Dietary Spray-Dried Porcine Plasma Reduces Neuropathological Alzheimer’s Disease Hallmarks in SAMP8 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design and Diets
2.2. Sample Collection
2.3. Sample Homogenization
2.4. Western Blot
2.5. ELISA Assay
2.6. Real-Time PCR
2.7. Statistical Analysis
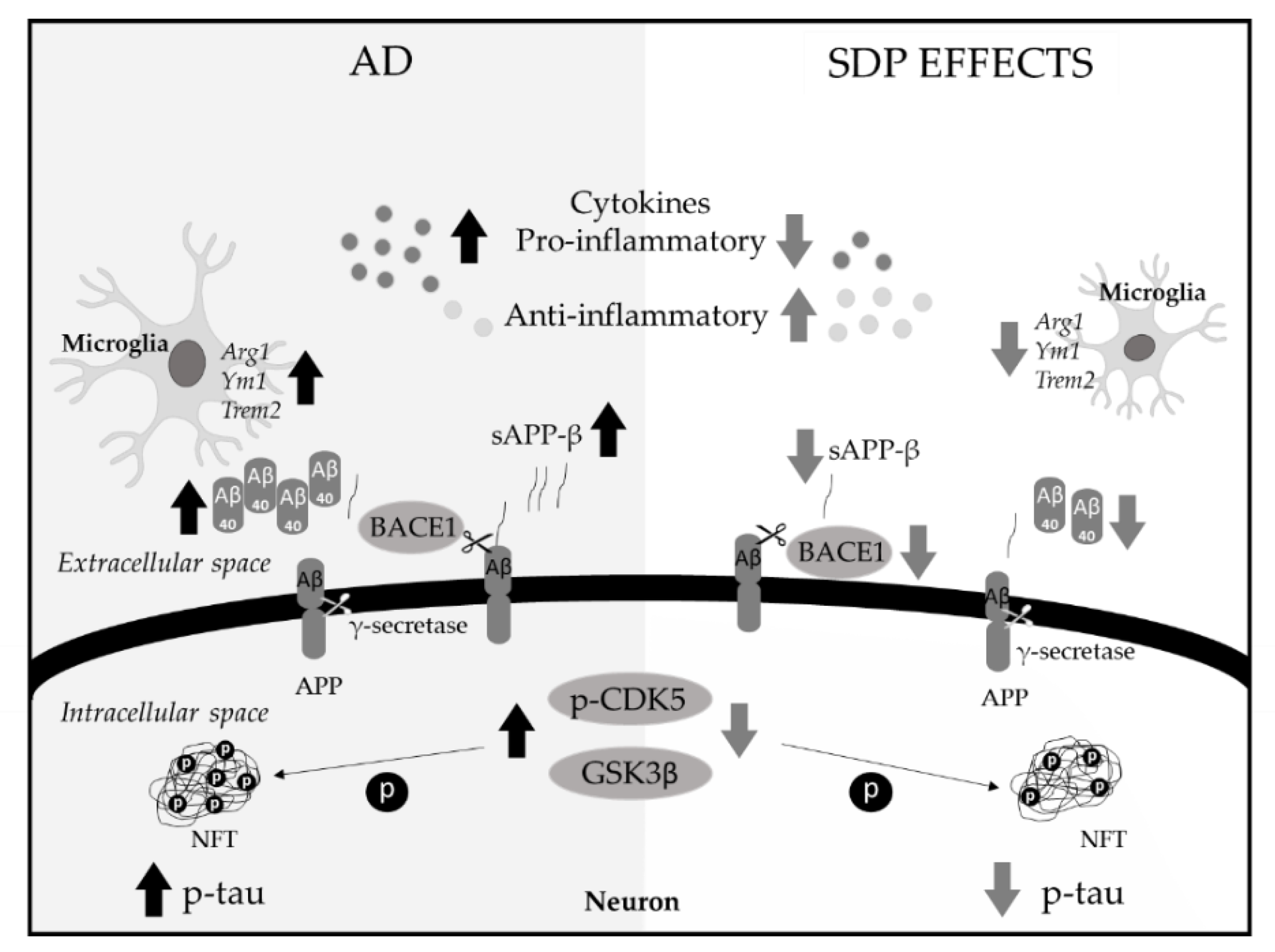
3. Results
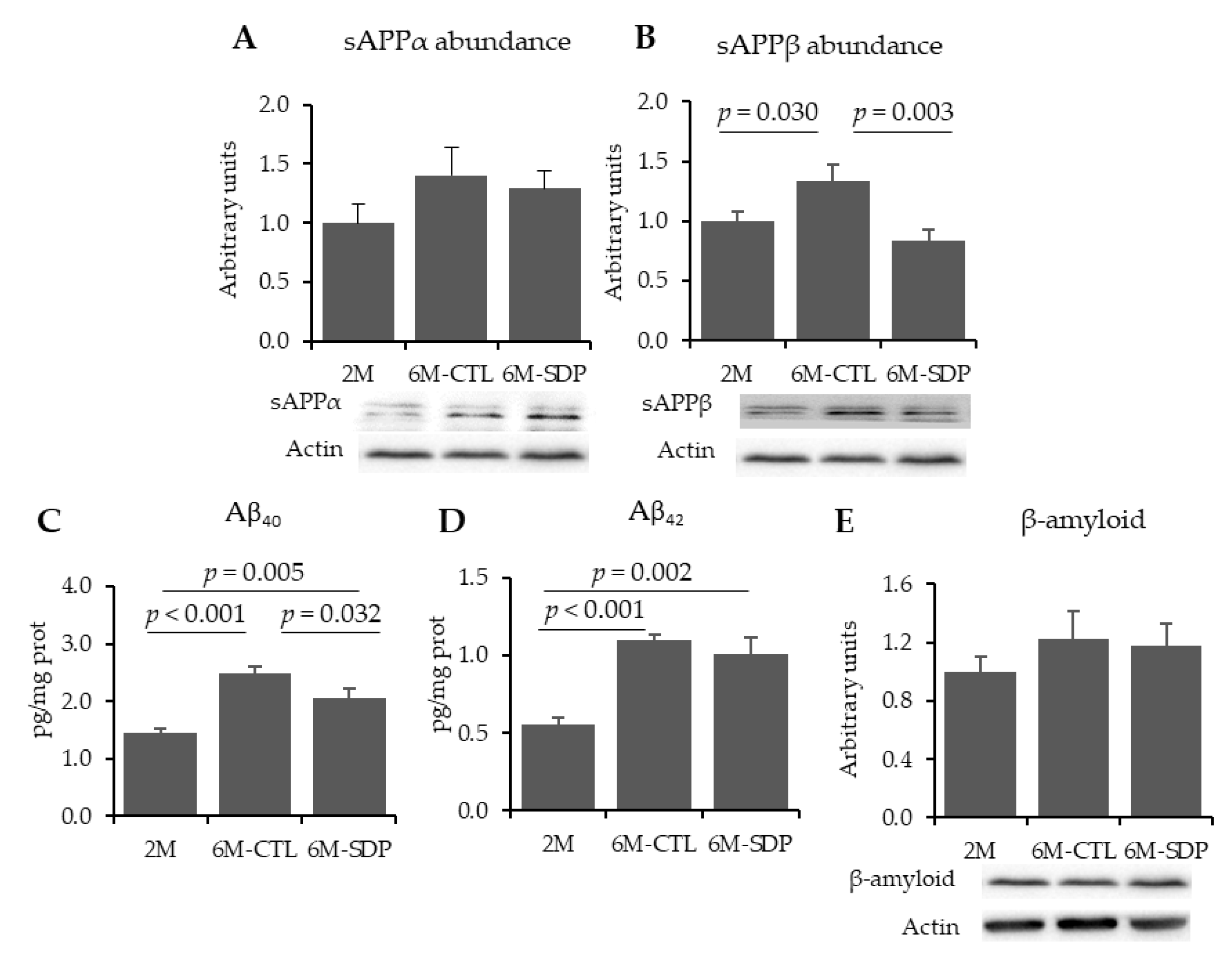
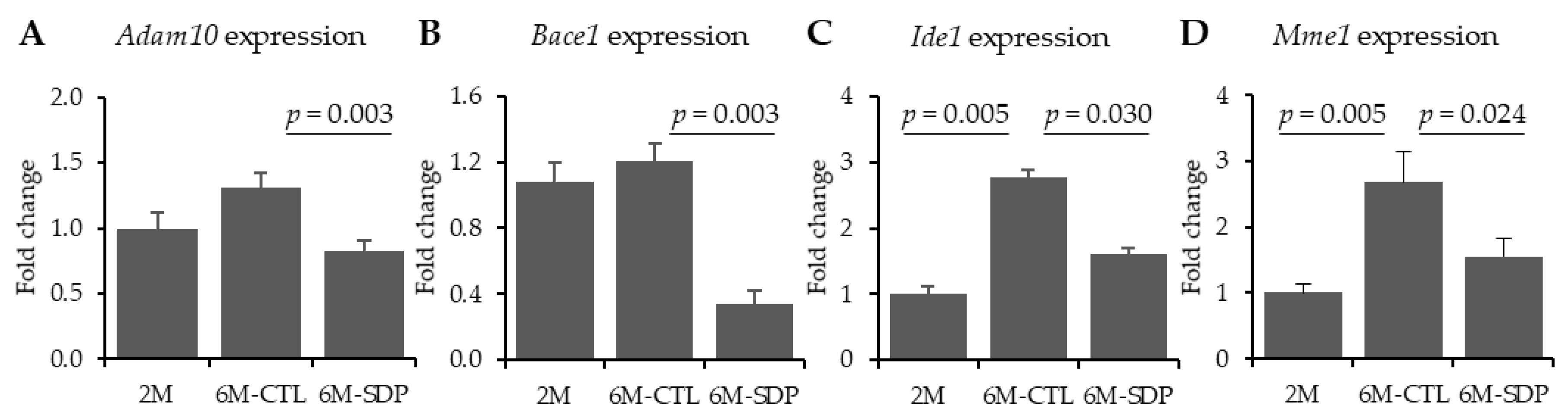
3.1. Amyloid Pathology
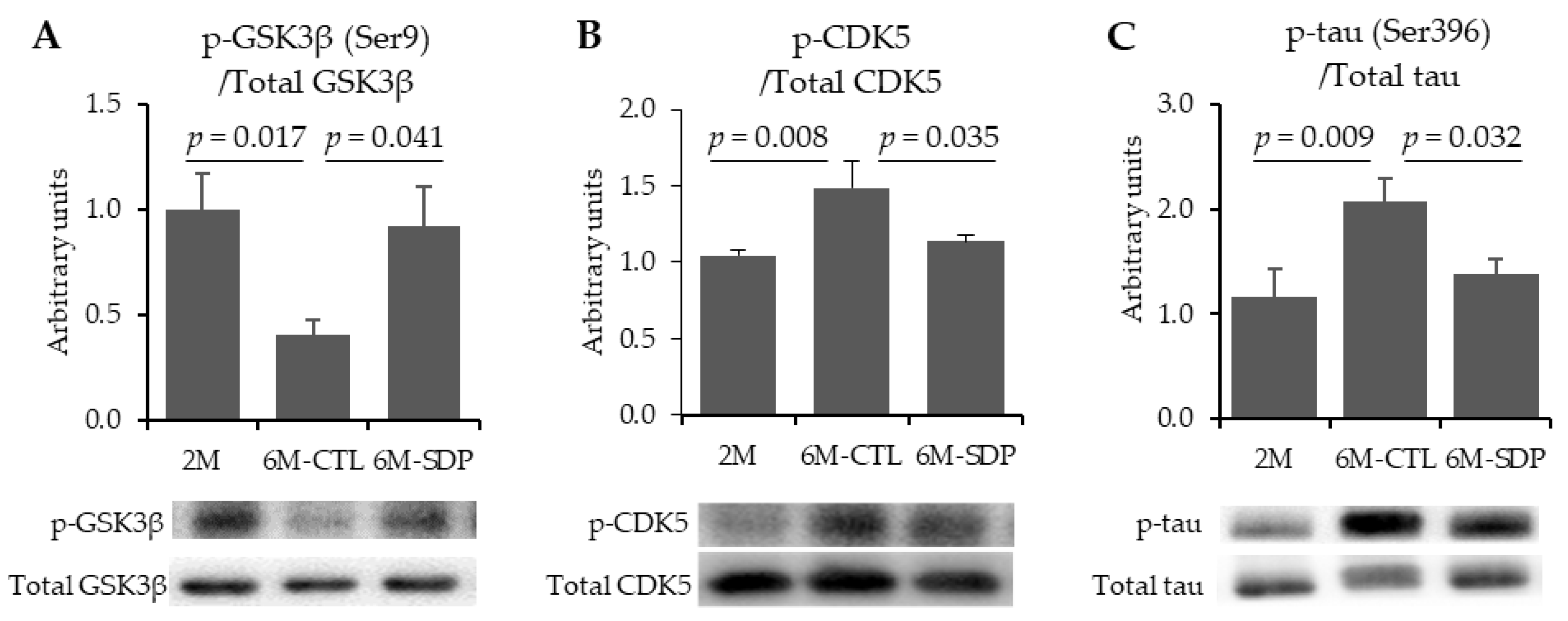
3.2. Neurofibrillary Tangles
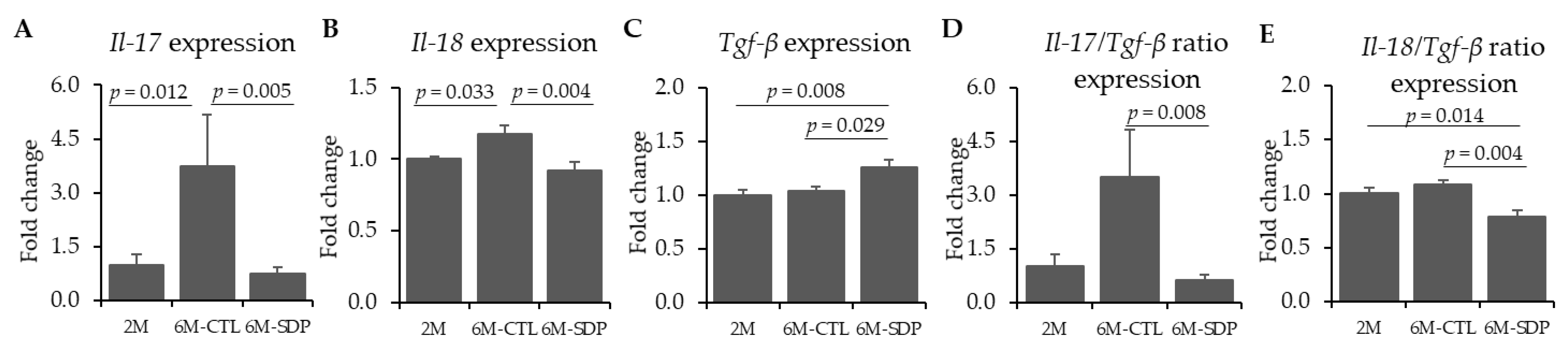
3.3. Neuroinflammatory Markers
3.4. Microglia Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soria-Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology, 3rd ed.; DeKosky, S.T., Asthana, S., Eds.; Elsevier: Boston, MA, USA, 2019; Volume 167, pp. 231–255. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Vassar, R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 89. [Google Scholar] [CrossRef]
- Chong, F.P.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Tau proteins and tauopathies in Alzheimer’s disease. Cell. Mol. Neurobiol. 2018, 38, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Souder, D.C.; Anderson, R.M. An expanding GSK3 network: Implications for aging research. Geroscience 2019, 41, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The role of Cdk5 in Alzheimer’s disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.T.; Wan, C.; Yang, W.; Cai, Z. Role of Cdk5 in amyloid-beta pathology of Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Ader, R.; Godbout, J.P.; Johnson, R.W. Aging, neuroinflammation and behavior. In Psychoneuroimmunology, 4th ed.; Ader, R., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 322–345. [Google Scholar]
