Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Caenorhabditis Elegans Strains and Treatment with Caffeine
2.2. Analysis of Intestinal Aging
2.3. Live Image Observation of Fluorescence-Tagged Transgenic Animals
2.4. Western Blot Analysis
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.6. Analysis of Reactive Oxygen Species (ROS) Production in Mitochondria
2.7. Analysis of the Mitochondrial Membrane Potential (MMP)
2.8. Motility Assay
2.9. Survival Assay
2.10. Survival Assay under Paraquat-Induced Oxidative Stress
2.11. Statistical Analysis
3. Results
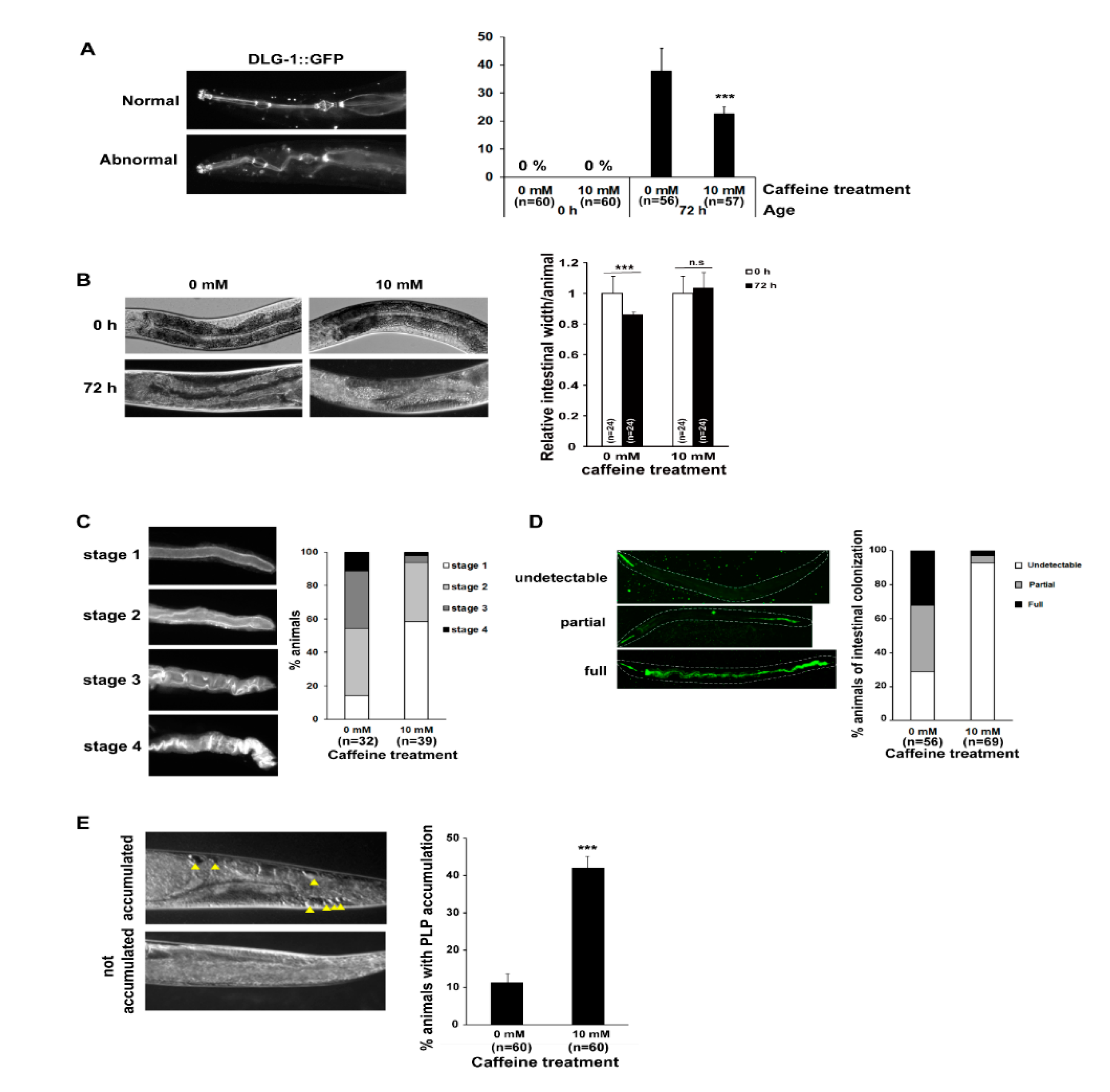
3.1. Long-Term Caffeine Intake Prevents Intestinal Aging in Caenorhabditis elegans
3.2. Long-Term Caffeine Intake Reduces Vitellogenesis in Aging Caenorhabditis elegans
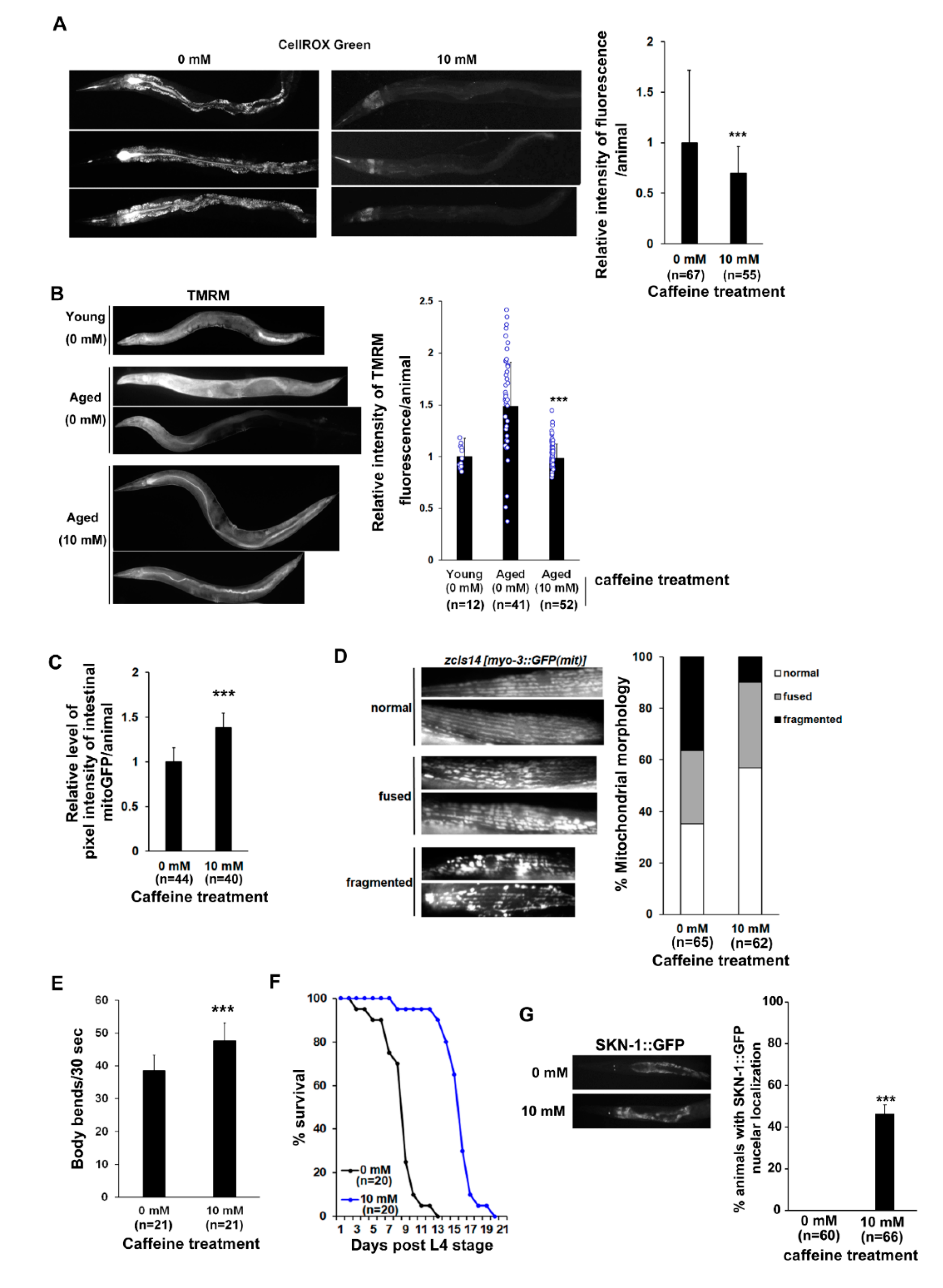
3.3. Long-Term Caffeine Intake Promotes Mitochondrial Function in Aging Caenorhabditis elegans
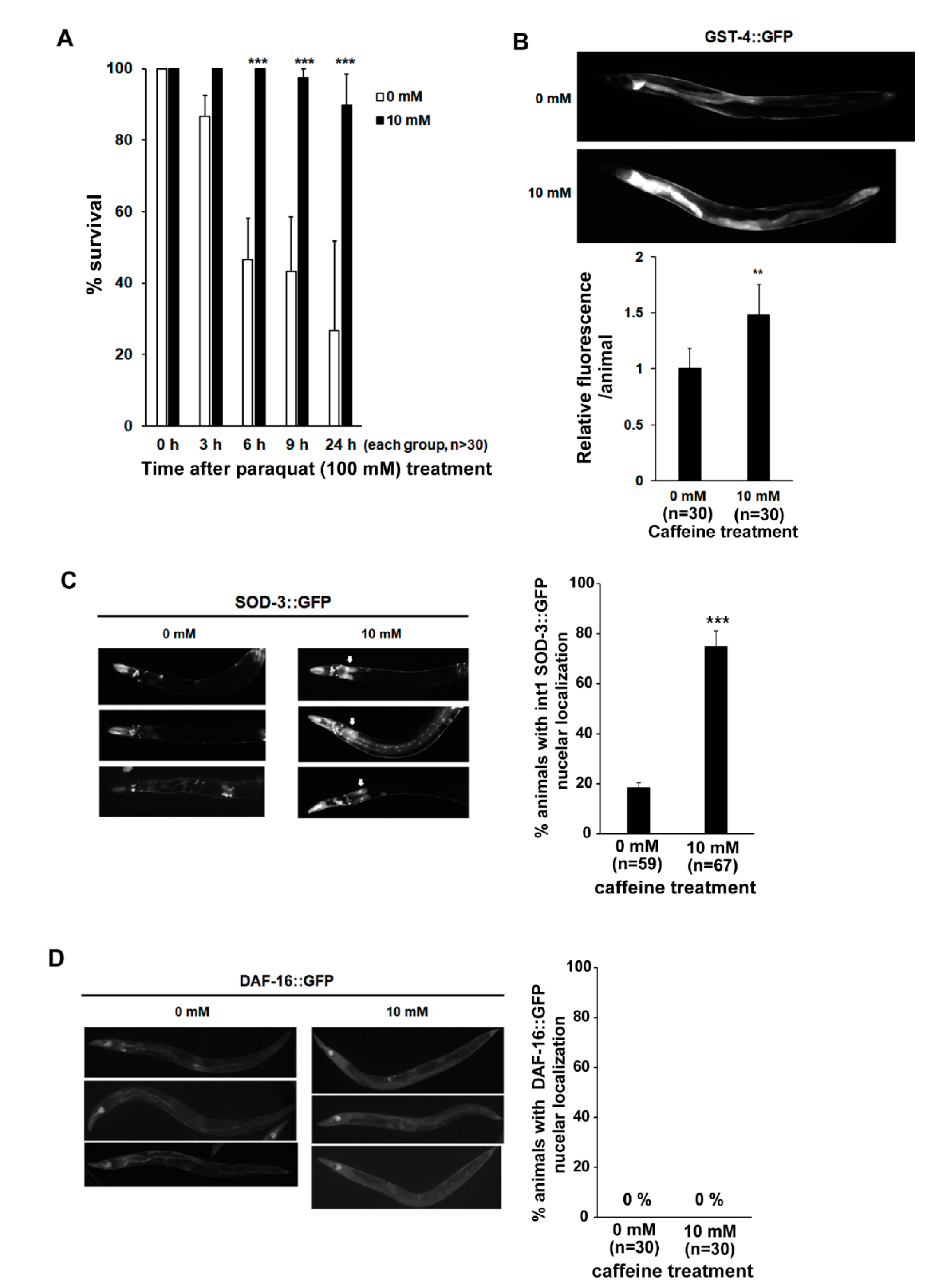
3.4. Long-Term Caffeine Intake Induces Oxidative Stress Response in Aging Caenorhabditis elegans
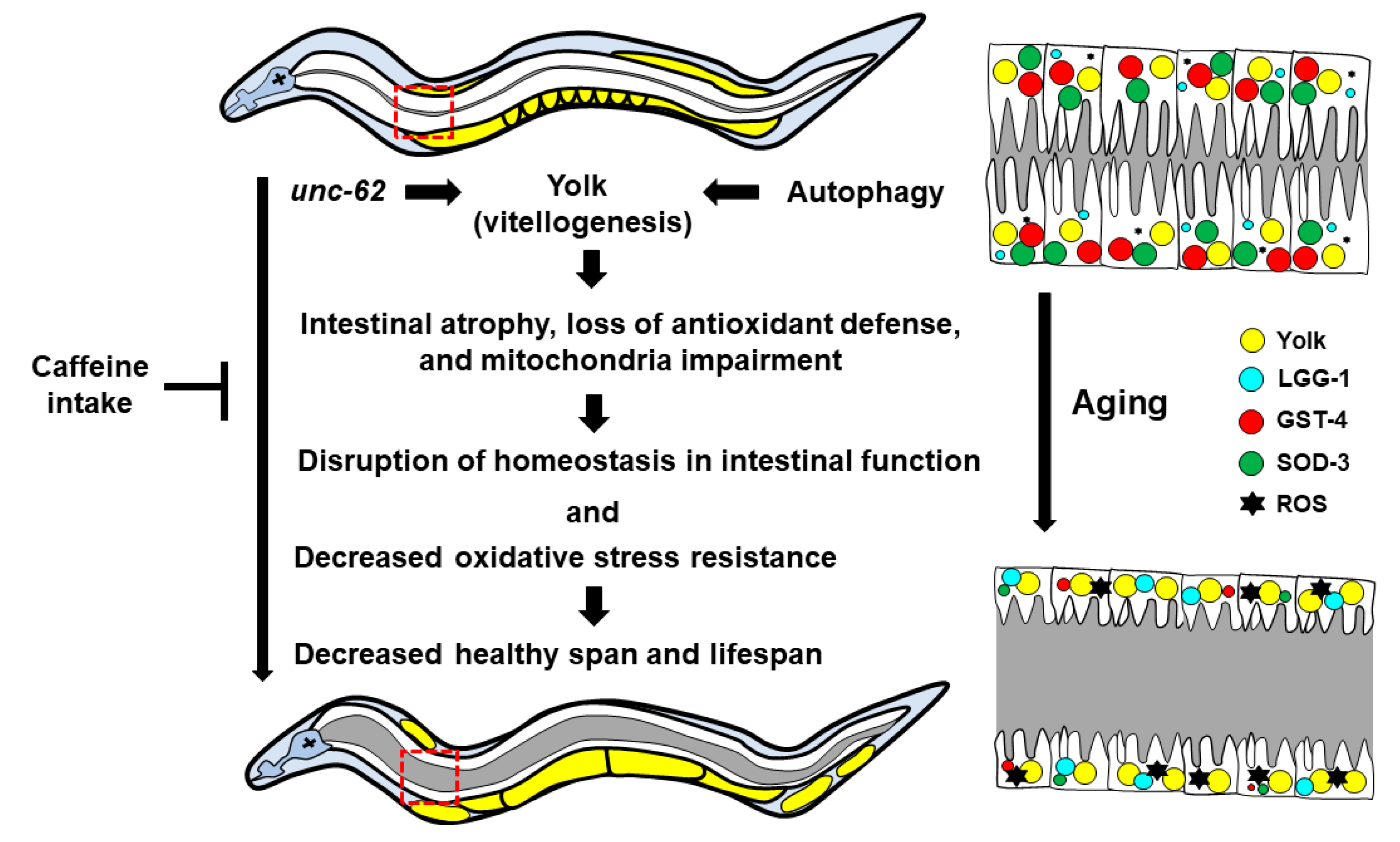
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Min, H.; Kawasaki, I.; Gong, J.; Shim, Y.H. Caffeine induces high expression of cyp-35A family genes and inhibits the early larval development in Caenorhabditis elegans. Mol. Cells. 2015, 38, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Guan, Y.; Huang, Q.; Lv, M.; He, X.; Yan, Y.; Hayashi, S.; Fang, C.; Wang, X.; Sheng, J. Low concentrations of caffeine and its analogs extend the lifespan of Caenorhabditis elegans by modulating IGF-1-Like pathway. Front. Aging Neurosci. 2018, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Roxo, M.; Cheng, X.; Zhang, S.; Cheng, H.; Wink, M. Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans. Food Chem. X 2019, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Youn, E.; Shim, Y.H. Maternal caffeine intake disrupts eggshell integrity and retards larval development by reducing yolk production in a Caenorhabditis elegans model. Nutrients 2020, 12, 1334. [Google Scholar] [CrossRef] [PubMed]
