Impact of a Geriatric Intervention to Improve Screening and Management of Undernutrition in Older Patients Undergoing Surgery for Colorectal Cancer: Results of the ANC Stepped-Wedge Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
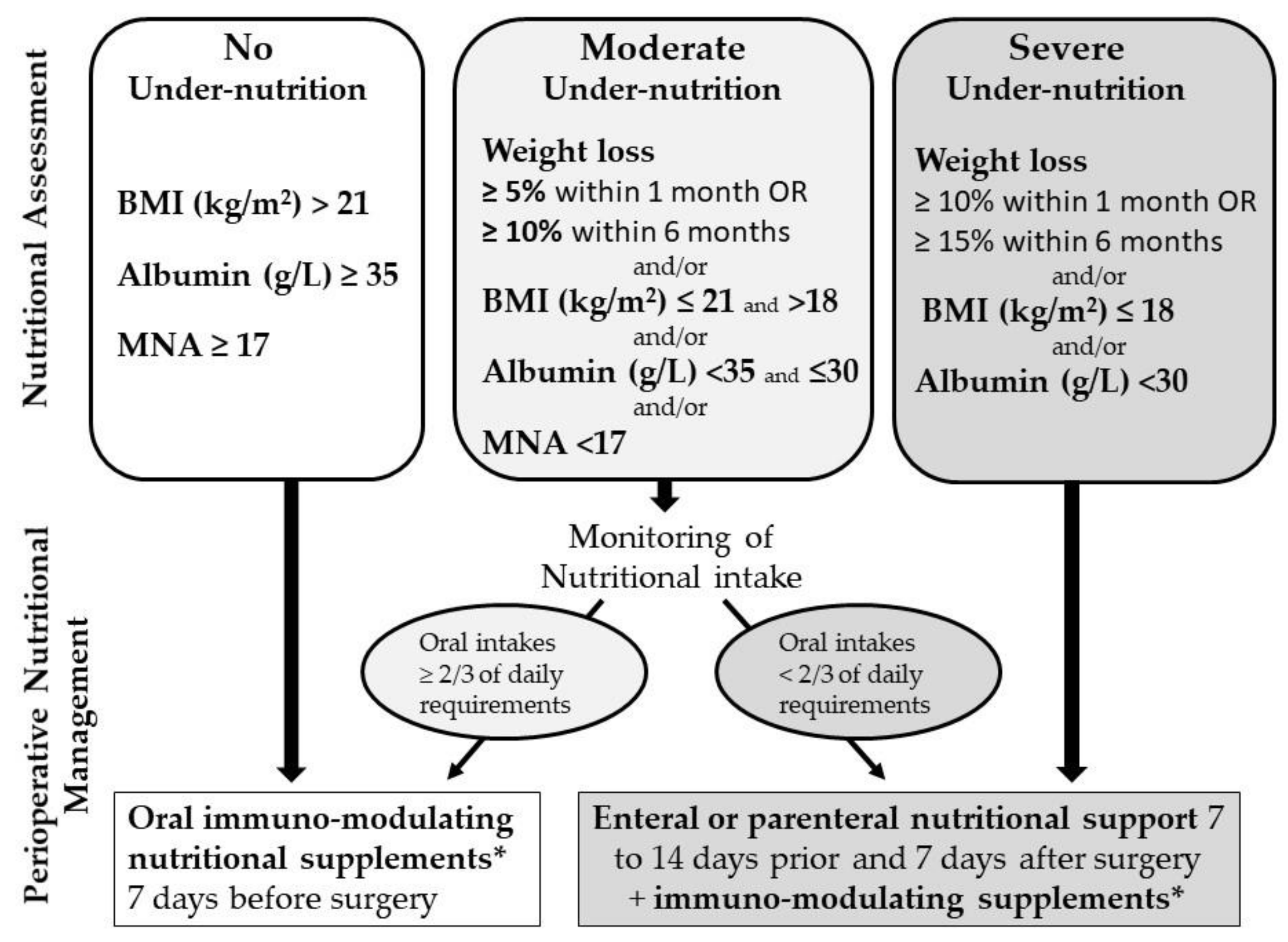
2.3. Study Intervention
2.4. Data Collection
2.5. Primary Outcome
2.6. Secondary Outcomes
2.7. Statistical Analysis
2.8. Ethics
3. Results
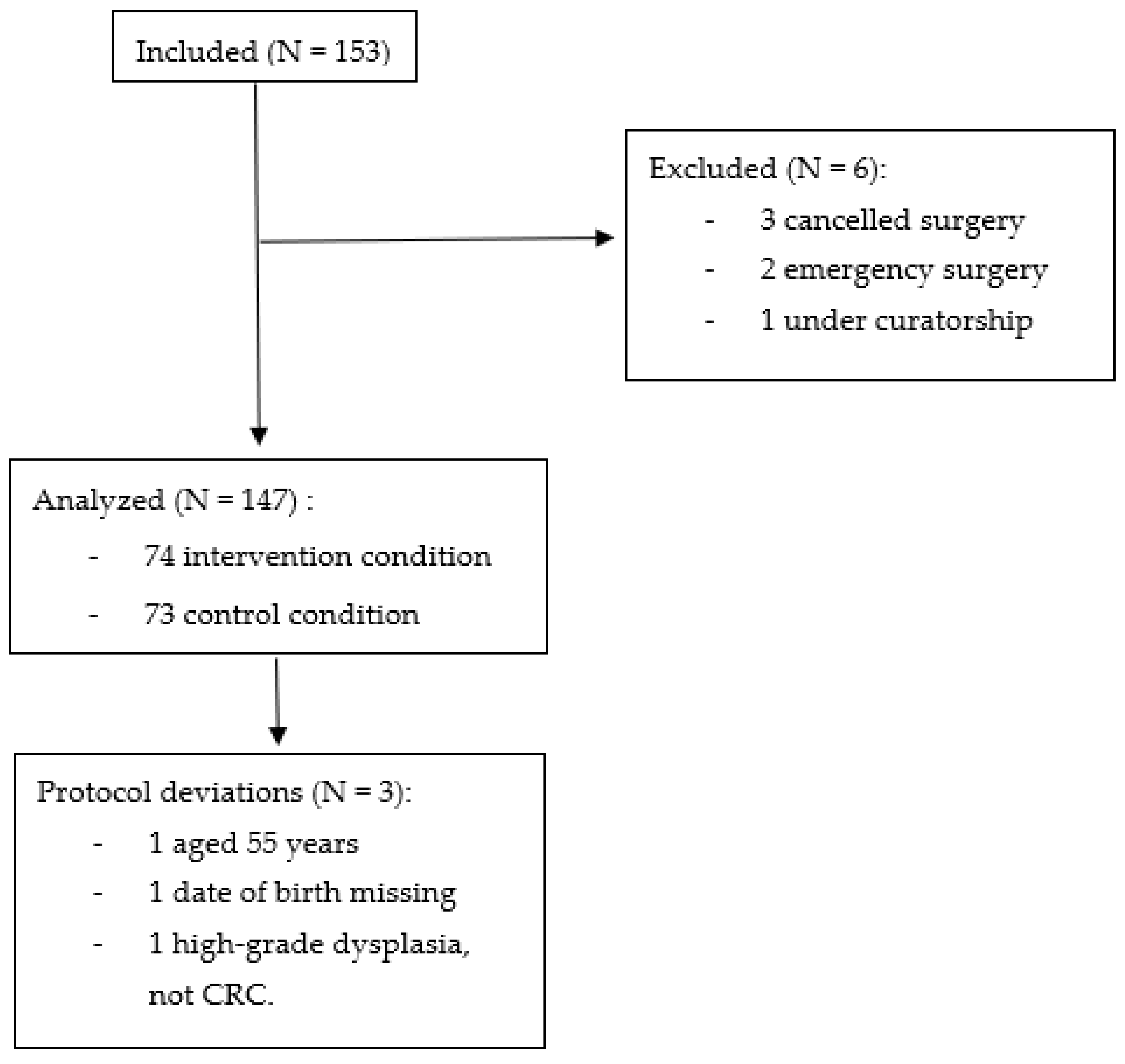
3.1. Participants
3.2. Results on the Primary Outcome
3.3. Evaluation (Nutritional Assessment)
3.4. Diagnosis (Nutritional Status)
3.5. Prescriptions (According to Nutritional Status)
3.6. Surgical Outcome
3.7. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
ANC Working Group
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Panis, Y.; Mathieu, P.; Mantion, G.; Kwiatkowski, F.; Slim, K.; Association Française de Chirurgie. Postoperative Mortality and Morbidity in French Patients Undergoing Colorectal Surgery: Results of a Prospective Multicenter Study. Arch. Surg. 2005, 140, 278–283; discussion 284. [Google Scholar] [CrossRef] [PubMed]
- Boakye, D.; Rillmann, B.; Walter, V.; Jansen, L.; Hoffmeister, M.; Brenner, H. Impact of Comorbidity and Frailty on Prognosis in Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2018, 64, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Bisson, C.; Fonck, M.; Rainfray, M.; Soubeyran, P.; Bourdel-Marchasson, I. Undernutrition in Elderly Patients with Cancer: Target for Diagnosis and Intervention. Crit. Rev. Oncol. Hematol. 2008, 67, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Schwegler, I.; von Holzen, A.; Gutzwiller, J.-P.; Schlumpf, R.; Mühlebach, S.; Stanga, Z. Nutritional Risk Is a Clinical Predictor of Postoperative Mortality and Morbidity in Surgery for Colorectal Cancer. Br. J. Surg. 2010, 97, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Morishima, T.; Sato, A.; Nakata, K.; Miyashiro, I. Geriatric Assessment Domains to Predict Overall Survival in Older Cancer Patients: An Analysis of Functional Status, Comorbidities, and Nutritional Status as Prognostic Factors. Cancer Med. 2020, 9, 5839–5850. [Google Scholar] [CrossRef]
- Balderas-Peña, L.-M.-A.; González-Barba, F.; Martínez-Herrera, B.-E.; Palomares-Chacón, U.-R.; Durán-Anguiano, O.; Salazar-Páramo, M.; Gómez-Sánchez, E.; Dávalos-Cobián, C.; Nava-Zavala, A.-H.; Hernández-Chávez, G.-A.; et al. Body Composition and Biochemical Parameters of Nutritional Status: Correlation with Health-Related Quality of Life in Patients with Colorectal Cancer. Nutrients 2020, 12, 2110. [Google Scholar] [CrossRef]