- Dansokho, C.; Heneka, M.T. Neuroinflammatory responses in Alzheimer’s disease. J. Neural Transm. 2018, 125, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; LaFerla, F.M. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013, 239, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Strohmeyer, R.; Kovelowski, C.J.; Li, R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia 2002, 40, 260–269. [Google Scholar] [CrossRef]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef]
- Torrallardona, D. Spray dried animal plasma as an alternative to antibiotics in weanling pigs—A review. Asian Australas. J. Anim. Sci. 2010, 23, 131–148. [Google Scholar] [CrossRef]
- Van Dijk, A.J.; Margry, R.J.C.F.; Van der Lee, A.G.; Hemke, G.; Beynen, A.C. Growth performance and health status in weanling piglets fed spray-dried porcine plasma under typical Northern European conditions. J. Anim. Physiol. Anim. Nutr. 2002, 86, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; González-Esquerra, R.; Polo, J. Impact of spray-dried plasma on intestinal health and broiler performance. Microorganisms 2019, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Miró, L.; Polo, J.; Russell, L.; Campbell, J.; Weaver, E.; Crenshaw, J.; Moretó, M. Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J. Nutr. 2010, 140, 25–30. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Amat, C.; Polo, J.; Moretó, M. The anti-inflammatory effect of spray-dried plasma is mediated by a reduction in mucosal lymphocyte activation and infiltration in a mouse model of intestinal inflammation. Nutrients 2016, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Miró, L.; Garcia-Just, A.; Amat, C.; Polo, J.; Moretó, M.; Pérez-Bosque, A. Dietary animal plasma proteins improve the intestinal immune response in senescent mice. Nutrients 2017, 9, 1346. [Google Scholar] [CrossRef] [PubMed]
- Miró, L.; Amat, C.; Rosell-Cardona, C.; Campbell, J.M.; Polo, J.; Pérez-Bosque, A.; Moretó, M. Dietary supplementation with spray-dried porcine plasma attenuates colon inflammation in a genetic mouse model of inflammatory bowel disease. Int. J. Mol. Sci. 2020, 21, 6760. [Google Scholar] [CrossRef]
