A Comparison of Gene Expression Profiles of Rat Tissues after Mild and Short-Term Calorie Restrictions
Abstract
:1. Introduction
2. Materials and Methods
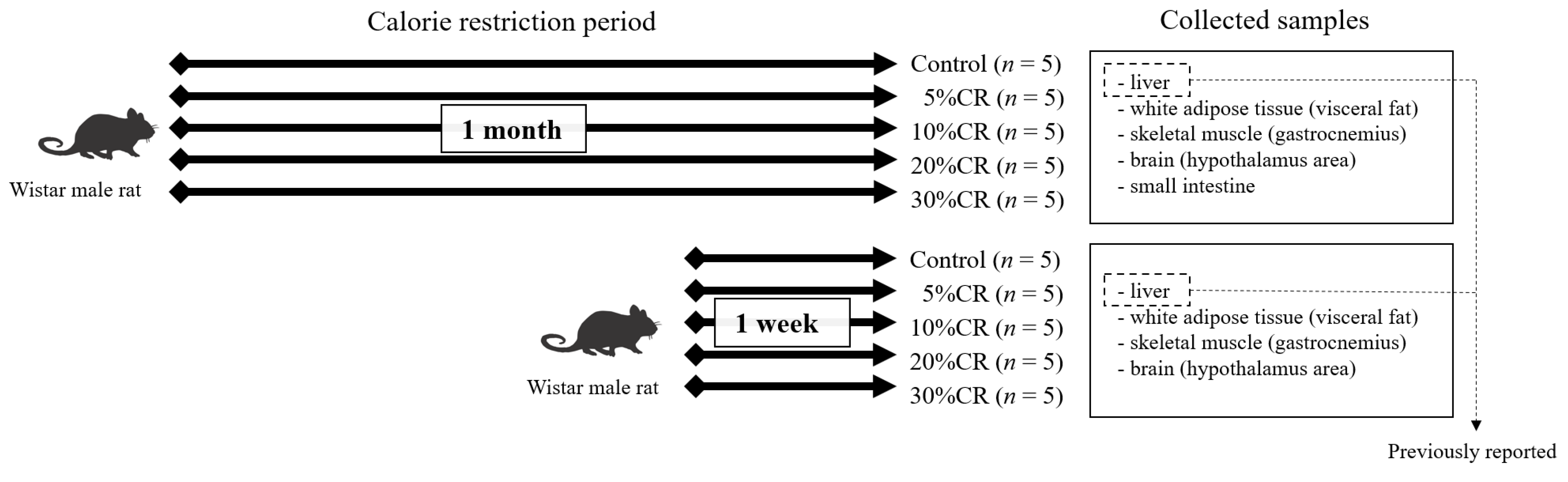
2.1. Animal Experiment
2.2. Microarray Experiments
2.3. Differentially Expressed Gene Probes
2.4. Gene Ontology Analysis
3. Results
3.1. The Number of CR Responsive Genes
3.2. CR Responsive Genes for Each Tissue
3.3. Up-Regulated Genes across All Tissues Studied
3.4. Down-Regulated Genes across All Tissues Studied
3.5. Comparison with a Meta-Analysis of the CR Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Austad, S.N.; Hoffman, J.M. Beyond calorie restriction: Aging as a biological target for nutrient therapies. Curr. Opin. Biotechnol. 2020, 70, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, B.; Baidoo, E.E.K. Omics-driven biotechnology for industrial applications. Front. Bioeng. Biotechnol. 2021, 9, 613307. [Google Scholar] [CrossRef]
- Aon, M.A.; Bernier, M.; Mitchell, S.J.; Di Germanio, C.; Mattison, J.A.; Ehrlich, M.R.; Colman, R.J.; Anderson, R.M.; de Cabo, R. Untangling determinants of enhanced health and lifespan through a multi-omics approach in mice. Cell Metab. 2020, 32, 100–116.e4. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Armengol, J.; Fajas, L.; Lopez-Mejia, I.C. Inter-organ communication: A gatekeeper for metabolic health. EMBO Rep. 2019, 20, e47903. [Google Scholar] [CrossRef]
- Wang, F.; So, K.-F.; Xiao, J.; Wang, H. Organ-organ communication: The liver’s perspective. Theranostics 2021, 11, 3317–3330. [Google Scholar] [CrossRef]
- Saito, K.; Ohta, Y.; Sami, M.; Kanda, T.; Kato, H. Effect of mild restriction of food intake on gene expression profile in the liver of young rats: Reference data for in vivo nutrigenomics study. Br. J. Nutr. 2010, 104, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [Green Version]
- Hoggard, N. Increase in circulating and adipose tissue expression of secretory leukocyte peptidase inhibitor (SLPI) with obesity and diabetes. Open Nutr. J. 2012, 6, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Laurens, C.; Badin, P.-M.; Louche, K.; Mairal, A.; Tavernier, G.; Marette, A.; Tremblay, A.; Weisnagel, S.J.; Joanisse, D.R.; Langin, D.; et al. G0/G1 Switch Gene 2 controls adipose triglyceride lipase activity and lipid metabolism in skeletal muscle. Mol. Metab. 2016, 5, 527–537. [Google Scholar] [CrossRef]
- Xu, Y.; O’Malley, B.W.; Elmquist, J.K. Brain nuclear receptors and body weight regulation. J. Clin. Investig. 2017, 127, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Cyr, N.E.; Toorie, A.M.; Steger, J.S.; Sochat, M.M.; Hyner, S.; Perello, M.; Stuart, R.; Nillni, E.A. Mechanisms by which the orexigen NPY regulates anorexigenic α-MSH and TRH. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E640–E650. [Google Scholar] [CrossRef] [Green Version]
- Alnouti, Y.; Klaassen, C.D. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol. Sci. 2006, 93, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Jarry, A.-C.; Merah, N.; Cisse, F.; Cayetanot, F.; Fiamma, M.-N.; Willemetz, A.; Gueddouri, D.; Barka, B.; Valet, P.; Guilmeau, S.; et al. Neuromedin U is a gut peptide that alters oral glucose tolerance by delaying gastric emptying via direct contraction of the pylorus and vagal-dependent mechanisms. FASEB J. 2019, 33, 5377–5388. [Google Scholar] [CrossRef]
