Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Monocyte Isolation and Macrophage Differentiation
2.3. Cytokine Measurements, Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. RNA Preparation and RT-PCR
2.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
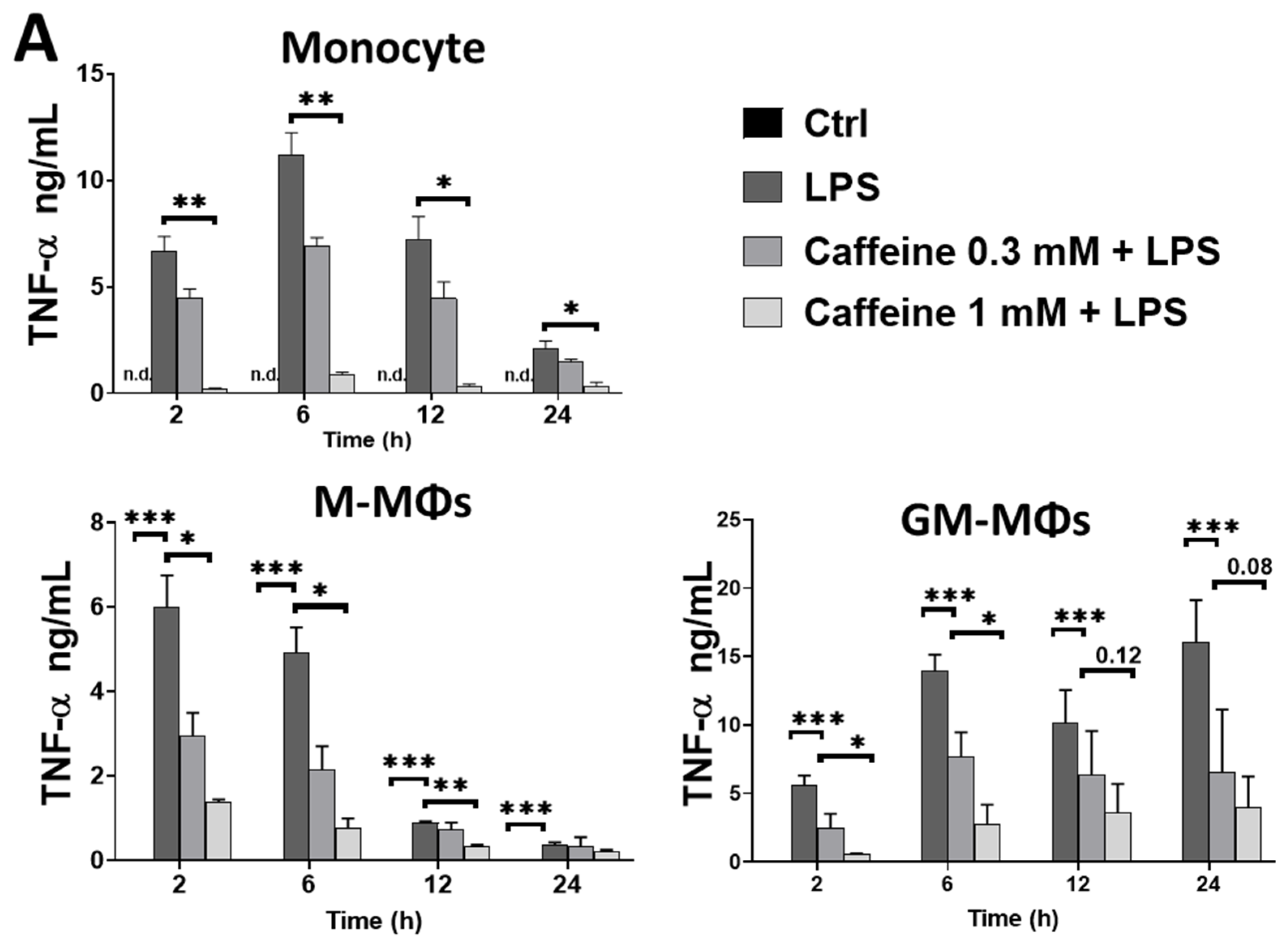