- Sutphin, G.L.; Bishop, E.; Yanos, M.E.; Moller, R.M.; Kaeberlein, M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Logev. Healthspan. 2012, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Bridi, J.C.; Barros, A.G.A.; Sampaio, L.R.; Ferreira, J.C.D.; Antunes Soares, F.A.; Romano-Silva, M.A. Lifespan extension induced by caffeine in Caenorhabditis elegans is partially dependent on adenosine signaling. Front. Aging Neurosci. 2015, 7, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Amin, M.; Kawasaki, I.; Gong, J.; Shim, Y.H. Caffeine induces the stress response and up-regulates heat shock proteins in Caenorhabditis elegans. Mol. Cells. 2016, 39, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Min, H.; Youn, E.; Kawasaki, I.; Shim, Y.H. Caffeine-induced food-avoidance behavior is mediated by neuroendocrine signals in Caenorhabditis elegans. BMB Rep. 2017, 50, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, E.J.; Greenstein, D. Introduction to the germ line. Wormbook 2005, 1–4. [Google Scholar] [CrossRef]
- Luo, S.; Murphy, C.T. Caenorhabditis elegans reproductive aging: Regulation and underlying mechanisms. Genesis 2011, 49, 53–65. [Google Scholar] [CrossRef]
- Hsin, H.; Kenyon, C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 1999, 399, 362–366. [Google Scholar] [CrossRef]
- Berman, J.R.; Kenyon, C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 2006, 124, 1055–1068. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezcurra, M.; Benedetto, A.; Sornda, T.; Gilliat, A.F.; Au, C.; Zhang, Q.; van Schelt, S.; Petrache, A.L.; Wang, H.; de la Guardia, Y.; et al. C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies. Curr. Biol. 2018, 28, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.D.; Weber, D.; Day, N.; Vitelli, C.; Crippen, D.; Herndon, L.A.; Hall, D.H.; Melov, S. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell 2011, 10, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdowski, L.; Thomson, A.B. Aging and the intestine. World J. Gastroenterol. 