Effect of Polyphenols Intake on Obesity-Induced Maternal Programming
Abstract
1. Introduction
2. Obesity and Maternal Programming
3. Adipose Tissue Programming
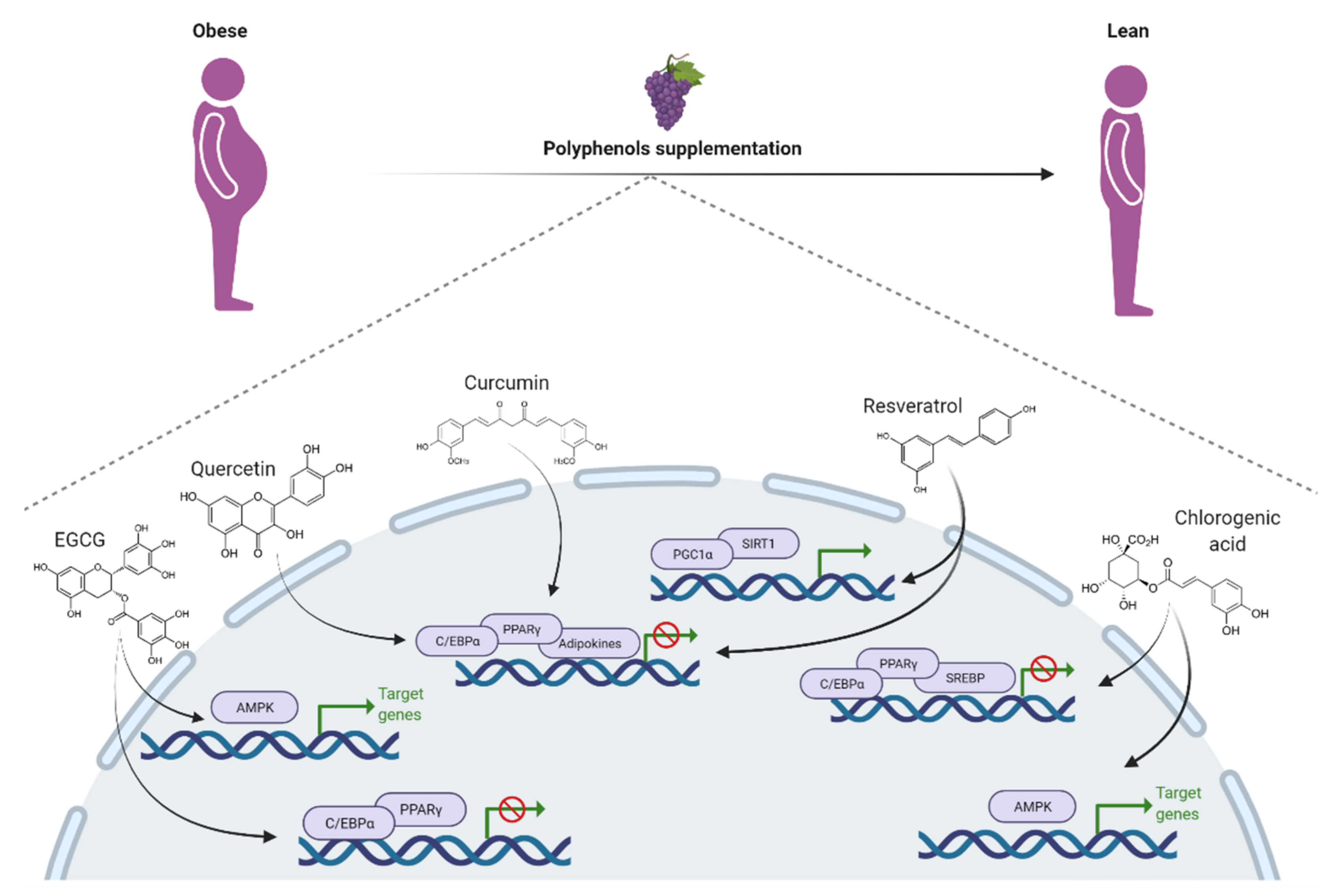
4. Polyphenol Effect on Obesity
5. Beneficial Impact of Polyphenols on Obesity-Induced Maternal Programming
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bentham, J.; Di Cesare, M.; Bilano, V.; Bixby, H.; Zhou, B.; Stevens, G.A.; Riley, L.M.; Taddei, C.; Hajifathalian, K.; Lu, Y.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Finer, N. Medical consequences of obesity. Medicine 2015, 43, 88–93. [Google Scholar] [CrossRef]
- De Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ayers, C.R.; Rohatgi, A.K.; Turer, A.T.; Berry, J.D.; Das, S.R.; Vega, G.L.; Khera, A.; McGuire, D.K.; Grundy, S.M.; et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity 2013, 21, E439–E447. [Google Scholar] [CrossRef]
- Barker, D.J.P. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- De Moura, E.G.; Passos, M.C.F. Neonatal programming of body weight regulation and energetic metabolism. Biosci. Rep. 2005, 25, 251–264. [Google Scholar] [CrossRef]
- Lecoutre, S.; Petrus, P.; Rydén, M.; Breton, C. Transgenerational Epigenetic Mechanisms in Adipose Tissue Development. Trends Endocrinol. Metab. 2018, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Developmental plasticity and human disease: Research directions. J. Intern. Med. 2007, 261, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Hales, C.N.; Fall, C.H.D.; Osmond, C.; Phipps, K.; Clark, P.M.S. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef]
- Da Silva Lima, N.; Gaspar De Moura, E.; Cottini Fonseca Passos, M.; Firmino Nogueira Neto, J.; Reis, A.M.; De Oliveira, E.; Lisboa, P.C. Early weaning causes undernutrition for a short period and programmes some metabolic syndrome components and leptin resistance in adult rat offspring. Br. J. Nutr. 2011, 105, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Desai, N.; Gupta, M.; Xue, X.; Chatterjee, P.K.; Rochelson, B.; Metz, C.N. The effects of prenatal metformin on obesogenic diet-induced alterations in maternal and fetal fatty acid metabolism. Nutr. Metab. 2016, 13, 55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veiga-Lopez, A.; Moeller, J.; Sreedharan, R.; Singer, K.; Lumeng, C.; Ye, W.; Pease, A.; Padmanabhan, V. Developmental programming: Interaction between prenatal bpa exposure and postnatal adiposity on metabolic variables in female sheep. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E238–E247. [Google Scholar] [CrossRef]
- Lecoutre, S.; Pourpe, C.; Butruille, L.; Marousez, L.; Laborie, C.; Guinez, C.; Lesage, J.; Vieau, D.; Eeckhoute, J.; Gabory, A.; et al. Reduced PPARγ2 expression in adipose tissue of male rat offspring from obese dams is associated with epigenetic modifications. FASEB J. 2018, 32, 2768–3778. [Google Scholar] [CrossRef]
- Catalano, P.M. Obesity and pregnancy—The propagation of a viscous cycle? J. Clin. Endocrinol. Metab. 2003, 88, 3505–3506. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-De-La-Vega-Monroy, M.L. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259. [Google Scholar] [CrossRef]
- Tan, H.C.; Roberts, J.; Catov, J.; Krishnamurthy, R.; Shypailo, R.; Bacha, F. Mother’s pre-pregnancy BMI is an important determinant of adverse cardiometabolic risk in childhood. Pediatr. Diabetes 2015, 16, 419–426. [Google Scholar] [CrossRef]