- Swindell, W.R. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics 2009, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plank, M.; Wuttke, D.; van Dam, S.; Clarke, S.A.; de Magalhães, J.P. A meta-analysis of caloric restriction gene expression profiles to infer common signatures and regulatory mechanisms. Mol. Biosyst. 2012, 8, 1339–1349. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Nakagawa, T. NAD metabolism: Implications in aging and longevity. Ageing Res. Rev. 2018, 47, 1–17. [Google Scholar] [CrossRef]
- Imai, S.; Yoshino, J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 26–33. [Google Scholar] [CrossRef] [Green Version]
- Stein, L.R.; Imai, S.-I. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Ke, S.-F.; Zhou, C.-C.; Zhang, S.-L.; Guan, Y.-F.; Xu, T.-Y.; Sheng, C.-Q.; Wang, P.; Miao, C.-Y. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Panaretou, B.; Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998, 17, 4829–4836. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D. Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle 2018, 17, 1048–1055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Pavlova, N.N.; Thompson, C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017, 36, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Johmura, Y.; Yamanaka, T.; Omori, S.; Wang, T.-W.; Sugiura, Y.; Matsumoto, M.; Suzuki, N.; Kumamoto, S.; Yamaguchi, K.; Hatakeyama, S.; et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 2021, 371, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Sparbier, C.E.; Chan, Y.-C.; Williamson, J.C.; Woods, K.; Beavis, P.A.; Lam, E.Y.N.; Henderson, M.A.; Bell, C.C.; Stolzenburg, S.; et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017, 549, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Mezzadra, R.; Sun, C.; Jae, L.T.; Gomez-Eerland, R.; de Vries, E.; Wu, W.; Logtenberg, M.E.W.; Slagter, M.; Rozeman, E.A.; Hofland, I.; et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017, 549, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Rostami, N.; Nikkhoo, A.; Ajjoolabady, A.; Azizi, G.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Yousefi, B.; Yousefi, M.; Jadidi-Niaragh, F. S1PR1 as a novel promising therapeutic target in cancer therapy. Mol. Diagn. Ther. 2019, 23, 467–487. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 2016, 207, 241–266. [Google Scholar]
- Fu, Z.D.; Klaassen, C.D. Short-term calorie restriction feminizes the mRNA profiles of drug metabolizing enzymes and transporters in livers of mice. Toxicol. Appl. Pharmacol. 2014, 274, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shen, Y.; Li, W.; Li, Q.; Miao, Y.; Zhong, Y. Upregulation of flavin-containing monooxygenase 3 mimics calorie restriction to retard liver aging by inducing autophagy. Aging 2020, 12, 931–944. [Google Scholar] [CrossRef]
- Rossner, R.; Kaeberlein, M.; Leiser, S.F. Flavin-containing monooxygenases in aging and disease: Emerging roles for ancient enzymes. J. Biol. Chem. 2017, 292, 11138–11146. [Google Scholar] [CrossRef] [Green Version]
