Effect of Preoperative Immunonutrition on Postoperative Major Morbidity after Cytoreductive Surgery and HIPEC in Patients with Peritoneal Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Preoperative and Surgical Management
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Surgery
3.2. Immunonutrition and Postoperative Complications
3.3. CRP Values
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugarbaker, P.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat. Rev. 2016, 48, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D.; et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef]
- Elias, D.; Goéré, D.; Dumont, F.; Honoré, C.; Dartigues, P.; Stoclin, A.; Malka, D.; Boige, V.; Ducreux, M. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur. J. Cancer 2014, 50, 332–340. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef]
- Zivanovic, O.; Chi, D.S.; Filippova, O.; Randall, L.M.; Bristow, R.E.; O’Cearbhaill, R.E. It’s time to warm up to hyperthermic intraperitoneal chemotherapy for patients with ovarian cancer. Gynecol. Oncol. 2018, 151, 555–561. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus peritoneal metastases (PRODIGE 7): A multicentre, randomised, open label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Newton, A.D.; Bartlett, E.K.; Karakousis, G.C. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A review of factors contributing to morbidity and mortality. J. Gastrointest. Oncol. 2016, 7, 99–111. [Google Scholar] [PubMed]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the Treatment of Peritoneal Carcinomatosis by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Still be Regarded as a Highly Morbid Procedure? A Systematic Review of Morbidity and Mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high¬risk surgical oncology procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascales Campos, P.; Gil, J.; Parrilla, P. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with primary and recurrent advanced ovarian cancer. Eur. J. Surg. Oncol. 2014, 40, 970–975. [Google Scholar] [CrossRef]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Predictors of Severe Morbidity after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Patients with Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2016, 23, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Serious Postoperative Complications Affect Early Recurrence after Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2015, 22, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Passot, G.; Bakrin, N.; Roux, A.S.; Vaudoyer, D.; Gilly, F.N.; Glehen, O.; Cotte, E. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: A prospective study of 216 patients. EJSO 2014, 40, 529–535. [Google Scholar] [CrossRef]
- Zhu, X.; Herrera, G.; Ochoa, J.B. Immunosupression and infection after major surgery: A nutritional deficiency. Crit. Care Clin. 2010, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Büchler, M.; Farhadi, J.; Berger, D.; Hüsler, J.; Schneider, H.; Krähenbühl, S.; Krähenbühl, L. Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery-a randomized controlled pilot study. Ann. Surg. Oncol. 2007, 14, 2798–7806. [Google Scholar] [CrossRef]
- Sandrucci, S.; Beets, G.; Braga, M.; Dejong, K.; Demartines, N. Perioperative nutrition and enhanced recovery after surgery in gastrointestinal cancer patients. A position paper by the ESSO task force in collaboration with the ERAS society (ERAS coalition). Eur. J. Surg. Oncol. 2018, 44, 509–514. [Google Scholar] [CrossRef]
- Buzquurz, F.; Bojesen, R.D.; Grube, C.; Madsen, M.T.; Gögenur, I. Impact of oral preoperative and perioperative immunonutrition on postoperative infection and mortality in patients undergoing cancer surgery: Systematic review and meta-analysis with trial sequential analysis. BJS Open 2020, 4, 764–775. [Google Scholar] [CrossRef]
- Zhang, B.; Najarali, Z.; Ruo, L.; Alhusaini, A.; Solis, N.; Valencia, M.; Sanchez, M.I.P.; Serrano, P.E. Effect of Perioperative Nutritional Supplementation on Postoperative Complications—Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2019, 23, 1682–1693. [Google Scholar] [CrossRef]
- Thornblade, L.W.; Varghese, T.K., Jr.; Shi, X.; Johnson, E.K.; Bastawrous, A.; Billingham, R.P.; Thirlby, R.; Fichera, A.; Flum, D.R. Preoperative Immunonutrition and Elective Colorectal Resection Outcomes. Dis. Colon. Rectum. 2017, 60, 68–75. [Google Scholar] [CrossRef]