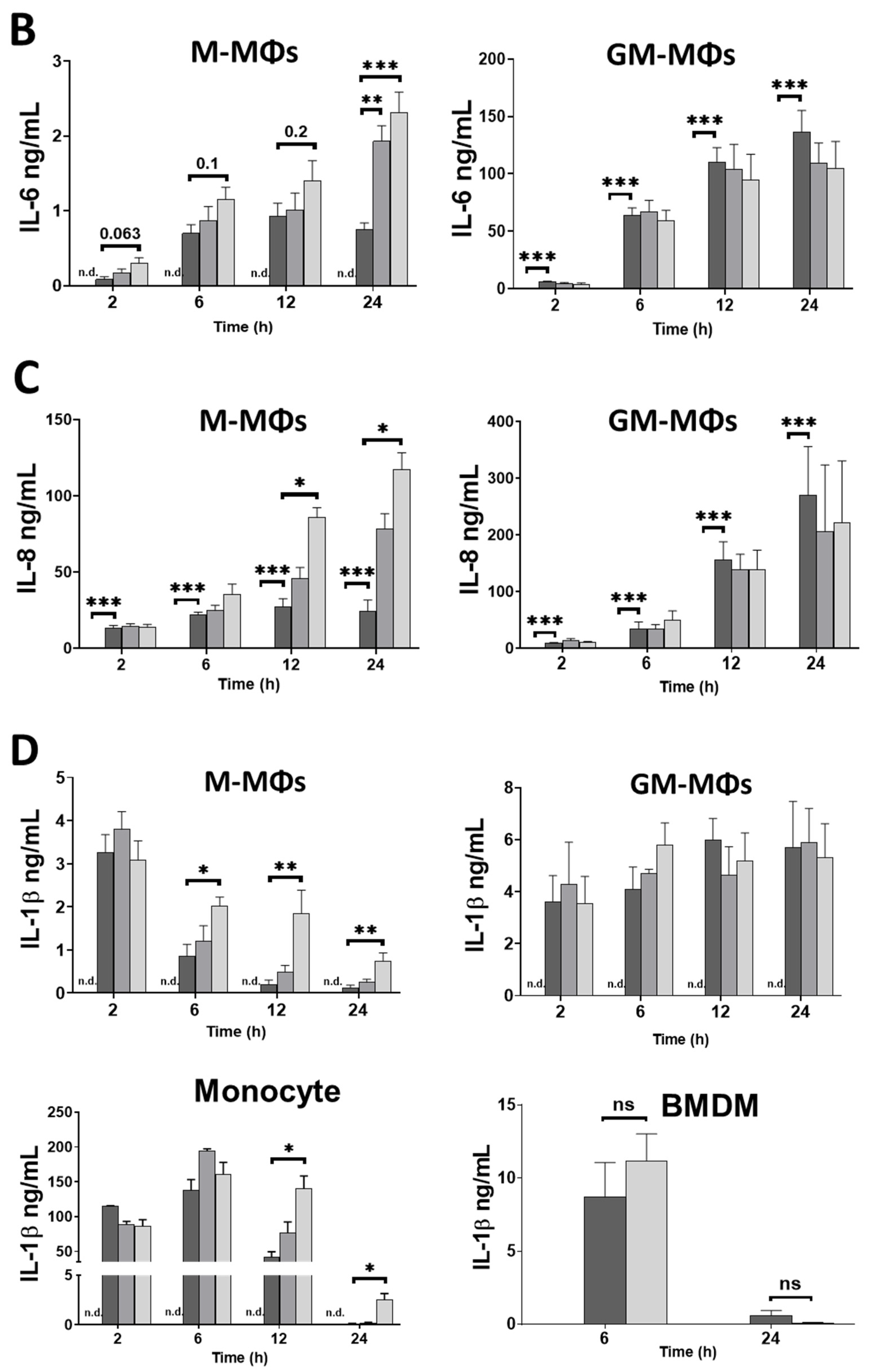
3.1. Caffeine Differently Modulates Cytokine Secretion by LPS-Activated Human Myeloid Cells
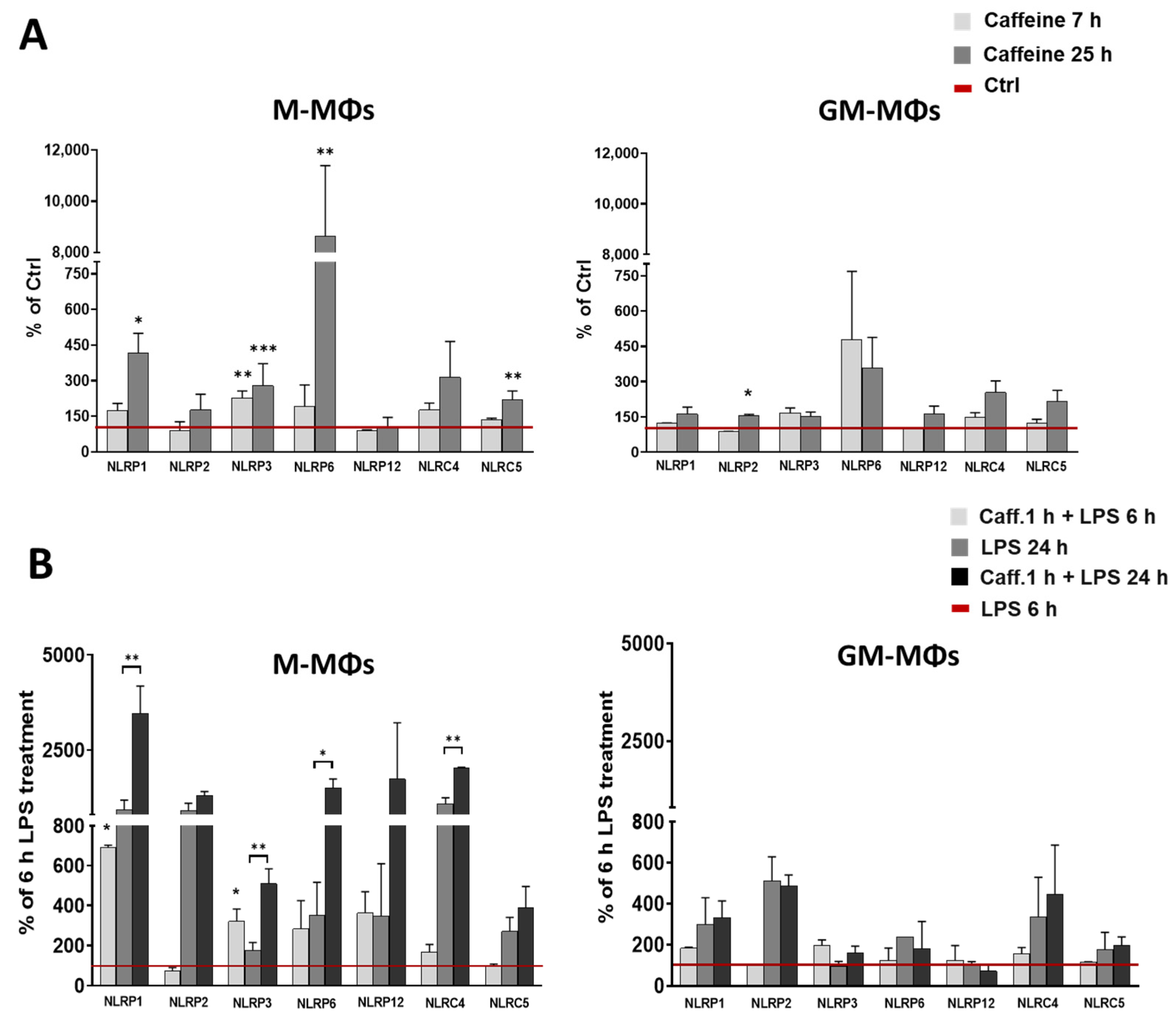
3.2. Caffeine Enhances the Expression of Many Intracellular Pattern-Recognition Receptors in M-MΦs but Not in GM-MΦs
3.3. Caffeine Pre-Treatment Enhances the Expression and Activation of NLRP3 Inflammasome in M-MΦs