- Oesch, F.; Glatt, H.; Schmassmann, H. The apparent ubiquity of epoxide hydratase in rat organs. Biochem. Pharmacol. 1977, 26, 603–607. [Google Scholar] [CrossRef]
- Jacob, L.; Freyn, M.; Kalder, M.; Dinas, K.; Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: A retrospective study of 422,010 patients followed for up to 30 years. Oncotarget 2018, 9, 17420–17429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempel, N.; Wang, H.; LeCluyse, E.L.; McManus, M.E.; Negishi, M. The human sulfotransferase SULT1A1 gene is regulated in a synergistic manner by Sp1 and GA binding protein. Mol. Pharmacol. 2004, 66, 1690–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.Y.; Park, S.; Kim, M.S.; Kim, H.-K.; Han, S.N. Effects of mild calorie restriction on lipid metabolism and inflammation in liver and adipose tissue. Biochem. Biophys. Res. Commun. 2017, 490, 636–642. [Google Scholar] [CrossRef]
- Okamoto, F.; Tanaka, T.; Sohmiya, K.; Kawamura, K. CD36 abnormality and impaired myocardial long-chain fatty acid uptake in patients with hypertrophic cardiomyopathy. Jpn. Circ. J. 1998, 62, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, T.; Dhahbi, J.M.; Cui, X.; Mote, P.L.; Bartke, A.; Spindler, S.R. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol. Genom. 2004, 17, 307–315. [Google Scholar] [CrossRef]
- Athinarayanan, S.; Fan, Y.-Y.; Wang, X.; Callaway, E.; Cai, D.; Chalasani, N.; Chapkin, R.S.; Liu, W. Fatty acid desaturase 1 influences hepatic lipid homeostasis by modulating the PPARα-FGF21 axis. Hepatol. Commun. 2020, 5, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Burris, T.P. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol. Endocrinol. 2008, 22, 1509–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, S.N.; Lau, P.; Burke, L.J.; Muscat, G.E.O. REV-ERBbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: Evidence for cross-talk between orphan nuclear receptors and myokines. J. Biol. Chem. 2005, 280, 8651–8659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, A.; Gupta, R.; Makwana, K.; Kondratov, R. Circadian clocks, diets and aging. Nutr. Healthy Aging 2017, 4, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raines, A.M.; Sunde, R.A. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genom. 2011, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Organs | Duration | 5% | 10% | 20% | 30% | Overlap † |
|---|---|---|---|---|---|---|
| liver | 1 week | 183 | 588 | 902 | 734 | 90 |
| 1 month | 495 | 454 | 572 | 570 | ||
| adipose | 1 week | 387 | 981 | 1084 | 1674 | 59 |
| 1 month | 304 | 1232 | 1624 | 1255 | ||
| muscle | 1 week | 684 | 663 | 1229 | 1517 | 101 |
| 1 month | 1041 | 1097 | 1074 | 1422 | ||
| brain (hypothalamus) | 1 week | 1346 | 1413 | 1429 | 1345 | 3 |
| 1 month | 76 | 58 | 119 | 148 | ||
| intestine | - | - | - | - | - | 70 |
| 1 month | 370 | 503 | 530 | 558 |
| 1 Week | 1 Month | Gene Title | Gene Symbol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Probe ID | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | |||