- Maijó, M.; Miro, L.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, L.; Weaver, E.; Moreto, M.; Pérez-Bosque, A. Dietary plasma proteins attenuate the innate immunity response in a mouse model of acute lung injury. Br. J. Nutr. 2012, 107, 867–875. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Lee, J.J.; Che, T.M.; Soares-Almeida, J.A.; Chun, J.L.; Campbell, J.M.; Polo, J.; Crenshaw, J.D.; Seo, S.W.; et al. Spray-dried plasma attenuates inflammation and improves pregnancy rate of mated female mice. J. Anim. Sci. 2015, 93, 298–305. [Google Scholar] [CrossRef]
- Moretó, M.; Miró, L.; Amat, C.; Polo, J.; Manichanh, C.; Pérez-Bosque, A. Dietary supplementation with spray-dried porcine plasma has prebiotic effects on gut microbiota in mice. Sci. Rep. 2020, 10, 2926. [Google Scholar] [CrossRef]
- Garcia-Just, A.; Miró, L.; Pérez-Bosque, A.; Amat, C.; Polo, J.; Pallàs, M.; Griñán-Ferré, C.; Moretó, M. Dietary spray-dried porcine plasma prevents cognitive decline in senescent mice and reduces neuroinflammation and oxidative stress. J. Nutr. 2020, 150, 303–311. [Google Scholar] [CrossRef]
- Morley, J.E.; Vijaya, S.A.; Kumar, B.; Armbrecht, H.J. The SAMP8 mouse: A model to develop therapeutic interventions for Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 1123–1130. [Google Scholar] [CrossRef]
- Griñan-Ferré, C.; Palomera-Ávalos, V.; Puigoriol-Illamola, D.; Camins, A.; Porquet, D.; Plá, V.; Aguado, F.; Pallàs, M. Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp. Gerontol. 2016, 80, 57–69. [Google Scholar] [CrossRef]
- Pallàs, M. Senescence-accelerated mice P8: A tool to study brain aging and Alzheimer’s disease in a mouse model. ISRN Cell Biol. 2012, 1–12. [Google Scholar] [CrossRef]
- Miyamoto, M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp. Gerontol. 1997, 32, 139–148. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Higuchi, K.; Hosono, M.; Akiguchi, I.; Katoh, H. A novel murine model of aging, Senescence-Accelerated Mouse (SAM). Arch. Gerontol. Geriatr. 1994, 19, 185–192. [Google Scholar] [CrossRef]