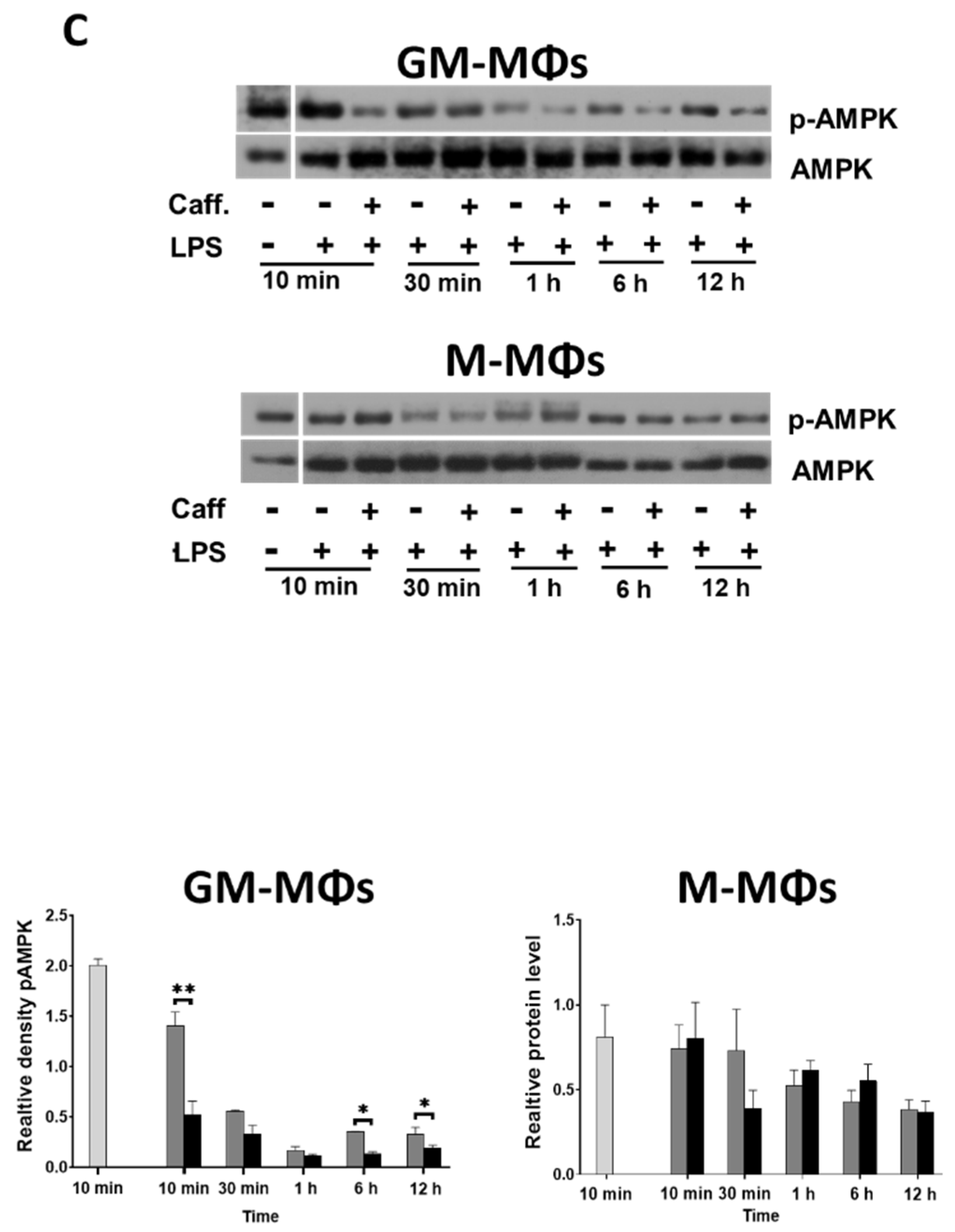
3.4. Caffeine Downregulates Akt Signaling, and Differently Regulates mTOR and AMPK Signaling Pathways in the M-MΦs and GM-MΦs
3.5. Caffeine Modifies Adenosine Receptor Expression
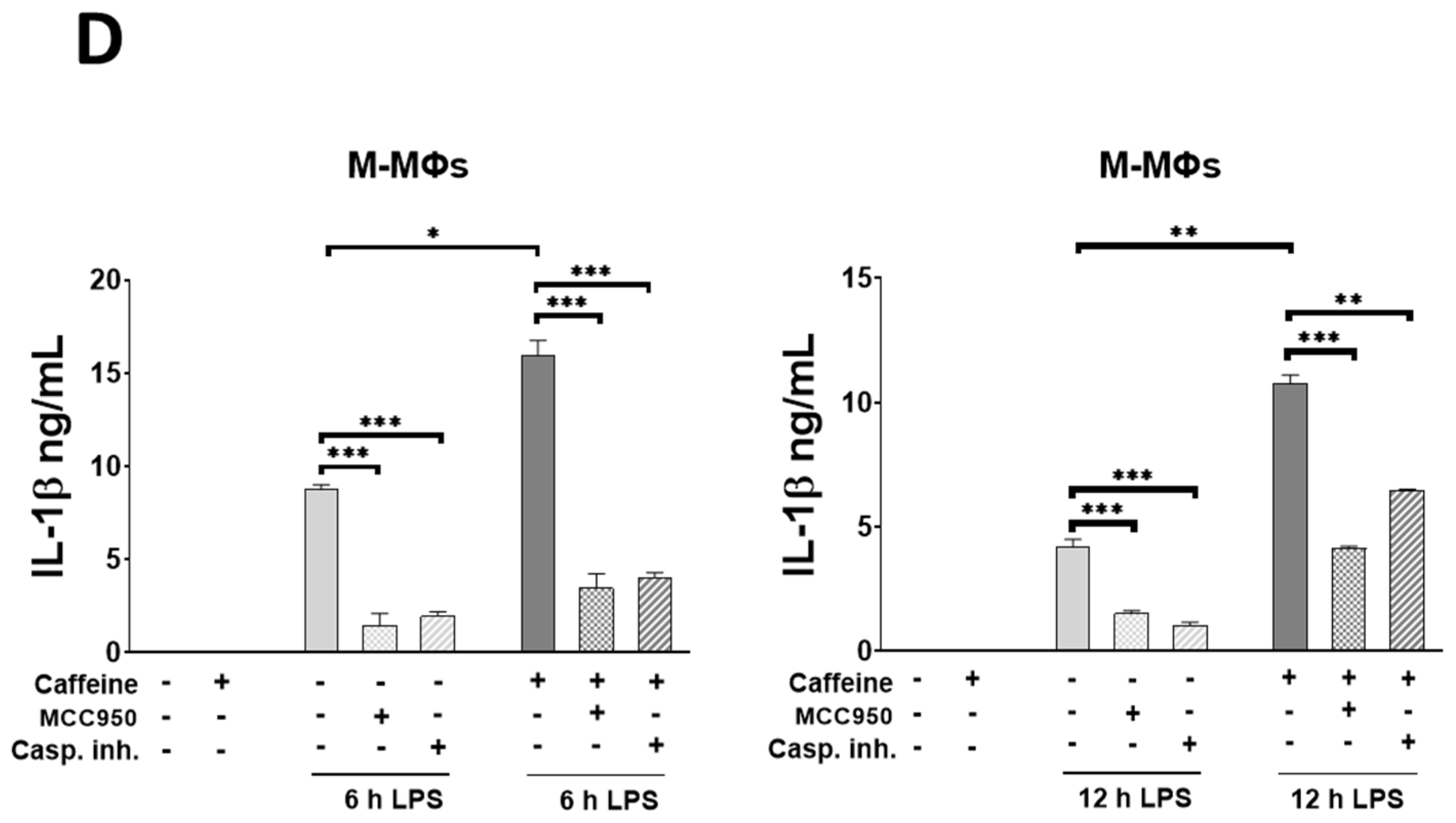