| liver (40 gene probes) | 1388271_at | 2.2 | 3.5 | 4.5 | 4.5 | 2.3 | 2.9 | 3.4 | 4.0 | metallothionein 2A | Mt2A |
| 1371237_a_at | 2.4 | 3.6 | 4.1 | 4.0 | 2.9 | 3.4 | 3.5 | 3.9 | metallothionein 1a///transthyretin | Mt1a///Ttr | |
| 1387336_at | 1.0 | 2.4 | 3.7 | 3.7 | 2.0 | 2.6 | 3.2 | 3.5 | N-acetyltransferase 8 | Nat8 | |
| 1387156_at | 3.9 | 4.4 | 4.4 | 4.1 | 3.9 | 4.1 | 3.8 | 3.3 | hydroxysteroid (17-beta) dehydrogenase 2 | Hsd17b2 | |
| 1368213_at | 0.8 | 1.5 | 2.5 | 3.1 | 1.5 | 2.0 | 2.5 | 2.8 | P450 (cytochrome) oxidoreductase | Por | |
| adipose (45 gene probes) | 1387874_at | 2.5 | 2.7 | 2.9 | 2.9 | 2.8 | 2.9 | 3.0 | 2.5 | D site of albumin promoter (albumin D-box) binding protein | Dbp |
| 1388039_a_at | 1.0 | 1.7 | 2.0 | 2.4 | 1.6 | 2.3 | 2.3 | 2.4 | gamma-aminobutyric acid (GABA) B receptor 1 | Gabbr1 | |
| 1368406_at | 1.2 | 1.6 | 2.3 | 2.4 | 1.4 | 1.7 | 2.2 | 2.3 | steroidogenic acute regulatory protein | Star | |
| 1372536_at | 0.8 | 1.2 | 1.9 | 2.3 | 0.9 | 1.4 | 1.9 | 2.2 | aarF domain containing kinase 3 | Adck3 | |
| 1387174_a_at | 1.0 | 1.5 | 1.9 | 2.0 | 1.5 | 1.8 | 2.2 | 2.2 | steroidogenic acute regulatory protein | Star | |
| muscle (50 gene probes) | 1378927_at | 0.8 | 1.0 | 1.6 | 1.6 | 1.4 | 1.5 | 1.8 | 1.8 | - | - |
| 1387053_at | 0.6 | 1.0 | 0.9 | 1.5 | 0.9 | 0.9 | 1.1 | 1.6 | flavin containing monooxygenase 1 | Fmo1 | |
| 1368971_a_at | 0.7 | 0.8 | 1.0 | 1.1 | 1.2 | 1.1 | 1.3 | 1.5 | synaptojanin 2 | Synj2 | |
| 1369150_at | 0.3 | 0.9 | 0.6 | 1.2 | 0.7 | 0.6 | 0.8 | 1.4 | pyruvate dehydrogenase kinase, isozyme 4 | Pdk4 | |
| 1370019_at | 0.7 | 0.9 | 1.2 | 1.4 | 1.0 | 1.1 | 1.2 | 1.4 | sulfotransferase family 1A member 1 | Sult1a1 | |
| brain | not identifed | ||||||||||
| intestine (47 gene probes) | 1370019_at | 1.2 | 1.3 | 1.3 | 1.8 | sulfotransferase family 1A member 1 | Sult1a1 | ||||
| 1371076_at | 1.3 | 1.1 | 2.0 | 1.8 | cytochrome P450, family 2, subfamily b, polypeptide 1///cytochrome P450, family 2, subfamily b, polypeptide 2 | Cyp2b1///Cyp2b2 | |||||
| 1368303_at | 1.3 | 1.3 | 1.6 | 1.4 | period circadian clock 2 | Per2 | |||||
| 1367774_at | 0.5 | 1.0 | 0.7 | 1.3 | glutathione S-transferase alpha 1///glutathione S-transferase alpha-3-like | Gsta1///LOC102550391 | |||||
| 1369455_at | 1.3 | 0.9 | 1.9 | 1.2 | ATP-binding cassette, subfamily G (WHITE), member 5 | Abcg5 | |||||
| 1 Week | 1 Month | Gene Title | Gene Symbol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Probe ID | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | |||
| liver (50 gene probes) | 1367707_at | −1.55 | −2.45 | −4.3 | −4.4 | −1.7 | −2.85 | −3.6 | −4.4 | fatty acid synthase | Fasn |
| 1367708_a_at | −1.2 | −2.15 | −4 | −4.3 | −1.6 | −2.75 | −3.5 | −3.9 | fatty acid synthase | Fasn | |
| 1373718_at | −1.6 | −2.9 | −3.75 | −4.05 | −2.55 | −3.15 | −3.45 | −3.4 | tubulin, beta 2A class IIa | Tubb2a | |
| 1370870_at | −1.5 | −1.9 | −2.95 | −2.95 | −1.75 | −2.25 | −2.55 | −2.85 | malic enzyme 1, NADP(+)-dependent, cytosolic | Me1 | |
| 1367854_at | −1.1 | −1.85 | −2.55 | −2.8 | −1.4 | −2 | −2.3 | −2.55 | ATP citrate lyase | Acly | |
| adipose (14 gene probes) | 1367998_at | −1.15 | −1.4 | −1.35 | −2.85 | −0.7 | −2.5 | −2.45 | −1.95 | secretory leukocyte peptidase inhibitor | Slpi |
