ± 0.99 | * | 6.08 ± 0.85 | |
| Adipogenesis marker | AOC3 | 30.68 ± 3.37 | 78.22 ± 7.58 | *** | 84.64 ± 7.10 |
| H2O2 Release (pmol/mg prot/min) | db+/- | Bza-db+/- | db-/- | Bza-db-/- |
|---|---|---|---|---|
| basal | 65 ± 13 | 102 ± 24 | 113 ± 19 * | 103 ± 9 |
| AOC3-dependent oxidation of Bza 0.1 mmol/L | 152 ± 10 | 171 ± 29 | 299 ± 47 * | 252 ± 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iffiú-Soltesz, Z.; Wanecq, E.; Tóthfalusi, L.; Szökő, É.; Carpéné, C. Oral Supplementation with Benzylamine Delays the Onset of Diabetes in Obese and Diabetic db-/- Mice. Nutrients 2021, 13, 2622. https://doi.org/10.3390/nu13082622
Iffiú-Soltesz Z, Wanecq E, Tóthfalusi L, Szökő É, Carpéné C. Oral Supplementation with Benzylamine Delays the Onset of Diabetes in Obese and Diabetic db-/- Mice. Nutrients. 2021; 13(8):2622. https://doi.org/10.3390/nu13082622
Chicago/Turabian StyleIffiú-Soltesz, Zsuzsa, Estelle Wanecq, László Tóthfalusi, Éva Szökő, and Christian Carpéné. 2021. "Oral Supplementation with Benzylamine Delays the Onset of Diabetes in Obese and Diabetic db-/- Mice" Nutrients 13, no. 8: 2622. https://doi.org/10.3390/nu13082622
APA StyleIffiú-Soltesz, Z., Wanecq, E., Tóthfalusi, L., Szökő, É., & Carpéné, C. (2021). Oral Supplementation with Benzylamine Delays the Onset of Diabetes in Obese and Diabetic db-/- Mice. Nutrients, 13(8), 2622. https://doi.org/10.3390/nu13082622