- Borg, B.S.; Campbell, J.M.; Russel, L.E.; Rodríguez, C.; Ródenas, J. Evaluation of the chemical and biological characteristics of spray-dried plasma protein collected from various locations around the world. Am. Assoc. Swine Vet. 2002, 33, 97–100. [Google Scholar]
- Blázquez, E.; Rodríguez, C.; Ródenas, J.; Segalés, J.; Pujols, J.; Polo, J. Biosafety steps in the manufacturing process of spray-dried plasma: A review with emphasis on the use of ultraviolet irradiation as a redundant biosafety procedure. Porc. Health Manag. 2020, 6, 16. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Laboratory Animals, 4th ed.; National Academy Press: Washington, DC, USA, 1995. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Filling the void for new Alzheimer’s disease therapy. Nat. Aging 2021. [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2018, 21, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.L.; Resnick, R.J.; De, S.; Acevedo, L.A.; Lu, K.P.; Schroeder, F.C.; Nicholson, L.K. Cyclic cis-locked phospho-dipeptides reduce entry of AβPP into amyloidogenic processing pathway. J. Alzheimer’s Dis. 2017, 55, 391–410. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Z.; Xiao, M.; Wang, C.; Tian, F. Chronic cerebral hypoperfusion promotes amyloid-beta pathogenesis via activating β/γ-secretases. Neurochem. Res. 2017, 42, 3446–3455. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, Z. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience against Alzheimer Neurodegeneration through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef]
- Lee, S.; Hall, G.F.; Shea, T.B. Potentiation of tau aggregation by cdk5 and GSK3β. J. Alzheimer’s Dis. 2011, 26, 355–364. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, L.; Su, S.C.; Tsai, L.H.; Zhu, J.J. Cdk5 is a new rapid synaptic homeostasis regulator capable of initiating the early Alzheimer-like pathology. Cereb. Cortex 2016, 26, 2937–2951. [Google Scholar] [CrossRef][Green Version]