2006, 12, 7578–7584. [Google Scholar] [CrossRef]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Tata, J.R.; Smith, D.F. Vitellogenesis: A versatile model for hormonal regulation of gene expression. Recent Prog. Horm. Res. 1979, 35, 47–95. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Vitellogenins—Yolk gene function and regulation in Caenorhabditis elegans. Front. Physiol. 2019, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E. Is vitellogenin an ancestor of apolipoprotein B-100 of human low-density lipoprotein and human lipoprotein lipase? Biochem. J. 1988, 255, 1057–1060. [Google Scholar] [CrossRef] [Green Version]
- Kimble, J.; Sharrock, W.J. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev. Biol. 1983, 96, 189–196. [Google Scholar] [CrossRef]
- Spieth, J.; MacMorris, M.; Broverman, S.; Greenspoon, S.; Blumenthal, T. Regulated expression of a vitellogenin fusion gene in transgenic nematodes. Dev. Biol. 1988, 130, 285–293. [Google Scholar] [CrossRef]
- Barbieri, M.; Bonafè, M.; Franceschi, C.; Paolisso, G. Insulin/IGF-I-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1064–E1071. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2010, 1204, 156–162. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.A.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcidueñas-Fimbres, T.E.; Paz-Graniel, I.; Nishi, S.K.; Salas-Salvadó, J.; Babio, N. Eating speed, eating frequency, and their relationships with diet quality, adiposity, and metabolic syndrome, or its components. Nutrients 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular health benefits of specific vegetable types: A narrative review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Karwowski, B.T. Nutrition can help DNA repair in the case of aging. Nutrients 2020, 12, 3364. [Google Scholar] [CrossRef] [PubMed]