- Soubeyran, P.; Bellera, C.; Goyard, J.; Heitz, D.; Curé, H.; Rousselot, H.; Albrand, G.; Servent, V.; Jean, O.S.; van Praagh, I.; et al. Screening for Vulnerability in Older Cancer Patients: The ONCODAGE Prospective Multicenter Cohort Study. PLoS ONE 2014, 9, e115060. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN Expert Group Recommendations for Action against Cancer-Related Malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Fettes, S.B.; Davidson, H.I.M.; Richardson, R.A.; Pennington, C.R. Nutritional Status of Elective Gastrointestinal Surgery Patients Pre- and Post-Operatively. Clin. Nutr. 2002, 21, 249–254. [Google Scholar] [CrossRef]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-Associated Malnutrition, Cachexia and Sarcopenia: The Skeleton in the Hospital Closet 40 Years Later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- Aaldriks, A.A.; van der Geest, L.G.M.; Giltay, E.J.; le Cessie, S.; Portielje, J.E.A.; Tanis, B.C.; Nortier, J.W.R.; Maartense, E. Frailty and Malnutrition Predictive of Mortality Risk in Older Patients with Advanced Colorectal Cancer Receiving Chemotherapy. J. Geriatr. Oncol. 2013, 4, 218–226. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A New Definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of Nutritional Prehabilitation, with and without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterology 2018, 155, 391–410.e4. [Google Scholar] [CrossRef]
- Leung, A.M.; Gibbons, R.L.; Vu, H.N. Predictors of Length of Stay Following Colorectal Resection for Neoplasms in 183 Veterans Affairs Patients. World J. Surg. 2009, 33, 2183–2188. [Google Scholar] [CrossRef]
- Sungurtekin, H.; Sungurtekin, U.; Balci, C.; Zencir, M.; Erdem, E. The Influence of Nutritional Status on Complications after Major Intraabdominal Surgery. J. Am. Coll. Nutr. 2004, 23, 227–232. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment Serum Albumin as a Predictor of Cancer Survival: A Systematic Review of the Epidemiological Literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Huhmann, M.B.; August, D.A. Perioperative Nutrition Support in Cancer Patients. Nutr. Clin. Pract. 2012, 27, 586–592. [Google Scholar] [CrossRef]