3.6. Caffeine Potentiates NLRP3 Inflammasome-Mediated IL-1β Secretion by Downregulating STAT1/IL-10 Axis in M-MΦs
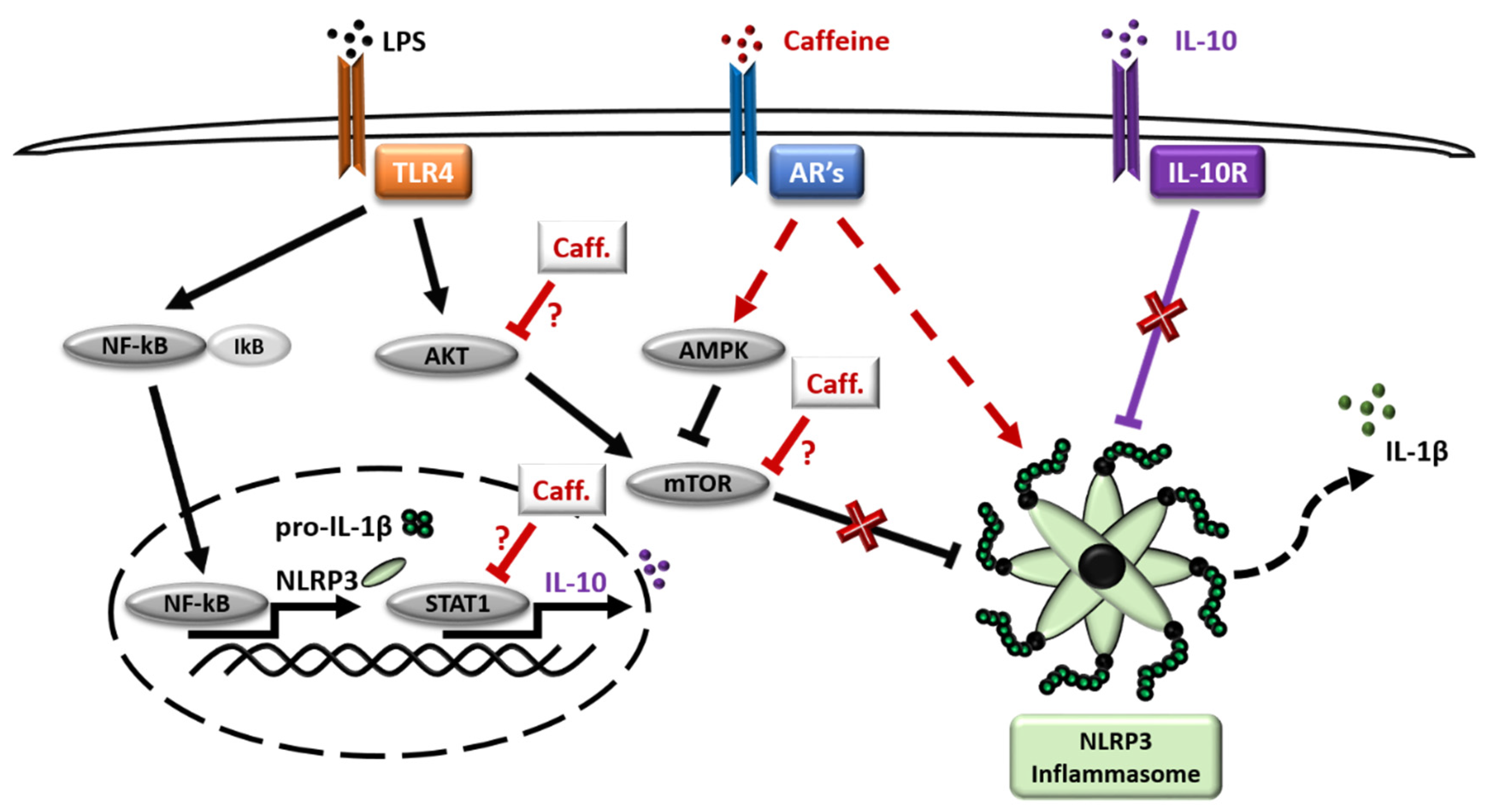
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Paula, J.; Farah, A. Caffeine consumption through coffee: Content in the beverage, metabolism, health benefits and risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-bioactivity relationships of methylxanthines: Trying to make sense of all the promises and the drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Model List of Essential Medicines: 18th List, April 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/93142/?sequence=1 (accessed on 14 July 2021).