- Hermida, M.A.; Kumar, J.D.; Leslie, N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 2017, 65, 5–15. [Google Scholar] [CrossRef]
- Del Valle, J.; Bayod, S.; Camins, A.; Beas-Zárate, C.; Velázquez-Zamora, D.A.; González-Burgos, I.; Pallàs, M. Dendritic spine abnormalities in hippocampal CA1 pyramidal neurons underlying memory deficits in the SAMP8 mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 32, 233–240. [Google Scholar] [CrossRef]
- Korzhevskii, D.E.; Kirik, O.V. Brain Microglia and microglial markers. Neurosci. Behav. Physiol. 2016, 46, 284–290. [Google Scholar] [CrossRef]
- Park, J.C.; Han, S.H.; Mook-Jung, I. Peripheral inflammatory biomarkers in Alzheimer’s disease: A brief review. BMB Rep. 2020, 53, 10–19. [Google Scholar] [CrossRef]
- Shen, X.N.; Niu, L.D.; Wang, Y.J.; Cao, X.P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Dewachter, I.; Landreth, G.E.; Willson, T.M.; Klockgether, T.; van Leuven, F.; Heneka, M.T. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J. Neurosci. 2003, 23, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zhong, Y. Interleukin-17A: The key cytokine in neurodegenerative diseases. Front. Aging Neurosci. 2020, 12, 566922. [Google Scholar] [CrossRef] [PubMed]
- White, C.S.; Lawrence, C.B.; Brough, D.; Rivers-Auty, J. Inflammasomes as therapeutic targets for Alzheimer’s disease. Brain Pathol. 2017, 27, 223–234. [Google Scholar] [CrossRef]
- Sugama, S.; Wirz, S.A.; Barr, A.M.; Conti, B.; Bartfai, T.; Shibasaki, T. Interleukin-18 null mice show diminished microglial activation and reduced dopaminergic neuron loss following acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Neuroscience 2004, 128, 451–458. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Codony, S.; Pujol, E.; Yang, J.; Leiva, R.; Escolano, C.; Puigoriol-Illamola, D.; Companys-Alemany, J.; Corpas, R.; Sanfeliu, C.; et al. Pharmacological inhibition of soluble epoxide hydrolase as a new therapy for Alzheimer’s disease. Neurotherapeutics 2020, 17, 1825–1835. [Google Scholar] [CrossRef]
