Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs)
2.2. Stimulation and Treatment of PBMCs
2.3. LEGENDplexTM Multiplex Cytokine Analysis
2.4. RNA Isolation and cDNA Synthesis
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction
2.6. Statistics
3. Results
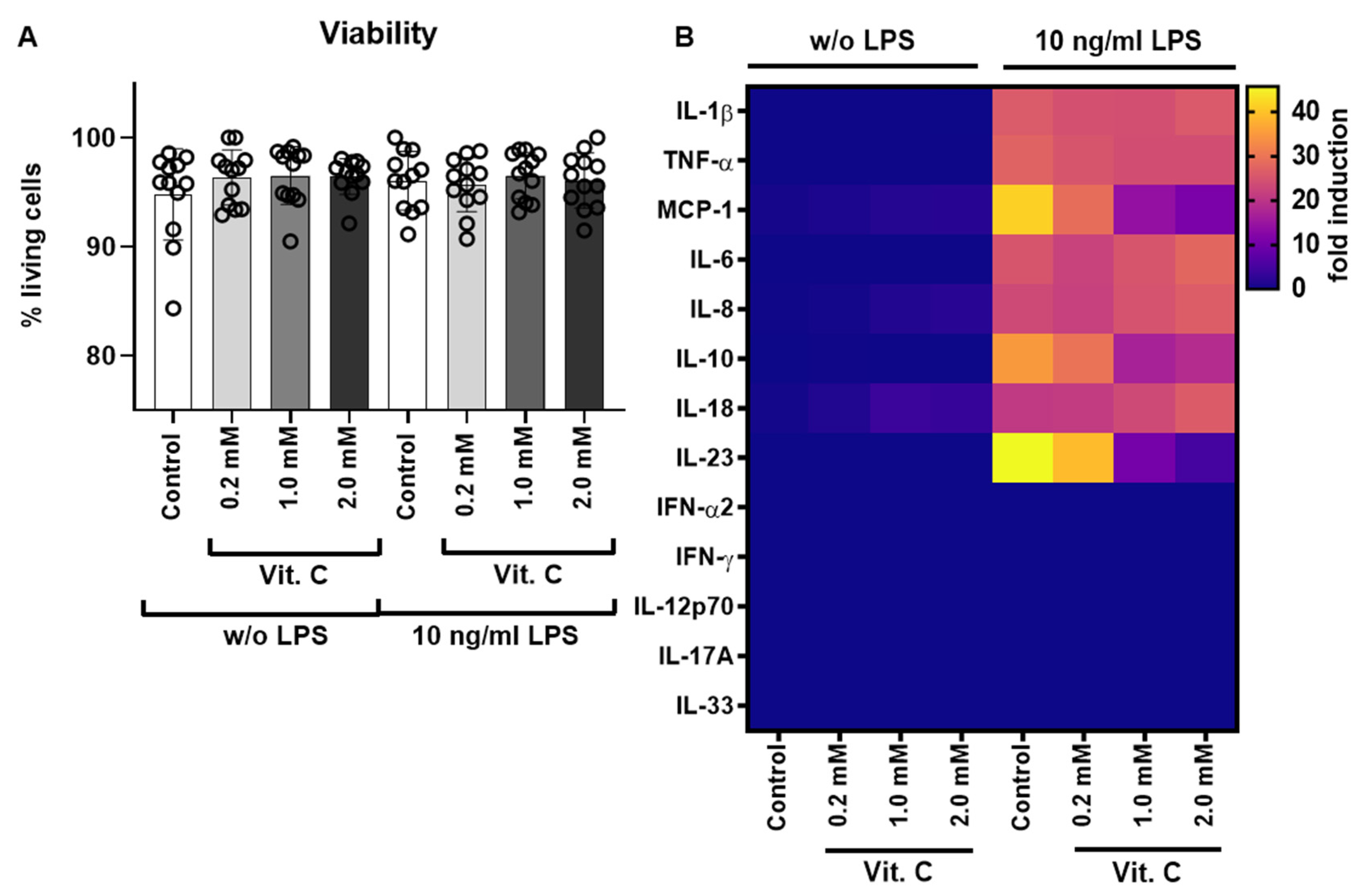
3.1. Testing of Different Vitamin C Concentrations during LPS Stimulation via Cytokine Release
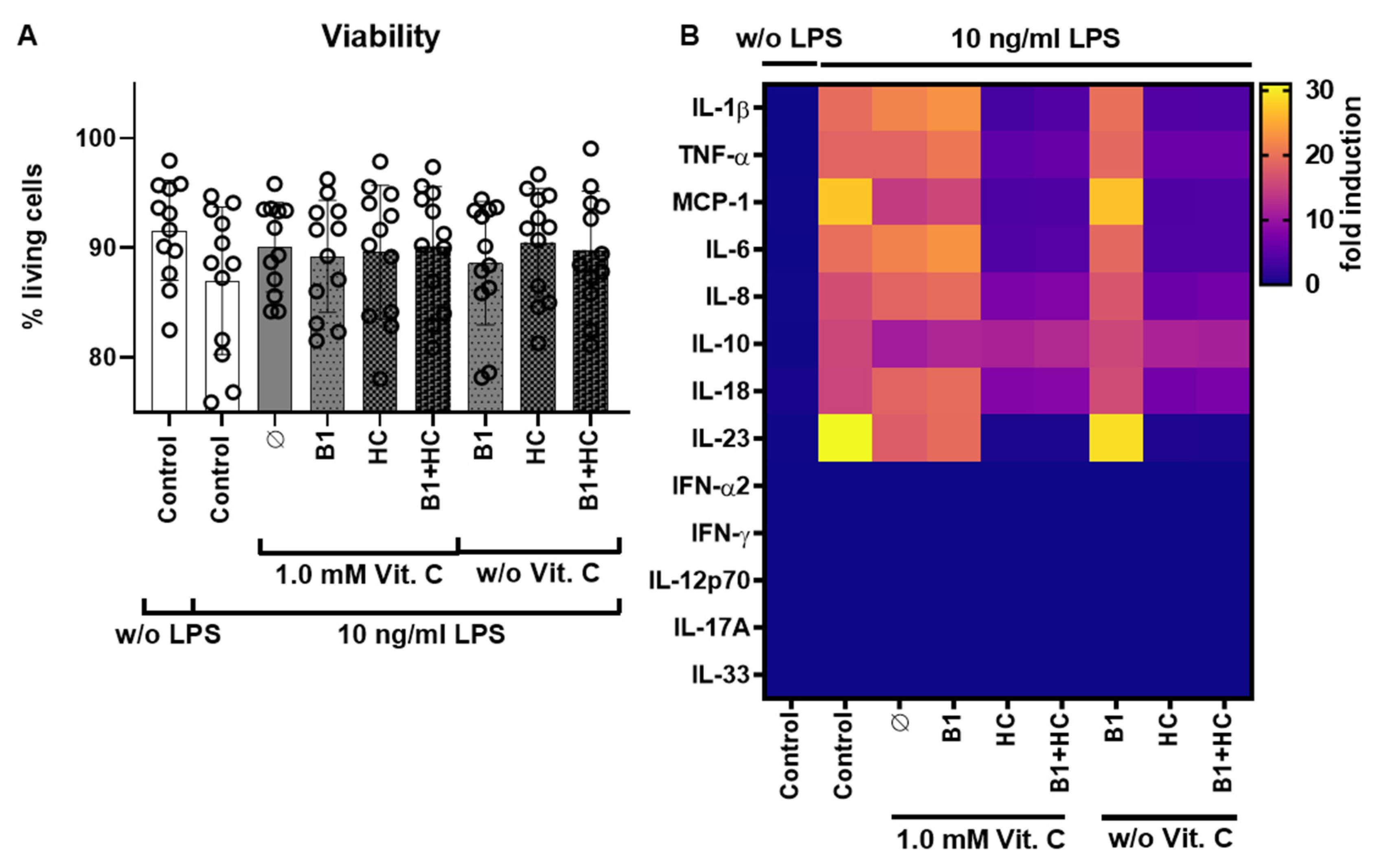
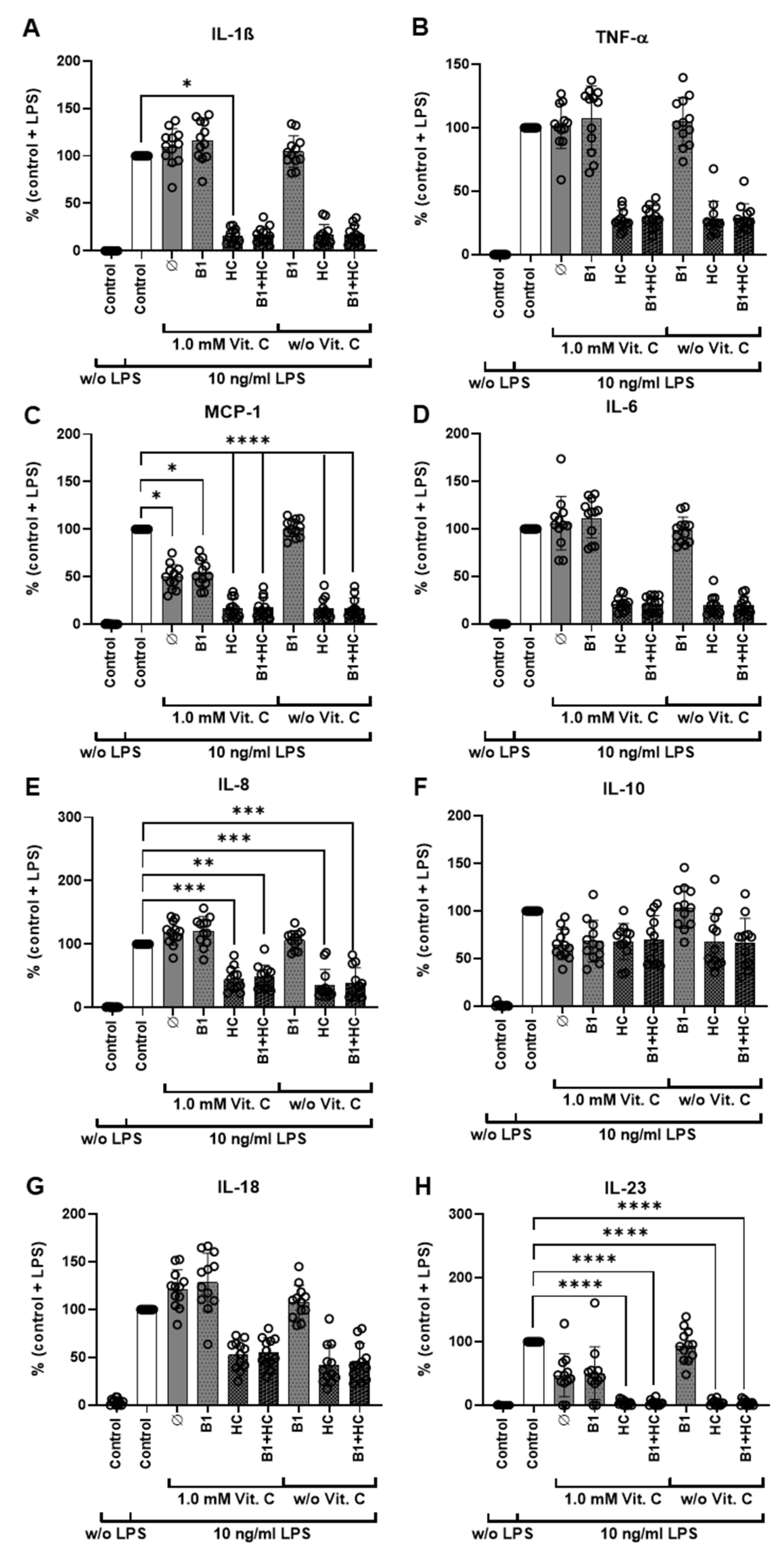
3.2. Combination of Vitamin C with Vitamin B1 and Hydrocortisone during LPS Stimulation via Cytokine Release
3.3. Analysis of Gene Expression of PBMCs after Exposure to Vitamin C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Graetz, T.J.; Hotchkiss, R.S. Sepsis: Preventing organ failure in sepsis—The search continues. Nat. Rev. Nephrol. 2017, 13, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; World Health Organization: Geneva, Switzerland, 2020; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Evans, T. Diagnosis and management of sepsis. Clin. Med. 2018, 18, 146–149. [Google Scholar] [CrossRef]
- Gauer, R.; Forbes, D.; Boyer, N. Sepsis: Diagnosis and Management. Am. Fam. Physician 2020, 101, 409–418. [Google Scholar]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Bollaert, P.-E.; Briegel, J.; Confalonieri, M.; de Gaudio, R.; Keh, D.; Kupfer, Y.; Oppert, M.; Meduri, G.U. Corticosteroids in the treatment of severe sepsis and septic shock in adults: A systematic review. JAMA 2009, 301, 2362–2375. [Google Scholar] [CrossRef] [Green Version]
- Sligl, W.I.; Milner, D.A.; Sundar, S.; Mphatswe, W.; Majumdar, S.R. Safety and efficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin. Infect. Dis. 2009, 49, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Keh, D.; Trips, E.; Marx, G.; Wirtz, S.P.; Abduljawwad, E.; Bercker, S.; Bogatsch, H.; Briegel, J.; Engel, C.; Gerlach, H.; et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA 2016, 316, 1775–1785. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.-F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524. [Google Scholar] [CrossRef]