- Wood-Bradley, R.J.; Barrand, S.; Giot, A.; Armitage, J.A. Understanding the role of maternal diet on kidney development; an opportunity to improve cardiovascular and renal health for future generations. Nutrients 2015, 7, 1881–1905. [Google Scholar] [CrossRef] [Green Version]
- Min, H.; Kim, J.S.; Ahn, J.; Shim, Y.H. Gliadin intake causes disruption of the intestinal barrier and an increase in germ cell apoptosis in a Caenorhabditis elegans model. Nutrients 2019, 11, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Egge, N.; Arneaud, S.L.B.; Wales, P.; Mihelakis, M.; McClendon, J.; Fonseca, R.S.; Savelle, C.; Gonzalez, I.; Ghorashi, A.; Yadavalli, S.; et al. Age-onset phosphorylation of a minor actin variant promotes intestinal barrier dysfunction. Dev. Cell. 2019, 51, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, N.J.; Meléndez, A. Detection of autophagy in Caenorhabditis elegans using GFP::LGG-1 as an autophagy marker. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Regmi, S.G.; Rolland, S.G.; Conradt, B. Age-dependent changes in mitochondrial morphology and volume are not predictors of lifespan. Aging 2014, 2, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Gatsi, R.; Schulze, B.; Rodríguez-Palero, M.J.; Hernando-Rodríguez, B.; Baumeister, R.; Artal-Sanz, M. Prohibitin-mediated lifespan and mitochondrial stress implicate SGK-1, insulin/IGF and mTORC2 in C. elegans. PLoS ONE 2014, 9, e107671. [Google Scholar] [CrossRef] [PubMed]
- Tullet., J.M.A.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity promoting factor SKN-1 by Insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palominos, M.F.; Calixto, A. Quantification of bacteria residing in Caenorhabditis elegans intestine. Bio Protoc. 2020, 10, e3605. [Google Scholar] [CrossRef]
- Min, H.; Lee, M.; Cho, K.S.; Lim, H.J.; Shim, Y.H. Nicotinamide supplementation improves oocyte quality and offspring development by modulating mitochondrial function in an aged Caenorhabditis elegans model. Antioxidants 2021, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.D.; Min, H.; Youn, E.; Kawasaki, I.; Shim, Y.H. Gliadin intake induces oxidative-stress responses in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2018, 503, 2139–2145. [Google Scholar] [CrossRef]