- Wang, J.; Tan, L.; Wang, H.F.; Tan, C.C.; Meng, X.F.; Wang, C.; Tang, S.W.; Yu, J.T. Anti-inflammatory drugs and risk of Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimer’s Dis. 2015, 44, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zotova, E.; Bharambe, V.; Cheaveau, M.; Morgan, W.; Holmes, C.; Harris, S.; Neal, J.W.; Love, S.; Nicoll, J.A.; Boche, D. Inflammatory components in human Alzheimer’s disease and after active amyloid-β42 immunization. Brain 2013, 136, 2677–2696. [Google Scholar] [CrossRef]
- Nilson, A.N.; English, K.C.; Gerson, J.E.; Whittle, T.B.; Crain, C.N.; Xue, J.; Sengupta, U.; Castillo-Carranza, D.L.; Zhang, W.; Gupta, P.; et al. Tau oligomers associate with inflammation in the brain and retina of tauopathy mice and in neurodegenerative diseases. J. Alzheimer’s Dis. 2017, 55, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Zhang, J.; Huang, S.; Liu, W.; Wang, Z.; Liang, S.; Tao, J.; Chen, L. Disease stage-associated alterations in learning and memory through the electroacupuncture modulation of the cortical microglial M1/M2 polarization in mice with Alzheimer’s disease. Neural Plast. 2020, 8836173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Cheng, B.; Li, Y.; Li, X.; Chen, X.; Zhang, Y.W. TREM2 in Alzheimer’s disease: Microglial survival and energy metabolism. Front. Aging Neurosci. 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Tan, M.S.; Gu, L.Z.; Zhang, Y.D.; Tan, L. Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiol. Aging 2014, 35, 1243–1251. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β-dependent neuroinflammation. J. Neuroinflamm. 