- Janssen, T.L.; Steyerberg, E.W.; Langenberg, J.C.M.; de Lepper, C.V.H.; Wielders, D.; Seerden, T.C.J.; de Lange, D.C.; Wijsman, J.H.; Ho, G.H.; Gobardhan, P.D.; et al. Multimodal Prehabilitation to Reduce the Incidence of Delirium and Other Adverse Events in Elderly Patients Undergoing Elective Major Abdominal Surgery: An Uncontrolled before-and-after Study. PLoS ONE 2019, 14, e0218152. [Google Scholar] [CrossRef]
- Gillis, C.; Fenton, T.R.; Sajobi, T.T.; Minnella, E.M.; Awasthi, R.; Loiselle, S.-È.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Trimodal Prehabilitation for Colorectal Surgery Attenuates Post-Surgical Losses in Lean Body Mass: A Pooled Analysis of Randomized Controlled Trials. Clin. Nutr. 2019, 38, 1053–1060. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Chambrier, C.; Sztark, F.; Groupe de Travail de la Société Francophone de Nutrition Clinique et Métabolisme (SFNEP) et de la Société Française D’anesthésie et Réanimation (Sfar). French clinical guidelines on perioperative nutrition. Update of the 1994 consensus conference on “Perioperative artificial nutrition after elective surgery in adults”. Ann. Fr. Anesth. Reanim. 2011, 30, 381–389. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Harsanyi, L.; Laviano, A.; Ljungqvist, O.; Soeters, P.; DGEM (German Society for Nutritional Medicine); Jauch, K.W.; Kemen, M.; Hiesmayr, J.M.; et al. ESPEN Guidelines on Enteral Nutrition: Surgery Including Organ Transplantation. Clin. Nutr. 2006, 25, 224–244. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Gillis, A.; Dixon, M.; Smith, A.; Law, C.; Coburn, N.G. A Patient-Centred Approach toward Surgical Wait Times for Colon Cancer: A Population-Based Analysis. Can. J. Surg. 2014, 57, 94–100. [Google Scholar] [CrossRef]
- Dupuis, M.; Kuczewski, E.; Villeneuve, L.; Bin-Dorel, S.; Haine, M.; Falandry, C.; Gilbert, T.; Passot, G.; Glehen, O.; Bonnefoy, M. Age Nutrition Chirugie (ANC) Study: Impact of a Geriatric Intervention on the Screening and Management of Undernutrition in Elderly Patients Operated on for Colon Cancer, a Stepped Wedge Controlled Trial. BMC Geriatr. 2017, 17, 10. [Google Scholar] [CrossRef][Green Version]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.H.; Mulsant, B.; Reynolds, C.F. Rating Chronic Medical Illness Burden in Geropsychiatric Practice and Research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Boyd, C.M.; Xue, Q.-L.; Simpson, C.F.; Guralnik, J.M.; Fried, L.P. Frailty, Hospitalization, and Progression of Disability in a Cohort of Disabled Older Women. Am. J. Med. 2005, 118, 1225–1231. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar] [PubMed]