- Lockwood, C.A.; Lynch, A.M.; Hardin, J. Dynamic analysis identifies novel roles for DLG-1 subdomains in AJM-1 recruitment and LET-413-dependent apical focusing. J. Cell Sci. 2008, 121, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Dowen, R.H.; Breen, P.C.; Tullius, T.; Conery, A.L.; Ruvkun, G. A microRNA program in the C. elegans hypodermis couples to intestinal mTORC2/PQM-1 signaling to modulate fat transport. Genes. Dev. 2016, 30, 1515–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowen, R.H. CEH-60/PBX and UNC-62/MEIS coordinate a metabolic switch that supports reproduction in C. elegans. Dev. Cell 2019, 49, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Chávez, V.; Mohri-Shiomi, A.; Maadani, A.; Vega, L.A.; Garsin, D.A. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 2007, 176, 1567–1577. [Google Scholar] [CrossRef] [Green Version]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or causal relationship? Antioxid. Redox Signal 2010, 13, 1911–1953. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, C.J.; Pollard, A.; Barratt, T.F.; Constantin-Teodosiu, D.; Greenhaff, P.L.; Szewczyk, N.J. Greater loss of mitochondrial function with ageing is associated with earlier onset of sarcopenia in C. elegans. Aging 2018, 10, 3382–3396. [Google Scholar] [CrossRef]
- Hsu, A.L.; Feng, Z.; Hsieh, M.Y.; Xu, X.Z.S. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol. Aging. 2009, 30, 1498–1503. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Hisamoto, N.; An, J.H.; Riva, P. Oliveira, R.P.; Nishida, E.; Blackwell, T.K.; Matsumoto, K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005, 19, 2278–2283. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.P.; Abate, J.P.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Robida-Stubbs, S.; Tullet, J.M.A.; Rual, J.F.; Vidal, M.; Blackwell, T.K. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 2010, 6, e1001048. [Google Scholar] [CrossRef]