2015, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Bagheri, H.; Barreto, G.E.; Read, M.I.; Sahebkar, A. Effects of Curcumin on Microglial Cells. Neurotox. Res. 2019, 36, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Chirlaque, C.; Aranda, C.J.; Ocón, B.; Polo, J.; Martínez-Augustin, O.; Sánchez de Medina, F. Immunoregulatory Effects of Porcine Plasma Protein Concentrates on Rat Intestinal Epithelial Cells and Splenocytes. Animals 2021, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Buchen, B.; Neurath, M.F.; Becker, C. Regulation and pathophysiological role of epithelial turnover in the gut. Semin. Cell Dev. Biol. 2014, 35, 40–50. [Google Scholar] [CrossRef]
- Menendez, A.; Willing, B.P.; Montero, M.; Wlodarska, M.; So, C.C.; Bhinder, G.; Vallance, B.A.; Finlay, B.B. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell alpha-defensins. J. Innate Immun. 2013, 5, 39–49. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhao, S.; Zhu, Y.; Fan, Z.; Wang, J.; Zhang, B.; Chen, Y. Microbiota-gut-brain axis and toll-like receptors in Alzheimer’s disease. Comput. Struct. Biotechnol. J. 2019, 17, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, S.; Zhang, X. Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Miró, L.; Moretó, M.; Amat, C.; Polo, J.; Pérez-Bosque, A. Aging effects on gut microbiota in SAMP8 mice. Proceedings 2020, 61, 25. [Google Scholar] [CrossRef]
- Askarova, S.; Umbayev, B.; Masoud, A.R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front. Cell. Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef]
- Torrallardona, D.; Conde, M.R.; Badiola, I.; Polo, J.; Brufau, J. Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J. Anim. Sci. 2003, 81, 1220–1226. [Google Scholar] [CrossRef]
- Caracciolo, B.; Xu, W.; Collins, S.; Fratiglioni, L. Cognitive decline, dietary factors and gut-brain interactions. Mech. Ageing Dev. 2014, 136–137, 59–69. [Google Scholar] [CrossRef]

| Ingredients | Control Diet | SDP 1 Diet |
|---|---|---|
| g/kg | ||
| SDP | - | 80 |
| Dried skim milk | 530.7 | 340.5 |
| Corn starch | 199.3 | 308.8 |