| 1368294_at | −1.1 | −0.5 | −1.4 | −2.3 | −0.9 | −1.45 | −1.8 | −1.8 | deoxyribonuclease 1-like 3 | Dnase1l3 | |
| 1389006_at | −0.75 | −0.7 | −1.2 | −2 | −0.7 | −1.2 | −1.6 | −1.6 | macrophage expressed 1 | Mpeg1 | |
| 1368189_at | −0.9 | −0.65 | −1.45 | −1.7 | −0.9 | −0.85 | −1.3 | −1.4 | 7-dehydrocholesterol reductase | Dhcr7 | |
| 1373718_at | −0.45 | −0.8 | −0.8 | −0.95 | −0.75 | −0.95 | −0.8 | −1.15 | tubulin, beta 2A class IIa | Tubb2a | |
| muscle (51 gene probes) | 1388395_at | −0.7 | −1.7 | −2.5 | −2.9 | −2.5 | −3.5 | −3 | −2.2 | G0/G1switch 2 | G0s2 |
| 1378423_at | −0.3 | −0.9 | −1.3 | −1.6 | −1.8 | −1.7 | −1.8 | −2.1 | nicotinamide riboside kinase 2 | Nmrk2 | |
| 1379416_at | −1.1 | −1.4 | −2 | −2.2 | −1.8 | −1.5 | −2.5 | −2 | autism susceptibility candidate 2-like | LOC100362819 | |
| 1378586_at | −2.5 | −2.2 | −2.2 | −2.2 | −2 | −1.7 | −0.6 | −1.9 | cytokine inducible SH2-containing protein | Cish | |
| 1374204_at | −1.4 | −1.1 | −1.8 | −1.9 | −2.5 | −2.6 | −2.5 | −1.4 | WD repeat and SOCS box-containing 1 | Wsb1 | |
| brain (3 gene probes) | 1375043_at | −2.3 | −2.4 | −2 | −2.1 | −1.3 | −1.4 | −1.2 | −1.4 | - | --- |
| 1369067_at | −1 | −1 | −0.9 | −1.1 | −0.9 | −1.2 | −1.2 | −1.1 | nuclear receptor subfamily 4, group A, member 3 | Nr4a3 | |
| 1368321_at | −0.7 | −0.7 | −0.4 | −0.7 | −1.2 | −1.4 | −1.2 | −0.9 | early growth response 1 | Egr1 | |
| intestine (23 gene probes) | 1369717_at | −1 | −1.1 | −1.1 | −1.7 | neuromedin U | Nmu | ||||
| 1387758_at | −0.5 | −1 | −0.2 | −1.7 | alkaline phosphatase 3, intestine, not Mn requiring | Akp3 | |||||
| 1378658_at | −0.9 | −1.6 | −1 | −1.4 | chloride channel accessory 4 | Clca4 | |||||
| 1368247_at | −1.4 | −1.1 | −1.1 | −1.2 | heat shock 70kD protein 1A///heat shock 70kD protein 1B (mapped) | Hspa1a///Hspa1b | |||||
| 1389986_at | −0.7 | −0.5 | −1.1 | −1.1 | - | --- | |||||
| Probe ID | Gene Symbol | Gene Title | # of Overlapped Gene Probes | Liver | Adipose | Muscle | Brain(hypothalamus) | Intestine | |||||||||||||||||||||||||||||||
| 1 Week | 1 Month | 1 Week | 1 Month | 1 Week | 1 Month | 1 Week | 1 Month | 1 Month | |||||||||||||||||||||||||||||||
| 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | ||||
| 1390430_at | Nr1d2 | nuclear receptor subfamily 1, group D, member 2 | 29 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||
| 1370708_a_at | Akr1c14 | aldo-keto reductase family 1, member C14 | 21 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||
| 1389014_at | Nampt | nicotinamide phosphoribosyltransferase | 21 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||
| 1367633_at | Glul | glutamate-ammonia ligase | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| 1370019_at | Sult1a1 | sulfotransferase family 1A member 1 | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| 1386870_at | Glul | glutamate-ammonia ligase | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| 1386901_at | Cd36 | CD36 molecule (thrombospondin receptor) | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| 1387053_at | Fmo1 | flavin containing monooxygenase 1 | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| 1387669_a_at | Ephx1 | epoxide hydrolase 1, microsomal (xenobiotic) | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| 1387874_at | Dbp | D site of albumin promoter (albumin D-box) binding protein | 20 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||