- Ornellas, F.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Programming of obesity and comorbidities in the progeny: Lessons from a model of diet-induced obese parents. PLoS ONE 2015, 10, e0124737. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Fernandes, T.P.; Rocha, C.P.D.; Calviño, C.; Pazos-Moura, C.C.; Lisboa, P.C.; Moura, E.G.; Trevenzoli, I.H. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J. Physiol. 2012, 590, 5503–5518. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237.e1–237.e13. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Berry, R.; Wang, T.; Ceyhan, O.; Tavazoie, S.; Friedman, J.M.; Rodeheffer, M.S. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development 2011, 138, 4709–4719. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Steger, D.J.; Grant, G.R.; Schupp, M.; Tomaru, T.; Lefterova, M.I.; Schug, J.; Manduchi, E.; Stoeckert, C.J.; Lazar, M.A. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010, 24, 1035–1044. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by Zfp. Nature 2010, 7188, 619–623. [Google Scholar] [CrossRef]
- Musri, M.M.; Párrizas, M. Epigenetic regulation of adipogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 342–349. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Holtrup, B.; Church, C.D.; Berry, R.; Colman, L.; Jeffery, E.; Bober, J.; Rodeheffer, M.S. Puberty is an important developmental period for the establishment of adipose tissue mass and metabolic homeostasis. Adipocyte 2017, 6, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jiang, Y.; Graff, J.M. Emerging Roles of Adipose Progenitor Cells in Tissue Development, Homeostasis, Expansion and Thermogenesis. Trends Endocrinol. Metab. 2016, 27, 574–585. [Google Scholar] [CrossRef]
- Radford, E.J.; Ito, M.; Shi, H.; Corish, J.A.; Yamazawa, K.; Isganaitis, E.; Seisenberger, S.; Hore, T.A.; Reik, W.; Erkek, S.; et al. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 2014, 345, 1255903. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, C.R.; Wei, Y.P.; Zhao, Z.A.; Hou, Y.; Schatten, H.; Sun, Q.Y. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kang, S. Functional implications of DNA methylation in adipose biology. Diabetes 2019, 68, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Borengasser, S.J.; Zhong, Y.; Kang, P.; Lindsey, F.; Ronis, M.J.J.; Badger, T.M.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013, 154, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Liang, J.F.; Rogers, C.J.; Zhao, J.X.; Zhu, M.J.; Du, M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 2013, 62, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Wang, B.; Pan, H.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 2016, 594, 4453–4466. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Kano, F.; Shiota, K.; Murata, M. Expression of the peroxisome proliferator activated receptor γ gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol. 2009, 7, 38. [Google Scholar] [CrossRef]
- Zwamborn, R.A.J.; Slieker, R.C.; Mulder, P.C.A.; Zoetemelk, I.; Verschuren, L.; Suchiman, H.E.D.; Toet, K.H.; Droog, S.; Slagboom, P.E.; Kooistra, T.; et al. Prolonged high-fat diet induces gradual and fat depot-specific DNA methylation changes in adult mice. Sci. Rep. 2017, 7, 43261. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Sawston, UK, 2018; ISBN 9780128147757. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanita 2007, 43, 394. [Google Scholar]
- Kalupahana, N.S.; Moustaid-Moussa, N. The adipose tissue renin-angiotensin system and metabolic disorders: A review of molecular mechanisms. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 379–390. [Google Scholar] [CrossRef]