- Behrens, E.M.; Koretzky, G.A. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017, 69, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef] [Green Version]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczyński, P.; Zhitkovich, A.; Rubiś, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with Community Acquired Pneumonia Exhibit Depleted Vitamin C Status and Elevated Oxidative Stress. Nutrients 2020, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Chiscano-Camón, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Le Goff, C.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum Levels of Vitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study. Med. Drug Discov. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Carr, A.C. Can a simple chemical help to both prevent and treat sepsis. Crit. Care 2018, 22, 247. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, A.; Donnino, M.W. Thiamine (vitamin B1) in septic shock: A targeted therapy. J. Thorac. Dis. 2020, 12, S78–S83. [Google Scholar] [CrossRef]
- Attaluri, P.; Castillo, A.; Edriss, H.; Nugent, K. Thiamine Deficiency: An Important Consideration in Critically Ill Patients. Am. J. Med. Sci. 2018, 356, 382–390. [Google Scholar] [CrossRef]
- Donnino, M.W.; Carney, E.; Cocchi, M.N.; Barbash, I.; Chase, M.; Joyce, N.; Chou, P.P.; Ngo, L. Thiamine deficiency in critically ill patients with sepsis. J. Crit. Care 2010, 25, 576–581. [Google Scholar] [CrossRef]
- Moskowitz, A.; Andersen, L.W.; Huang, D.T.; Berg, K.M.; Grossestreuer, A.V.; Marik, P.E.; Sherwin, R.L.; Hou, P.C.; Becker, L.B.; Cocchi, M.N.; et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: A review of the biologic rationale and the present state of clinical evaluation. Crit. Care 2018, 22, 283. [Google Scholar] [CrossRef] [Green Version]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Puri, P.; Massey, H.D.; Idowu, M.O.; Brophy, D.F.; Voelkel, N.F.; Fowler, A.A.; et al. Attenuation of sepsis-induced organ injury in mice by vitamin C. JPEN J. Parenter. Enter. Nutr. 2014, 38, 825–839. [Google Scholar] [CrossRef]