- Shore, D.E.; Ruvkun, G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013, 23, 409–420. [Google Scholar] [CrossRef] [Green Version]
- De Pooter-Stijnman, L.M.M.; Vrijkotte, S.; Smalbrugge, M. Effect of caffeine on sleep and behaviour in nursing home residents with dementia. Eur. Geriatr. Med. 2018, 9, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Vercambre, M.N.; Berr, C.; Ritchie, K.; Kang, J.H. Caffeine and cognitive decline in elderly women at high vascular risk. J. Alzheimer’s Dis. 2013, 35, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Gunter, M.J.; Murphy, N.; Cross, A.J.; Dossus, L.; Dartois, L.; Fagherazzi, G.; Kaaks, R.; Kühn, T.; Boeing, H.; Aleksandrova, K.; et al. Coffee drinking and mortality in 10 european countries: A multinational cohort study. Ann. Intern. Med. 2017, 167, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Van Nostrand, E.L.; Sánchez-Blanco, A.; Wu, B.; Nguyen, A.; Kim, S.K. Roles of the developmental regulator unc-62/Homothorax in limiting longevity in Caenorhabditis elegans. PLoS Genet. 2013, 9, e1003325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyash, V.; Geier, C.; Henske, A.; Mukherjee, S.; Hirsh, D.; Thiele, C.; Grant, B.; Maxfield, F.R.; Kurzchalia, T.V. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol. Biol. Cell. 2001, 12, 1725–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosada, A.; Kassner, U.; Weidemann, F.; König, M.; Buchmann, N.; Steinhagen-Thiessen, E.; Spira, D. Hyperlipidemias in elderly patients: Results from the Berlin Aging Study II (BASEII), a cross-sectional study. Lipids Health Dis. 2020, 19, 92. [Google Scholar] [CrossRef]
- Greaves, L.C.; Barron, M.J.; Plusa, S.; Kirkwood, T.B.; Mathers, J.C.; Taylor, R.W.; Turnbull, D.M. Defects in multiple complexes of the respiratory chain are present in ageing human colonic crypts. Exp. Gerontol. 2010, 45, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly, -Y.M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Vermulst, M.; Wanagat, J.; Kujoth, G.C.; Bielas, J.H.; Rabinovitch, P.S.; Prolla, T.A.; Loeb, L.A. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat. Genet. 2008, 40, 392–394. [Google Scholar] [CrossRef]