- Moschino, L.; Zivanovic, S.; Hartley, C.; Trevisanuto, D.; Baraldi, E.; Roehr, C.C. Caffeine in preterm infants: Where are we in 2020? ERJ Open Res. 2020, 6, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichelt, U.; Cay, R.; Schmitz, T.; Strauss, E.; Sifringer, M.; Bührer, C.; Endesfelder, S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 2013, 41, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Caffeine suppresses TNF-α production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharmacol. 2004, 4, 1409–1417. [Google Scholar] [CrossRef]
- Ritter, M.; Hohenberger, K.; Alter, P.; Herzum, M.; Tebbe, J.; Maisch, M. Caffeine inhibits cytokine expression in lymphocytes. Cytokine 2005, 30, 177–181. [Google Scholar] [CrossRef]
- Al Reef, T.; Ghanem, E. Caffeine: Well-known as psychotropic substance, but little as immunomodulator. Immunobiology 2018, 223, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Crișan, T.O.; Netea, M.G.; Joosten, L.A.B. Innate immune memory: Implications for host responses to damage-associated molecular patterns. Eur. J. Immunol. 2016, 46, 817–828. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Motta, V.; Soares, F.; Sun, T.; Philpott, D.J. NOD-like receptors: Versatile cytosolic sentinels. Physiol. Rev. 2015, 95, 149–178. [Google Scholar] [CrossRef] [Green Version]
- Alatshan, A.; Benkő, S. Nuclear Receptors as Multiple Regulators of NLRP3 Inflammasome Function. Front. Immunol. 2021, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Szamosi, S.; Kovács, G.E.; Kocsis, E.; Benkő, S. The NLRP3 inflammasome-interleukin 1 pathway as a therapeutic target in gout. Arch. Biochem. Biophys. 2019, 670, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Wani, K.; AlHarthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public Health 2021, 18, 511. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Alatshan, A.; Kovács, G.E.; Aladdin, A.; Czimmerer, Z.; Tar, K.; Benkő, S. All-Trans Retinoic Acid Enhances both the Signaling for Priming and the Glycolysis for Activation of NLRP3 Inflammasome in Human Macrophage. Cells 2020, 9, 1591. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Silva, I.G.; Prokhorov, A.; Abooali, M.; Yasinska, I.M.; Casely-Hayford, M.A.; Berger, S.M.; Fasler-Kan, E.; Sumbayev, V.V. Caffeine affects the biological responses of human hematopoietic cells of myeloid lineage via downregulation of the mTOR pathway and xanthine oxidase activity. Oncotarget 2015, 6, 2474–2487. [Google Scholar] [CrossRef] [Green Version]
- Säemann, M.D.; Haidinger, M.; Hecking, M.; Hörl, W.H.; Weichhart, T. The multifunctional role of mTOR in innate immunity: Implications for transplant immunity. Am. J. Transplant. 2009, 9, 2655–2661. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and on exercise performance. Nutr. Res. 2020, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Li, B.; Malireddi, R.K.S.; Lamkanfi, M.; Geiger, T.L.; Kanneganti, T.-D. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, A.; Curti, V.; C Tenore, G.; M Nabavi, S.; Daglia, M. Effects of tea and coffee consumption on cardiovascular diseases and relative risk factors: An update. Curr. Pharm. Des. 2017, 23, 2474–2487. [Google Scholar] [CrossRef]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Immunomodulatory effects of caffeine: Friend or foe? Pharmacol. Ther. 2006, 111, 877–892. [Google Scholar] [CrossRef]
- Kantamala, D.; Vongsakul, M.; Satayavlvad, J. The in vivo and in vitro effects of caffeine on rat immune cells activities: B, T and NK cells. Asian Pac. J. Allergy Immunol. 1990, 8, 77–82. [Google Scholar]
- Rosenthal, L.A.; Taub, D.D.; Moors, M.A.; Blank, K.J. Methylxanthine-induced inhibition of the antigen-and superantigen-specific activation of T and B lymphocytes. Immunopharmacology 1992, 24, 203–217. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Lee, C.-S.; Chae, H.-J.; Kim, H.-R.; Baek, S.-H.; An, N.-H.; Kim, H.-M. Inhibitory effect of anaphylactic shock by caffeine in rats. Int. J. Immunopharmacol. 2000, 22, 411–418. [Google Scholar] [CrossRef]