- Ribaudo, J.M.; Cella, D.; Hahn, E.A.; Lloyd, S.R.; Tchekmedyian, N.S.; Von Roenn, J.; Leslie, W.T. Re-Validation and Shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) Questionnaire. Qual. Life Res. 2000, 9, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.L.K.; Mantoo, S.K.; Tan, K.Y. “Start to Finish Trans-Institutional Transdisciplinary Care”: A Novel Approach Improves Colorectal Surgical Results in Frail Elderly Patients. Colorectal Dis. 2016, 18, O43–O50. [Google Scholar] [CrossRef]
- Mazzola, M.; Bertoglio, C.; Boniardi, M.; Magistro, C.; De Martini, P.; Carnevali, P.; Morini, L.; Ferrari, G. Frailty in Major Oncologic Surgery of Upper Gastrointestinal Tract: How to Improve Postoperative Outcomes. Eur. J. Surg. Oncol. 2017, 43, 1566–1571. [Google Scholar] [CrossRef]
- Milder, D.A.; Pillinger, N.L.; Kam, P.C.A. The Role of Prehabilitation in Frail Surgical Patients: A Systematic Review. Acta Anaesthesiol. Scand. 2018, 62, 1356–1366. [Google Scholar] [CrossRef]
- Burden, S.; Todd, C.; Hill, J.; Lal, S. Pre-operative Nutrition Support in Patients Undergoing Gastrointestinal Surgery. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bruns, E.R.J.; Argillander, T.E.; Van Den Heuvel, B.; Buskens, C.J.; Van Duijvendijk, P.; Winkels, R.M.; Kalf, A.; Van Der Zaag, E.S.; Wassenaar, E.B.; Bemelman, W.A.; et al. Oral Nutrition as a Form of Pre-Operative Enhancement in Patients Undergoing Surgery for Colorectal Cancer: A Systematic Review. Surg. Infect. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Looijaard, S.M.L.M.; Slee-Valentijn, M.S.; Otten, R.H.J.; Maier, A.B. Physical and Nutritional Prehabilitation in Older Patients with Colorectal Carcinoma: A Systematic Review. J. Geriatr. Phys. Ther. 2018, 41, 236–244. [Google Scholar] [CrossRef]
- Hijazi, Y.; Gondal, U.; Aziz, O. A Systematic Review of Prehabilitation Programs in Abdominal Cancer Surgery. Int. J. Surg. 2017, 39, 156–162. [Google Scholar] [CrossRef]