- Fisher, B.J.; Seropian, I.M.; Kraskauskas, D.; Thakkar, J.N.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit. Care Med. 2011, 39, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Dickson, K.; Lehmann, C. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int. J. Mol. Sci. 2019, 20, 4341. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Greco, S.; Nguyen, H.H.T.; Ho, J.T.; Lewis, J.G.; Torpy, D.J.; Inder, W.J. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr. Disord. 2014, 14, 91. [Google Scholar] [CrossRef] [Green Version]
- Zempleni, J.; Hagen, M.; Hadem, U.; Vogel, S.; Kübler, W. Utilization of intravenously infused thiamin hydrochloride in healthy adult males. Nutr. Res. 1996, 16, 1479–1485. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Latifi, S.Q.; O’Riordan, M.A.; Levine, A.D. Interleukin-10 controls the onset of irreversible septic shock. Infect. Immun. 2002, 70, 4441–4446. [Google Scholar] [CrossRef] [Green Version]
- Kawai, S.; Sakayori, S.; Watanabe, H.; Nakagawa, T.; Inoue, G.; Kobayashi, H. The Role of Interleukin-10 in Systemic Inflammatory Response Syndrome with Sepsis. J. Infect. Chemother. 1998, 4, 121–127. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Pang, X.; Huang, Y.; Yang, B.; Yang, Y.; Chen, K.; Liu, X.; Mao, P.; Li, Y. Interleukin-10/lymphocyte ratio predicts mortality in severe septic patients. PLoS ONE 2017, 12, e0179050. [Google Scholar] [CrossRef] [Green Version]
- Cauvi, D.M.; Williams, M.R.; Bermudez, J.A.; Armijo, G.; de Maio, A. Elevated expression of IL-23/IL-17 pathway-related mediators correlates with exacerbation of pulmonary inflammation during polymicrobial sepsis. Shock 2014, 42, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosmann, M.; Ward, P.A. Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clin. Transl. Med. 2012, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Chen, Y.; Lin, Y.; Zhang, W.; Cai, Y.; Chen, F.; Liao, Q.; Yin, Z.; Wang, Y.; Tao, S.; et al. Association study of MCP-1 promoter polymorphisms with the susceptibility and progression of sepsis. PLoS ONE 2017, 12, e0176781. [Google Scholar] [CrossRef]