- Dragan, S.; Andrica, F.; Serban, M.-C.; Timar, R. Polyphenols-Rich Natural Products for Treatment of Diabetes. Curr. Med. Chem. 2014, 22, 14–22. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Meydani, M.; Hasan, S.T. Dietary polyphenols and obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Wei, L.; Castro-Muñozledo, F.; Koo, W.L. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011, 89, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef]
- Moon, H.S.; Chung, C.S.; Lee, H.G.; Kim, T.G.; Choi, Y.J.; Cho, C.S. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity 2007, 15, 2571–2582. [Google Scholar] [CrossRef]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Forney, L.A.; Lenard, N.R.; Stewart, L.K.; Henagan, T.M. Dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int. J. Mol. Sci. 2018, 19, 895. [Google Scholar] [CrossRef]
- Nettore, I.C.; Rocca, C.; Mancino, G.; Albano, L.; Amelio, D.; Grande, F.; Puoci, F.; Pasqua, T.; Desiderio, S.; Mazza, R.; et al. Quercetin and its derivative Q2 modulate chromatin dynamics in adipogenesis and Q2 prevents obesity and metabolic disorders in rats. J. Nutr. Biochem. 2019, 69, 151–162. [Google Scholar] [CrossRef]
- Leiherer, A.; Stoemmer, K.; Muendlein, A.; Saely, C.H.; Kinz, E.; Brandtner, E.M.; Fraunberger, P.; Drexel, H. Quercetin impacts expression of metabolism-and obesity-associated genes in SGBS adipocytes. Nutrients 2016, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Tzeng, T.F.; Liou, S.S.; Chang, Y.S.; Liu, I.M. Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPAR levels. Planta Med. 2011, 77, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, Q.; Zhao, T. Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice. Biomed. Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kwon, M.; Choi, J.S.; Jeong, H.O.; Chung, H.Y.; Kim, H.R. Kaempferol Isolated from Nelumbo nucifera Inhibits Lipid Accumulation and Increases Fatty Acid Oxidation Signaling in Adipocytes. J. Med. Food 2015, 18, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Torres-Villarreal, D.; Camacho, A.; Castro, H.; Ortiz-Lopez, R.; de la Garza, A.L. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J. Physiol. Biochem. 2019, 75, 83–88. [Google Scholar] [CrossRef]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar]
- Aguirre, L.; Fernández-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar] [PubMed]
- Chen, S.; Xiao, X.; Feng, X.; Li, W.; Zhou, N.; Zheng, L.; Sun, Y.; Zhang, Z.; Zhu, W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J. Nutr. Biochem. 2012, 23, 1100–1112. [Google Scholar] [CrossRef]
- Lasa, A.; Schweiger, M.; Kotzbeck, P.; Churruca, I.; Simón, E.; Zechner, R.; del Puy Portillo, M. Resveratrol regulates lipolysis via adipose triglyceride lipase. J. Nutr. Biochem. 2012, 23, 379–384. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K., II; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Santos, R.M.M.; Lima, D.R.A. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016, 55, 1345–1358. [Google Scholar] [CrossRef]
- Nordestgaard, A.T.; Thomsen, M.; Nordestgaard, B.G. Coffee intake and risk of obesity, metabolic syndrome and type 2 diabetes: A Mendelian randomization study. Int. J. Epidemiol. 2015, 44, 551–565. [Google Scholar] [CrossRef]
- Koyama, T.; Maekawa, M.; Ozaki, E.; Kuriyama, N.; Uehara, R. Daily consumption of coffee and eating bread at breakfast time is associated with lower visceral adipose tissue and with lower prevalence of both visceral obesity and metabolic syndrome in Japanese populations: A cross-sectional study. Nutrients 2020, 12, 3090. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Samadi, M.; Mohammadshahi, M.; Jalali, M.T.; Engali, K.A. Energy restriction combined with green coffee bean extract affects serum adipocytokines and the body composition in obese women. Asia Pac. J. Clin. Nutr. 2017, 26, 1048–1054. [Google Scholar]