- Burden, S.T.; Gibson, D.J.; Lal, S.; Hill, J.; Pilling, M.; Soop, M.; Ramesh, A.; Todd, C. Pre-Operative Oral Nutritional Supplementation with Dietary Advice versus Dietary Advice Alone in Weight-Losing Patients with Colorectal Cancer: Single-Blind Randomized Controlled Trial. J. Cachexia Sarcopenia Muscle 2017, 8, 437–446. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colonic Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. Clin. Nutr. 2012, 31, 783–800. [Google Scholar] [CrossRef]
- Achilli, P.; Mazzola, M.; Bertoglio, C.L.; Magistro, C.; Origi, M.; Carnevali, P.; Gervasi, F.; Mastellone, C.; Guanziroli, N.; Corradi, E.; et al. Preoperative Immunonutrition in Frail Patients with Colorectal Cancer: An Intervention to Improve Postoperative Outcomes. Int. J. Colorectal Dis. 2020, 35, 19–27. [Google Scholar] [CrossRef]
- Braga, M.; Wischmeyer, P.E.; Drover, J.; Heyland, D.K. Clinical Evidence for Pharmaconutrition in Major Elective Surgery. JPEN J. Parenter. Enter. Nutr. 2013, 37, 66S–72S. [Google Scholar] [CrossRef]
- Hegazi, R.A.; Hustead, D.S.; Evans, D.C. Preoperative Standard Oral Nutrition Supplements vs Immunonutrition: Results of a Systematic Review and Meta-Analysis. J. Am. Coll. Surg. 2014, 219, 1078–1087. [Google Scholar] [CrossRef]
- Thornblade, L.W.; Varghese, T.K.; Shi, X.; Johnson, E.K.; Bastawrous, A.; Billingham, R.P.; Thirlby, R.; Fichera, A.; Flum, D.R. Preoperative Immunonutrition and Elective Colorectal Resection Outcomes. Dis. Colon Rectum 2017, 60, 68–75. [Google Scholar] [CrossRef]
- Heyland, D.K.; Novak, F.; Drover, J.W.; Jain, M.; Su, X.; Suchner, U. Should Immunonutrition Become Routine in Critically Ill Patients? A Systematic Review of the Evidence. JAMA 2001, 286, 944–953. [Google Scholar] [CrossRef]
- Buzquurz, F.; Bojesen, R.D.; Grube, C.; Madsen, M.T.; Gögenur, I. Impact of Oral Preoperative and Perioperative Immunonutrition on Postoperative Infection and Mortality in Patients Undergoing Cancer Surgery: Systematic Review and Meta-Analysis with Trial Sequential Analysis. BJS Open 2020, 4, 764–775. [Google Scholar] [CrossRef]
- Xu, J.; Sun, X.; Xin, Q.; Cheng, Y.; Zhan, Z.; Zhang, J.; Wu, J. Effect of Immunonutrition on Colorectal Cancer Patients Undergoing Surgery: A Meta-Analysis. Int. J. Colorectal Dis. 2018, 33, 273–283. [Google Scholar] [CrossRef]
- Coiera, E. Why System Inertia Makes Health Reform so Difficult. BMJ 2011, 342, d3693. [Google Scholar] [CrossRef]
- Ellis, G.; Whitehead, M.A.; Robinson, D.; O’Neill, D.; Langhorne, P. Comprehensive Geriatric Assessment for Older Adults Admitted to Hospital: Meta-Analysis of Randomised Controlled Trials. BMJ 2011, 343, d6553. [Google Scholar] [CrossRef]