- Bossink, A.W.; Paemen, L.; Jansen, P.M.; Hack, C.E.; Thijs, L.G.; van Damme, J. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood 1995, 86, 3841–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, A.; Hogaboam, C.M.; Lukacs, N.W.; Lincoln, P.M.; Strieter, R.M.; Kunkel, S.L. Endogenous MCP-1 influences systemic cytokine balance in a murine model of acute septic peritonitis. Exp. Mol. Pathol. 2000, 68, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. CCL2, a product of mice early after systemic inflammatory response syndrome (SIRS), induces alternatively activated macrophages capable of impairing antibacterial resistance of SIRS mice. J. Leukoc. Biol. 2004, 76, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-H.; Chang, C.-H.; Ko, W.-J.; Lin, C.-F.; Liu, H.-H.; Chow, L.-P.; Huang, C.-T.; Yu, S.-L.; Chen, Y.-S. Biomarkers of early sepsis may be correlated with outcome. J. Transl. Med. 2014, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Ramnath, R.D.; Ng, S.W.; Guglielmotti, A.; Bhatia, M. Role of MCP-1 in endotoxemia and sepsis. Int. Immunopharmacol. 2008, 8, 810–818. [Google Scholar] [CrossRef]
- Donnino, M.W.; Andersen, L.W.; Chase, M.; Berg, K.M.; Tidswell, M.; Giberson, T.; Wolfe, R.; Moskowitz, A.; Smithline, H.; Ngo, L.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit. Care Med. 2016, 44, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.; Andersen, L.W.; Cocchi, M.N.; Karlsson, M.; Patel, P.V.; Donnino, M.W. Thiamine as a Renal Protective Agent in Septic Shock. A Secondary Analysis of a Randomized, Double-Blind, Placebo-controlled Trial. Ann. Am. Thorac. Soc. 2017, 14, 737–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, Y.; Aso, S.; Iwagami, M.; Yasunaga, H.; Matsui, H.; Fushimi, K.; Hamasaki, Y.; Nangaku, M.; Doi, K. Association Between IV Thiamine and Mortality in Patients With Septic Shock: A Nationwide Observational Study. Crit. Care Med. 2020, 48, 1135–1139. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Reanalysis of the Effect of Vitamin C on Mortality in the CITRIS-ALI Trial: Important Findings Dismissed in the Trial Report. Front. Med. 2020, 7, 590853. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C. Is the VITAMINS RCT indicating potential redundancy between corticosteroids and vitamin C? Crit. Care 2020, 24, 129. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Ryoo, S.M.; Park, J.E.; Jo, Y.H.; Jang, D.-H.; Suh, G.J.; Kim, T.; Kim, Y.