- Van Furth, A.M.; Seijmonsbergen, E.M.; Langermans, J.A.; Van Der Meide, P.H.; Van Furth, R. Effect of xanthine derivates and dexamethasone on Streptococcus pneumoniae-stimulated production of tumor necrosis factor alpha, interleukin-1 beta (IL-1 beta), and IL-10 by human leukocytes. Clin. Diagn. Lab. Immunol. 1995, 2, 689–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiners, I.; Hauschildt, S.; Nieber, K.; Münch, G. Pentoxyphylline and propentophylline are inhibitors of TNF-α release in monocytes activated by advanced glycation endproducts. J. Neural Transm. 2004, 111, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. The perspective of caffeine and caffeine derived compounds in therapy. Bratisl. Lek. Listy 2015, 116, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Paiva, C.; Beserra, B.T.S.; Reis, C.E.G.; Dorea, J.G.; Da Costa, T.H.M.; Amato, A.A. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular antioxidant and anti-inflammatory effects of coffee extracts with different roasting levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Valdez, R.; Ahlawat, R.; Wills-Karp, M.; Gauda, E.B. Mechanisms of modulation of cytokine release by human cord blood monocytes exposed to high concentrations of caffeine. Pediatric Res. 2016, 80, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Chen, Z.; Li, J.; Zhang, L.; Liu, H.; Huang, C.; Zhu, P. Caffeine protects against alcoholic liver injury by attenuating inflammatory response and oxidative stress. Inflamm. Res. 2010, 59, 635–645. [Google Scholar] [CrossRef]
- Boia, R.; Elvas, F.; Madeira, M.H.; Aires, I.D.; Rodrigues-Neves, A.C.; Tralhão, P.; Szabó, E.C.; Baqi, Y.; Müller, C.E.; Tomé, Â.R. Treatment with A 2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis. 2017, 8, 3065. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-H.; Kim, K.-J.; Ryu, S.-J.; Lee, B.-Y. Caffeine prevents LPS-induced inflammatory responses in RAW264. 7 cells and zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Iris, M.; Tsou, P.-S.; Sawalha, A.H. Caffeine inhibits STAT1 signaling and downregulates inflammatory pathways involved in autoimmunity. Clin. Immunol. 2018, 192, 68–77. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Tunc, T.; Aydemir, G.; Karaoglu, A.; Cekmez, F.; Kul, M.; Aydinoz, S.; Babacan, O.; Yaman, H.; Sarici, S.U. Toll-like receptor levels and caffeine responsiveness in rat pups during perinatal period. Regul. Pept. 2013, 182, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Yeretssian, G. NOD-like receptors: Master regulators of inflammation and cancer. Front. Immunol. 2014, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Benko, S.; Magalhaes, J.G.; Philpott, D.J.; Girardin, S.E. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 2010, 185, 1681–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkő, S.; Kovács, E.G.; Hezel, F.; Kufer, T.A. NLRC5 functions beyond MHC I regulation—what do we know so far? Front. Immunol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Rosenthal, L.A.; Blank, K.J. Pentoxifylline-and caffeine-induced modulation of major histocompatibility complex class I expression on murine tumor cell lines. Immunopharmacology 1993, 25, 145–161. [Google Scholar] [CrossRef]
- Budai, M.M.; Tőzsér, J.; Benkő, S. Different dynamics of NLRP3 inflammasome-mediated IL-1β production in GM-CSF–and M-CSF–differentiated human macrophages. J. Leukoc. Biol. 2017, 101, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. Int. J. Biol. Sci. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Cunha, R.A.; Svenningsson, P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr. Top. Med. Chem. 2003, 3, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [Green Version]
- Ongini, E.; Fredholm, B.B. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996, 17, 364–372. [Google Scholar] [CrossRef]
- Francikowski, J.; Baran, B.; Płachetka-Bożek, A.; Krzyżowski, M.; Augustyniak, M. Caffeine effects on AdoR mRNA expression in Drosophila melanogaster. Open Life Sci. 2016, 11, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.K.; Gubitz, A.K.; Sirinathsinghji, D.J.S.; Richardson, P.J.; Freeman, T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996, 118, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.U.O.; Gao, L.; Cui, Y.; Zhang, Y.I.; Zhou, X.I.N. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery. Mol. Med. Rep. 2016, 13, 3715–3723. [Google Scholar] [CrossRef] [Green Version]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [Green Version]
- Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine monophosphate (AMP)-activated protein kinase: A new target for nutraceutical compounds. Int. J. Mol. Sci. 2017, 18, 288. [Google Scholar] [CrossRef] [Green Version]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.-S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and adenosine. J. Alzheimer’s Dis. 2010, 20, S3–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Yilmaz, Ö. Unfolding role of a danger molecule adenosine signaling in modulation of microbial infection and host cell response. Int. J. Mol. Sci. 2018, 19, 199. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, F.; Heit, A.; Dreher, S.; Eisenächer, K.; Mages, J.; Haas, T.; Krug, A.; Janssen, K.; Kirschning, C.J.; Wagner, H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur. J. Immunol. 2008, 38, 2981–2992. [Google Scholar] [CrossRef]
- Saiki, S.; Sasazawa, Y.; Imamichi, Y.; Kawajiri, S.; Fujimaki, T.; Tanida, I.; Kobayashi, H.; Sato, F.; Sato, S.; Ishikawa, K.-I. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 2011, 7, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Miwa, S.; Sugimoto, N.; Yamamoto, N.; Shirai, T.; Nishida, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Igarashi, K.; Yachie, A. Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-κB and MAPK pathways. Anticancer Res. 2012, 32, 3643–3649. [Google Scholar]
- Pattison, M.J.; MacKenzie, K.F.; Arthur, J.S.C. Inhibition of JAKs in macrophages increases lipopolysaccharide-induced cytokine production by blocking IL-10–mediated feedback. J. Immunol. 2012, 189, 2784–2792. [Google Scholar] [CrossRef]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar]
- Conti, P.; Kempuraj, D.; Kandere, K.; Di Gioacchino, M.; Barbacane, R.C.; Castellani, M.L.; Felaco, M.; Boucher, W.; Letourneau, R.; Theoharides, T.C. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 2003, 86, 123–129. [Google Scholar] [CrossRef]
- Yen, J.; Kong, W.; Hooper, K.M.; Emig, F.; Rahbari, K.M.; Kuo, P.; Scofield, B.A.; Ganea, D. Differential effects of IFN-β on IL-12, IL-23, and IL-10 expression in TLR-stimulated dendritic cells. J. Leukoc. Biol. 2015, 98, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudick, R.A.; Ransohoff, R.M.; Peppler, R.; Medendorp, S.V.; Lehmann, P.; Alam, J. Interferon beta induces interleukin-10 expression: Relevance to multiple sclerosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1996, 40, 618–627. [Google Scholar] [CrossRef]
- Baker, A.K.; Wang, R.; Mackman, N.; Luyendyk, J.P. mTOR-dependent IL-10 expression inhibits LPS induction of tissue factor and cytokines in macrophages. FASEB J. 2009, 23, 570–575. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, C.; Zhang, G.; Sun, C. The transcription factor cMaf is targeted by mTOR, and regulates the inflammatory response via the TLR4 signaling pathway. Int. J. Mol. Med. 2018, 41, 2935–2942. [Google Scholar] [CrossRef] [PubMed]





| Gene | Assay ID |
|---|---|
| Ppia (cyclophilin) | IDT (33731568) (33731567) (33731569) |
| IL-1β | Hs00174097_m1 |
| NLRP1 | Hs00248187_m1 |
| NLRP2 | Hs01546932_m1 |
| NLRP3 | Hs00918082_m1 |
| NLRP6 | Hs00373246_m1 |
| NLRP12 | Hs00536435_m1 |
| NLRC4 | Hs00368369_m1 |
| NLRC5 | Hs00260008_m1 |
| AR2a | Hs00169123_m1 |
| AR2b | Hs00386497_m1 |
| AR3 | Hs00181232_m1 |
| Antibody | CAT Number |
|---|---|
| Pro-IL-1β | 12,703, Cell Signaling |
| IL-1β | 83,186, Cell Signaling |
| Pro-caspase-1 | 3866, Cell Signaling |
| Caspase-1 | 4199, Cell Signaling |
| NLRP3 | 15,101, Cell Signaling |
| ASC | sc-30153, Santa Cruz Biotechnology |
| p-Akt/(S473) | 9271, Cell Signaling |
| p-mTOR (Ser2448) | 2971, Cell Signaling |
| p-STAT1 (Tyr701) | 9167, Cell Signaling |
| p-AMPK | 2535, Cell Signaling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, E.G.; Alatshan, A.; Budai, M.M.; Czimmerer, Z.; Bíró, E.; Benkő, S. Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations. Nutrients 2021, 13, 2409. https://doi.org/10.3390/nu13072409
Kovács EG, Alatshan A, Budai MM, Czimmerer Z, Bíró E, Benkő S. Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations. Nutrients. 2021; 13(7):2409. https://doi.org/10.3390/nu13072409
Chicago/Turabian StyleKovács, Elek Gergő, Ahmad Alatshan, Marietta Margit Budai, Zsolt Czimmerer, Eduárd Bíró, and Szilvia Benkő. 2021. "Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations" Nutrients 13, no. 7: 2409. https://doi.org/10.3390/nu13072409
APA StyleKovács, E. G., Alatshan, A., Budai, M. M., Czimmerer, Z., Bíró, E., & Benkő, S. (2021). Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations. Nutrients, 13(7), 2409. https://doi.org/10.3390/nu13072409