- Yen, T.C.; Chen, Y.S.; King, K.L.; Yeh, S.H.; Wei, Y.H. Liver mitochondrial respiratory functions decline with age. Biochem. Biophys. Res. Commun. 1989, 165, 944–1003. [Google Scholar] [CrossRef]
- Urbauer, E.; Rath, E.; Haller, D. Mitochondrial metabolism in the intestinal stem cell niche-sensing and signaling in health and disease. Front. Cell Dev. Biol. 2021, 8, 602814. [Google Scholar] [CrossRef]
- Schneider, A.M.; Özsoy, M.; Zimmermann, F.A.; Feichtinger, R.G.; Mayr, J.A.; Kofler, B.; Sperl, W.; Weghuber, D.; Mörwald, K. Age-related deterioration of mitochondrial function in the intestine. Oxidative Med. Cell Longev. 2020, 2020, 4898217. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poupet, C.; Saraoui, T.; Veisseire, P.; Bonnet, M.; Dausset, C.; Gachinat, M.; Camarès, O.; Chassard, C.; Nivoliez, A.; Bornes, S. Lactobacillus rhamnosus Lcr35 as an effective treatment for preventing Candida albicans infection in the invertebrate model Caenorhabditis elegans: First mechanistic insights. PLoS ONE 2019, 14, e0216184. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, H.; Youn, E.; Shim, Y.-H. Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model. Nutrients 2021, 13, 2517. https://doi.org/10.3390/nu13082517
Min H, Youn E, Shim Y-H. Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model. Nutrients. 2021; 13(8):2517. https://doi.org/10.3390/nu13082517
Chicago/Turabian StyleMin, Hyemin, Esther Youn, and Yhong-Hee Shim. 2021. "Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model" Nutrients 13, no. 8: 2517. https://doi.org/10.3390/nu13082517
APA StyleMin, H., Youn, E., & Shim, Y.-H. (2021). Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model. Nutrients, 13(8), 2517. https://doi.org/10.3390/nu13082517