-J.; Kim, S.; Cho, H.; et al. Combination therapy of vitamin C and thiamine for septic shock: A multi-centre, double-blinded randomized, controlled study. Intensive Care Med. 2020, 46, 2015–2025. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Oudemans-van Straaten, H.M.; Berger, M.M. Adjuvant vitamin C for sepsis: Mono or triple? Crit. Care 2019, 23, 425. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, H.; Lin, B.-W.; Lin, J.-D. Effects of different ascorbic acid doses on the mortality of critically ill patients: A meta-analysis. Ann. Intensive Care 2019, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, A.; Huang, D.T.; Hou, P.C.; Gong, J.; Doshi, P.B.; Grossestreuer, A.V.; Andersen, L.W.; Ngo, L.; Sherwin, R.L.; Berg, K.M.; et al. Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial. JAMA 2020, 324, 642–650. [Google Scholar] [CrossRef]
- Ge, Z.; Huang, J.; Liu, Y.; Xiang, J.; Gao, Y.; Walline, J.H.; Lu, X.; Yu, S.; Zhao, L.; Li, Y. Thiamine combined with vitamin C in sepsis or septic shock: A systematic review and meta-analysis. Eur. J. Emerg. Med. 2021, 28, 189–195. [Google Scholar] [CrossRef]
- Scholz, S.S.; Borgstedt, R.; Ebeling, N.; Menzel, L.C.; Jansen, G.; Rehberg, S. Mortality in septic patients treated with vitamin C: A systematic meta-analysis. Crit. Care 2021, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, S. Serum 25-Hydroxyvitamin D and the risk of mortality in adult patients with Sepsis: A meta-analysis. BMC Infect. Dis. 2020, 20, 189. [Google Scholar] [CrossRef] [Green Version]
- Tosoni, A.; Cossari, A.; Paratore, M.; Impagnatiello, M.; Passaro, G.; Vallone, C.V.; Zaccone, V.; Gasbarrini, A.; Addolorato, G.; de Cosmo, S.; et al. Delta-Procalcitonin and Vitamin D Can Predict Mortality of Internal Medicine Patients with Microbiological Identified Sepsis. Medicina 2021, 57, 331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Gao, L.; Cao, Z.; Wang, Q. Effects of a single dose of vitamin D in septic children: A randomized, double-blinded, controlled trial. J. Int. Med. Res. 2020, 48, 300060520926890. [Google Scholar] [CrossRef]