| Sucrose | 94.5 | 94.5 |
| Soybean oil | 70 | 70 |
| Cellulose | 50 | 50 |
| AIN-93-G-MX (94046) 2 | 35 | 35 |
| AIN-93 VX (94047) 2 | 15 | 15 |
| Choline bitartrate | 3 | 3 |
| Methionine | 2.5 | 3.2 |
| Primer | Forward (5′–3′) | Reverse (5′–3′) | Size (bp) |
|---|---|---|---|
| Arg1 | GACAGGGCTCCTTTCAGGAC | GCCAAGGTTAAAGCCACTGC | 108 |
| Il-17 | AGCAGCGATCATCCCTCAA | TTGCGCCAAGGGAGTTAAA | 104 |
| Il-18 | GTTTACAAGCATCCAGGCACAG | GAAGGTTTGAGGCGGCTTTC | 151 |
| Inos | CCCAACAATACAAGATGACCCT | AGGGATTCTGGAACATTCTGTG | 87 |
| Tgf-b | CAGGGTGAAGGGGAAAACTC | AGTTCGGTCATTAGTCTCGC | 204 |
| Trem2 | CCTGAAGAAGCGGAATGGGA | CTTGATTCCTGGAGGTGCTGT | 269 |
| Ym1 | GTGGAGAAAGACATTCCAAGGC | CAGTTCAGGGATCTTGTACCCA | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosell-Cardona, C.; Griñan-Ferré, C.; Pérez-Bosque, A.; Polo, J.; Pallàs, M.; Amat, C.; Moretó, M.; Miró, L. Dietary Spray-Dried Porcine Plasma Reduces Neuropathological Alzheimer’s Disease Hallmarks in SAMP8 Mice. Nutrients 2021, 13, 2369. https://doi.org/10.3390/nu13072369
Rosell-Cardona C, Griñan-Ferré C, Pérez-Bosque A, Polo J, Pallàs M, Amat C, Moretó M, Miró L. Dietary Spray-Dried Porcine Plasma Reduces Neuropathological Alzheimer’s Disease Hallmarks in SAMP8 Mice. Nutrients. 2021; 13(7):2369. https://doi.org/10.3390/nu13072369
Chicago/Turabian StyleRosell-Cardona, Cristina, Christian Griñan-Ferré, Anna Pérez-Bosque, Javier Polo, Mercè Pallàs, Concepció Amat, Miquel Moretó, and Lluïsa Miró. 2021. "Dietary Spray-Dried Porcine Plasma Reduces Neuropathological Alzheimer’s Disease Hallmarks in SAMP8 Mice" Nutrients 13, no. 7: 2369. https://doi.org/10.3390/nu13072369
APA StyleRosell-Cardona, C., Griñan-Ferré, C., Pérez-Bosque, A., Polo, J., Pallàs, M., Amat, C., Moretó, M., & Miró, L. (2021). Dietary Spray-Dried Porcine Plasma Reduces Neuropathological Alzheimer’s Disease Hallmarks in SAMP8 Mice. Nutrients, 13(7), 2369. https://doi.org/10.3390/nu13072369