- Zhang, H.F.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Wang, J.H.; Wang, J.H.; Zhang, Z. Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol. Sin. 2018, 39, 1855–1864. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, Y.; Shi, X.; Zhang, N.; Zu, G.; Li, Z.; Zhou, J.; Gao, D.; Lv, L.; Tian, X.; et al. New insights into salvianolic acid A action: Regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 2016, 6, 28743. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Qian, Q.; Ding, Q.; Zhou, L.; Fu, A.; Du, Z.; Wang, C.; Song, Z.; Li, S.; Dou, X. Activation of AMP-Activated Protein Kinase-Sirtuin 1 Pathway Contributes to Salvianolic Acid A-Induced Browning of White Adipose Tissue in High-Fat Diet Fed Male Mice. Front. Pharmacol. 2021, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Yan, Z.; Xu, T.T.; Wu, X.; Pi, A.; Liu, Q.; Chai, H.; Li, S.; Dou, X. Inhibition of TLR4/MAPKs Pathway Contributes to the Protection of Salvianolic Acid A Against Lipotoxicity-Induced Myocardial Damage in Cardiomyocytes and Obese Mice. Front. Pharmacol. 2021, 12, 76. [Google Scholar]
- Luna-Vital, D.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Loarca-Piña, G.; Gonzalez de Mejia, E. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Ilavenil, S.; Hwang, I.H.; Kim, D.; Choi, K.C. Ferulic acid stimulates adipocyte-specific secretory proteins to regulate adipose homeostasis in 3T3-L1 adipocytes. Molecules 2021, 26, 1984. [Google Scholar] [CrossRef] [PubMed]
- Chavanelle, V.; Otero, Y.F.; Le Joubioux, F.; Ripoche, D.; Bargetto, M.; Vluggens, A.; Montaurier, C.; Pickering, G.; Ducheix, G.; Dubray, C.; et al. Effects of Totum-63 on glucose homeostasis and postprandial glycemia: A translational study. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1119–E1137. [Google Scholar] [CrossRef] [PubMed]
- Dupuit, M.; Chavanelle, V.; Chassaing, B.; Perriere, F.; Etienne, M.; Plissonneau, C.; Boscaro, A.; Barnich, N.; Pialoux, V.; Maugard, T.; et al. The totum-63 supplement and high-intensity interval training combination limits weight gain, improves glycemic control, and influences the composition of gut mucosa-associated bacteria in rats on a high fat diet. Nutrients 2021, 13, 1569. [Google Scholar] [CrossRef]
- Van der Zande, H.J.P.; Lambooij, J.M.; Chavanelle, V.; Zawistowska-Deniziak, A.; Otero, Y.; Otto, F.; Lantier, L.; McGuinness, O.P.; Le Joubioux, F.; Giera, M.; et al. Effects of a novel polyphenol-rich plant extract on body composition, inflammation, insulin sensitivity, and glucose homeostasis in obese mice. Int. J. Obes. 2021. [Google Scholar] [CrossRef]
- Muhlhausler, B.; Smith, S.R. Early-life origins of metabolic dysfunction: Role of the adipocyte. Trends Endocrinol. Metab. 2009, 20, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Murabayashi, N.; Sugiyama, T.; Zhang, L.; Kamimoto, Y.; Umekawa, T.; Ma, N.; Sagawa, N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 39–44. [Google Scholar] [CrossRef]
- Lecoutre, S.; Breton, C. The cellularity of offspring’s adipose tissue is programmed by maternal nutritional manipulations. Adipocyte 2014, 3, 256–262. [Google Scholar] [CrossRef][Green Version]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Hauguel-de Mouzon, S. Obesity in Pregnancy Stimulates Macrophage Accumulation and Inflammation in the Placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef]
- Denison, F.C.; Roberts, K.A.; Barr, S.M.; Norman, J.E. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010, 140, 373–385. [Google Scholar] [CrossRef]