- Kocman, D.; Regen, E.; Phelps, K.; Martin, G.; Parker, S.; Gilbert, T.; Conroy, S. Can Comprehensive Geriatric Assessment Be Delivered without the Need for Geriatricians? A Formative Evaluation in Two Perioperative Surgical Settings. Age Ageing 2019. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Sulo, S.; VanDerBosch, G.; Kozmic, S.; Sokolowski, M.; Summerfelt, W.T.; Partridge, J.; Hegazi, R.; Nikolich, S. Nutrition-Focused Quality Improvement Program Results in Significant Readmission and Length of Stay Reductions for Malnourished Surgical Patients. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Freijer, K.; Bours, M.J.L.; Nuijten, M.J.C.; Poley, M.J.; Meijers, J.M.M.; Halfens, R.J.G.; Schols, J.M.G.A. The Economic Value of Enteral Medical Nutrition in the Management of Disease-Related Malnutrition: A Systematic Review. J. Am. Med. Dir. Assoc. 2014, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.P.; Awasthi, R.; Sweet, S.N.; Minnella, E.M.; Bergdahl, A.; Santa Mina, D.; Carli, F.; Scheede-Bergdahl, C. Four-Week Prehabilitation Program Is Sufficient to Modify Exercise Behaviors and Improve Preoperative Functional Walking Capacity in Patients with Colorectal Cancer. Support Care Cancer 2017, 25, 33–40. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
| Period 1 | Period 2 | Period 3 | Period 4 | Period 5 | Period 6 | |
|---|---|---|---|---|---|---|
| Center | 8 October 2013–1 April 2014 | 1 April 2014–1 October 2014 | 1 October 2014–1 April 2015 | 1 April 2015–1 October 2015 | 1 October 2015–1 April 2016 | 1 April 2016–13 December 2016 |
| 1 | 5 | 5 | 3 | 4 | 7 | 5 |
| 2 | 5 | 6 | 4 | 5 | 5 | 4 |
| 3 | 4 | 5 | 4 | 3 | 9 | 5 |
| 4 | 4 | 7 | 1 | 6 | 5 | 6 |
| 5 | 4 | 6 | 3 | 7 | 5 | 5 |
| N = | Global | Control | Intervention | p-Value 4 | ||
|---|---|---|---|---|---|---|
| Median age (min–max) | 146 | 79.6 (55.5–92.8) | 79.2 (55.5–89.2) | 80.5 (70–92.8) | 0.1738 * | |
| Female (%) | 147 | 73 (49.7) | 39 (53.4) | 34 (45.9) | 0.3645 | |
| Laboratory results | Median hemoglobin (g/L) | 137 | 11.8 (7–40) | 11.5 (7–40) | 12 (7.2–16.1) | 0.6935 * |
| Median albumin (g/L) (min–max) | 115 | 37 (21–48.2) | 38 (22–44) | 36.9 (21–48.2) | 0.7274 * | |
| Median pre-albumin (g/L) (min–max) | 98 | 0.23 (0.05–3) | 0.24 (0.11–1.9) | 0.22 (0.05–3) | 0.6608 * | |