| Probe ID | Gene Symbol | Gene Title | # of Overlapped Gene Probes | Liver | Adipose | Muscle | Brain(hypothalamus) | Intestine | |||||||||||||||||||||||||||||||
| 1 Week | 1 Month | 1 weEk | 1 Month | 1 Week | 1 Month | 1 Week | 1 Month | 1 Month | |||||||||||||||||||||||||||||||
| 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | 5% | 10% | 20% | 30% | ||||
| 1372056_at | Cmtm6 | CKLF-like MARVEL transmembrane domain containing 6 | 25 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||
| 1367979_s_at | Cyp51 | cytochrome P450, family 51 | 23 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||
| 1388850_at | Hsp90aa1 | heat shock protein 90, alpha (cytosolic), class A member 1 | 21 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||
| 1371327_a_at | Actg1 | actin, gamma 1 | 20 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||||||||
| 1367857_at | Fads1 | fatty acid desaturase 1 | 19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||
| 1371390_at | Tubb4b | tubulin, beta 4B class IVb | 19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||
| 1371653_at | Tpm4 | tropomyosin 4 | 19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||
| 1371840_at | S1pr1 | sphingosine-1-phosphate receptor 1 | 19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||
| 1372727_at | - | - | 19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||
| 1373718_at | Tubb2a | tubulin, beta 2A class IIa | 19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||||
| Gene Symbol | Function | Our Study | Ref #1 | Ref #2 |
|---|---|---|---|---|
| Nampt | NAD metabolism | up | up | |
| Glul | glutamate metabolism | up | up | |
| Sult1a1 | xenobiotic metabolism | up | up | up |
| Fmo1 | xenobiotic metabolism | up | up | |
| Dbp | circadian rhythm | up | up | down |
| Cmtm6 | immune system | down | down | |
| Hsp90aa1 | chaperone protein | down | down | |
| Actg1 | structural protein | down | down | down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, K.; Ito, M.; Chiba, T.; Jia, H.; Kato, H. A Comparison of Gene Expression Profiles of Rat Tissues after Mild and Short-Term Calorie Restrictions. Nutrients 2021, 13, 2277. https://doi.org/10.3390/nu13072277
Saito K, Ito M, Chiba T, Jia H, Kato H. A Comparison of Gene Expression Profiles of Rat Tissues after Mild and Short-Term Calorie Restrictions. Nutrients. 2021; 13(7):2277. https://doi.org/10.3390/nu13072277
Chicago/Turabian StyleSaito, Kenji, Maiko Ito, Takuya Chiba, Huijuan Jia, and Hisanori Kato. 2021. "A Comparison of Gene Expression Profiles of Rat Tissues after Mild and Short-Term Calorie Restrictions" Nutrients 13, no. 7: 2277. https://doi.org/10.3390/nu13072277
APA StyleSaito, K., Ito, M., Chiba, T., Jia, H., & Kato, H. (2021). A Comparison of Gene Expression Profiles of Rat Tissues after Mild and Short-Term Calorie Restrictions. Nutrients, 13(7), 2277. https://doi.org/10.3390/nu13072277