- Kataoka, S.; Norikura, T.; Sato, S. Maternal green tea polyphenol intake during lactation attenuates kidney injury in high-fat-diet-fed male offspring programmed by maternal protein restriction in rats. J. Nutr. Biochem. 2018, 56, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. Am. J. Obstet. Gynecol. 2010, 203, 75.e1–75.e6. [Google Scholar] [CrossRef][Green Version]
- Bonacasa, B.; Siow, R.C.M.; Mann, G.E. Impact of Dietary Soy Isoflavones in Pregnancy on Fetal Programming of Endothelial Function in Offspring. Microcirculation 2011, 18, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, S.; Yu, X.; Li, Y. Dietary epigallocatechin 3-gallate supplement improves maternal and neonatal treatment outcome of gestational diabetes mellitus: A double-blind randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 753–758. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Developmental programming of the metabolic syndrome: Can we reprogram with resveratrol? Int. J. Mol. Sci. 2018, 19, 2584. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef]
- De Brito Oliveira, A.L.; Monteiro, V.V.S.; Navegantes-Lima, K.C.; Reis, J.F.; de Souza Gomes, R.; Rodrigues, D.V.S.; de França Gaspar, S.L.; Monteiro, M.C. Resveratrol role in autoimmune disease—A mini-review. Nutrients 2017, 9, 1306. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Kim, O.Y.; Chung, J.Y.; Song, J. Effect of resveratrol on adipokines and myokines involved in fat browning: Perspectives in healthy weight against obesity. Pharmacol. Res. 2019, 148, 104411. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.H.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Tiao, M.M.; Tain, Y.L.; Huang, L.T. Effects of maternal resveratrol on maternal high-fat diet/obesity with or without postnatal high-fat diet. Int. J. Mol. Sci. 2020, 21, 3428. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Yu, H.R.; Tsai, C.C.; Huang, L.T.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lai, Y.J.; et al. Resveratrol intake during pregnancy and lactation re-programs adiposity and ameliorates leptin resistance in male progeny induced by maternal high-fat/high sucrose plus postnatal high-fat/high sucrose diets via fat metabolism regulation. Lipids Health Dis. 2020, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Brawerman, G.M.; Kereliuk, S.M.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal resveratrol administration protects against gestational diabetes-induced glucose intolerance and islet dysfunction in the rat offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Pound, L.D.; Thorn, S.R.; Gillingham, M.B.; Thornburg, K.L.; Friedman, J.E.; Frias, A.E.; Grove, K.L. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014, 28, 2466–2477. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, I.M.; dos Santos, T.W.; Ferraz, L.F.C.; Santos, J.C.; Ribeiro, M.L. Effect of Polyphenols Intake on Obesity-Induced Maternal Programming. Nutrients 2021, 13, 2390. https://doi.org/10.3390/nu13072390
Fortunato IM, dos Santos TW, Ferraz LFC, Santos JC, Ribeiro ML. Effect of Polyphenols Intake on Obesity-Induced Maternal Programming. Nutrients. 2021; 13(7):2390. https://doi.org/10.3390/nu13072390
Chicago/Turabian StyleFortunato, Isabela Monique, Tanila Wood dos Santos, Lucio Fábio Caldas Ferraz, Juliana Carvalho Santos, and Marcelo Lima Ribeiro. 2021. "Effect of Polyphenols Intake on Obesity-Induced Maternal Programming" Nutrients 13, no. 7: 2390. https://doi.org/10.3390/nu13072390
APA StyleFortunato, I. M., dos Santos, T. W., Ferraz, L. F. C., Santos, J. C., & Ribeiro, M. L. (2021). Effect of Polyphenols Intake on Obesity-Induced Maternal Programming. Nutrients, 13(7), 2390. https://doi.org/10.3390/nu13072390