| Median CRP (g/L) | 94 | 5.4 (0.4–212) | 6.05 (1.1–119) | 5.4 (0.4–212) | 0.4457 * | |
| Median BMI (Kg/m2) (min–max) | 146 | 24.7 (15.8–39.3) | 25.3 (17.9–39.3) | 24.1 (15.8–38.9) | 0.0927 | |
| Patients with weight loss (%) | 134 | 76 (56.7) | 30 (49.2) | 46 (63) | 0.1075 | |
| Median % weight loss over 6 months (min–max) | 50 | 6.58 (2.06–23.33) | 6.56 (2.27–23.33) | 6.8 (2.06–20.51) | 0.7769 * | |
| Moderate to severe undernutrition (%) | 99 | 61 (61.6) | 28 (82.4) | 33 (50.8) | 0.0022 | |
| Cachexia (%) | 106 | 6 (5.7) | 1 (2.4) | 5 (7.7) | 0.4016 | |
| Anorexia (%) | 93 | 11 (11.8) | 5 (21.7) | 6 (8.6) | 0.1321 * | |
| Median MNA (min–max) 1 | 59 | 21.5 (4–26) | 21.5 (16–25) | 21.8 (4–26) | 0.6138 * | |
| Median CIRS-G comorbidity score (min–max) | 92 | 6 (0–19) | 6 (1–15) | 6 (0-19) | 0.8754 * | |
| Number of medications (%) | >6 | 136 | 30 (22.1) | 18 (29) | 12 (16.2) | 0.1248 |
| 3–6 | 73 (53.7) | 28 (45.2) | 45 (60.8) | |||
| <3 | 73 (53.7) | 28 (45.2) | 45 (60.8) | |||
| Mean fatigue score (SD) | 75 | 6.3 (2.1) | 6.3 (2.2) | 6.3(2.1) | 0.7536 * | |
| Mean handgrip strength (SD) 2 | 65 | 24.3 (11.4) | 21.3 (5.5) | 24.5 (11.6) | 0.6472 | |
| Mean SPPB score (SD) 3 | 75 | 8.12 (2.87) | 7.17 (2.71) | 8.2 (2.89) | 0.3061 * | |
| Median duration of hospitalization (min–max) | 142 | 10.5 (1–94) | 12 (4–94) | 9 (1–70) | 0.0220 * | |
| Control n (%) | Intervention n (%) | p-Value | |
|---|---|---|---|
| Primary outcome | |||
| Appropriate nutritional management 1 | 1 (1.4) | 29 (39.2) | <0.0001 |
| Mixed model for stepped-wedge design | |||
| Time from first inclusion in cluster (time-period effect) | 0.9897 | ||
| Time from beginning of intervention phase (learning effect) | 0.2498 | ||
| Control/intervention phase | 0.0002 | ||
| Details of appropriate nutritional management | |||
| Screened for under-nutrition | |||
| At least 7 days prior to surgery | 11 (15.1) | 60 (81.1) | <0.0001 |
| BMI measured | 73 (100) | 74 (100) | - |
| Serum albumin measured | 52 (71.2) | 63 (85.1) | 0.0411 |
| Weight loss assessed | 60 (82.2) | 73 (98.6) | 0.0007 |
| MNA performed | 14 (19.2) | 72 (97.3) | <0.0001 |
| Adequate diagnosis of nutritional status | 13 (17.8) | 36 (48.6) | <0.0001 |
| Prescriptions in accordance with nutritional status 2 | 3 (4.1) | 31 (41.9) | <0.0001 |
| Control n (%) | Intervention n (%) | p-Value 4 | ||
|---|---|---|---|---|