- Hagag, A.A.; El Frargy, M.S.; Houdeeb, H.A. Therapeutic Value of Vitamin D as an Adjuvant Therapy in Neonates with Sepsis. Infect. Disord. Drug Targets 2020, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose Ascorbic acid on vasopressor’s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Yang, H.; Yang, W.; Li, M.; Chang, X.; Chen, Y. Effect of vitamin C in critically ill patients with sepsis and septic shock: A meta-analysis. Sci. Prog. 2021, 104, 36850421998175. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence |
|---|---|
| IL6_fo | 5′-cacagacagccactcacctc |
| IL6_rev | 5′-ttttctgccagtgcctcttt |
| TNFa_fo | 5′-ctcttctgcctgctgcactttg |
| TNFa_rev | 5′-atgggctacaggcttgtcactc |
| MCP1_fo | 5′-agaatcaccagcagcaagtgtcc |
| MCP1_rev | 5′-tcctgaacccacttctgcttgg |
| IL23A_fo | 5′-gagccttctctgctccctgata |
| IL23A_rev | 5′-gactgaggcttggaatctgctg |
| TBP_fo | 5′-tgcacaggagccaagagtgaa |
| TBP_rev | 5′-cacatcacagctccccacca |
| ActinB_fo | 5′-ttgttacaggaagtcccttgcc |
| ActinB_rev | 5′-atgctatcacctcccctgtgtg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, A.; Burkard, M.; Niessner, H.; Leischner, C.; Renner, O.; Vollbracht, C.; Michels, H.; Busch, C.; Sinnberg, T.; Venturelli, S. Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model. Nutrients 2021, 13, 2366. https://doi.org/10.3390/nu13072366
Lauer A, Burkard M, Niessner H, Leischner C, Renner O, Vollbracht C, Michels H, Busch C, Sinnberg T, Venturelli S. Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model. Nutrients. 2021; 13(7):2366. https://doi.org/10.3390/nu13072366
Chicago/Turabian StyleLauer, Annie, Markus Burkard, Heike Niessner, Christian Leischner, Olga Renner, Claudia Vollbracht, Holger Michels, Christian Busch, Tobias Sinnberg, and Sascha Venturelli. 2021. "Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model" Nutrients 13, no. 7: 2366. https://doi.org/10.3390/nu13072366
APA StyleLauer, A., Burkard, M., Niessner, H., Leischner, C., Renner, O., Vollbracht, C., Michels, H., Busch, C., Sinnberg, T., & Venturelli, S. (2021). Ex Vivo Evaluation of the Sepsis Triple Therapy High-Dose Vitamin C in Combination with Vitamin B1 and Hydrocortisone in a Human Peripheral Blood Mononuclear Cells (PBMCs) Model. Nutrients, 13(7), 2366. https://doi.org/10.3390/nu13072366