| Patients with at least one postsurgical complication (%) | 10 (13.7) | 28 (38.9) | 0.0006 | |
| Mortality (%) | - | 4 (6.8) | 0.11991 | |
| Reoperation (%) | 5 (35.7) | 9 (15.3) | 0.1256 * | |
| Complication type (%) | Fistula/peritonitis | 3 (21.4) | 8 (14) | 0.2563 * |
| Hematoma/hemorrhage 1 | 2 (14.3) | 11 (19.3) | ||
| Ileus/occlusion | 4 (28.6) | 6 (10.5) | ||
| Infection/sepsis | 1 (7.1) | 8 (14) | ||
| Wound dehiscence | 1 (7.1) | 3 (5.3) | ||
| diarrhea | 1 (7.1) | 3 (5.3) | ||
| Nausea/vomiting | 4 (7) | |||
| Bowel ischemia | 1 (7.1) | - | ||
| General complication or geriatric syndrome 2 | 1 (7.1) | 14 (24.6) | ||
| Grade of complications 3 (%) | I | 3 (21.4) | 25 (42.4) | 0.0321 * |
| II | 4 (28.6) | 19 (32.2) | ||
| IIIa | 5 (35.7) | 3 (5.1) | ||
| IIIb | 2 (14.3) | 8 (13.6) | ||
| IV | - | - | ||
| V | - | 4 (6.8) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, T.; Bernard, L.; Alexandre, M.; Bin-Dorel, S.; Villeneuve, L.; Decullier, E.; Bonnefoy, M.; ANC Working Group. Impact of a Geriatric Intervention to Improve Screening and Management of Undernutrition in Older Patients Undergoing Surgery for Colorectal Cancer: Results of the ANC Stepped-Wedge Trial. Nutrients 2021, 13, 2347. https://doi.org/10.3390/nu13072347
Gilbert T, Bernard L, Alexandre M, Bin-Dorel S, Villeneuve L, Decullier E, Bonnefoy M, ANC Working Group. Impact of a Geriatric Intervention to Improve Screening and Management of Undernutrition in Older Patients Undergoing Surgery for Colorectal Cancer: Results of the ANC Stepped-Wedge Trial. Nutrients. 2021; 13(7):2347. https://doi.org/10.3390/nu13072347
Chicago/Turabian StyleGilbert, Thomas, Lorraine Bernard, Marine Alexandre, Sylvie Bin-Dorel, Laurent Villeneuve, Evelyne Decullier, Marc Bonnefoy, and ANC Working Group. 2021. "Impact of a Geriatric Intervention to Improve Screening and Management of Undernutrition in Older Patients Undergoing Surgery for Colorectal Cancer: Results of the ANC Stepped-Wedge Trial" Nutrients 13, no. 7: 2347. https://doi.org/10.3390/nu13072347
APA StyleGilbert, T., Bernard, L., Alexandre, M., Bin-Dorel, S., Villeneuve, L., Decullier, E., Bonnefoy, M., & ANC Working Group. (2021). Impact of a Geriatric Intervention to Improve Screening and Management of Undernutrition in Older Patients Undergoing Surgery for Colorectal Cancer: Results of the ANC Stepped-Wedge Trial. Nutrients, 13(7), 2347. https://doi.org/10.3390/nu13072347