- Challine, A.; Rives-Langes, C.; Danoussou, D.; Katsahian, S.; Boudaoud, A.A.; Gaujoux, S.; Dousset, B.; Carette, C.; Lazzati, A.; Czernichow, S. Impact of Oral Immunonutrition on Postoperative Morbidity in Digestive Oncologic Surgery A Nation-Wide Cohort Study. Ann. Surg. 2021, 273, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Deballon, P.; Radais, F.; Facy, O.; d’Athis, P.; Masson, D.; Charles, P.E.; Cheynel, N.; Favre, J.-P.; Rat, P. C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J. Surg. 2010, 34, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.P.; Zeng, I.S.; Srinivasa, S.; Lemanu, D.P.; Connolly, A.B.; Hill, A.G. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br. J. Surg. 2014, 101, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.; Ortega-Deballon, P.; Bourredjem, A.; Doussot, A.; Giaccaglia, V.; Fournel, I. Diagnostic Accuracy of Procalcitonin and C-reactive Protein for the Early Diagnosis of Intra-abdominal Infection After Elective Colorectal Surgery. A Meta-analysis. Ann. Surg. 2016, 264, 252–256. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J. Exp. Clin. Cancer Res. 1996, 15, 49–58. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Mehta, S.S.; Gelli, M.; Agarwal, D.; Goéré, D. Complications of Cytoreductive Surgery and HIPEC in the Treatment of Peritoneal Metastases. Indian J. Surg. Oncol. 2016, 7, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moya, P.; Soriano-Irigaray, L.; Ramirez, J.M.; Garcea, A.; Blasco, O.; Blanco, F.J.; Brugiotti, C.; Miranda, E.; Arroyo, A. Perioperative Standard Oral Nutrition Supplements Versus Immunonutrition in Patients Undergoing Colorectal Resection in an Enhanced Recovery (ERAS) Protocol: A Multicenter Randomized Clinical Trial (SONVI Study). Medicine 2016, 95, e3704. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Trivax, B.; Tandon, P.; Alkam, B.; Hanouneh, I.; Steiger, E. Should perioperative immunonutrition for elective surgery be the current standard of care? Gastroenterol. Rep. 2016, 4, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Guiral, D.; Mohamed, F.; Glehen, O.; Passot, G. Prehabilitation of patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Eur. J. Surg. Oncol. 2021, 47, 60–64. [Google Scholar] [CrossRef]
- Facy, O.; Paquette, B.; Orry, D.; Brinquet, C.; Masson, D.; Bouvier, A.; Fournel, I.; Charles, P.E.; Rat, P.; Ortega-Deballon, P.; et al. Diagnostic Accuracy of Inflammatory Markers As Early Predictors of Infection After Elective Colorectal Surgery. Results from the IMACORS Study. Ann. Surg. 2015, 263, 961–966. [Google Scholar] [CrossRef] [PubMed]
- van Kooten, J.P.; Oemrawsingh, A.; de Boer, N.L.; Verhoef, C.; Burger, J.W.A.; Madsen, E.V.E.; Brandt-Kerkhof, A.R.M. Predictive Ability of C-Reactive Protein in Detecting Short-Term Complications After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Retrospective Cross-Sectional Study. Ann. Surg. Oncol. 2021, 28, 233–243. [Google Scholar] [CrossRef] [PubMed]

| Overall | No Immunonutrition | Immunonutrition | p | |
|---|---|---|---|---|
| n = 107 | n = 59 | n = 48 | ||
| Age | 60 (54–68) | 60 (52–68) | 61 (55–67) | 0.329 |
| Sex | 0.26 | |||
| Men | 28 (26%) | 18 (31%) | 10 (21%) | |
| Women | 79 (74%) | 41 (69%) | 38 (79%) | |
| ASA score | 0.579 | |||
| I | 10 (9.3%) | 7 (11.9%) | 3 (6.3%) | |
| II | 63 (58.9%) | 33 (55.9%) | 30 (62.5%) | |
| III | 34 (31.8%) | 19 (32.2%) | 15 (31.3%) | |
| Neoadjuvant therapy | 0.047 | |||
| No | 51 (48.1%) | 33 (56.9%) | 18 (37.5%) | |
| Yes | 55 (51.9%) | 25 (43.1%) | 30 (62.5%) | |
| Carcinomatosis | 0.037 | |||
| Ovarian | 53 (49.5%) | 24 (40.7%) | 29 (60.4%) | |
| Colorectal | 32 (29.9%) | 22 (37.3%) | 10 (20.8%) | |
| Pseudomyxoma | 13 (12.1%) | 9 (15.3%) | 4 (8.3%) | |
| Gastric | 2 (1.9%) | 2 (3.4%) | 0 | |
| Endometrial | 2 (1.9%) | 2 (3.4%) | 0 | |
| Mesothelioma | 1 (0.9%) | 0 | 1(2.1%) | |
| Primary | 1 (0.9%) | 0 | 1 (2.1%) | |
| Others | 3 (2.8%) | 0 | 3 (6.3%) | |
| PCI median | 8 (6–15) | 8 (5–13) | 10 (6–15) | 0.157 |
| PCI | 0.077 | |||
| 1–5 | 24 (22.4%) | 18 (30.5%) | 6 (12.5%) | |
| 6–15 | 63 (58.9%) | 32 (54.2%) | 31 (64.6%) | |
| >15 | 20 (18.7%) | 9 (15.3%) | 11 (22.9%) | |
| Visceral resections (median) | 2 (1–2) | 1 (0–2) | 2 (1–3) | 0.015 |
| Number visceral resections | 0.002 | |||
| 0 | 20 (18.7%) | 15 (25.4%) | 5 (10.4%) | |
| 1 | 31 (29%) | 23 (39%) | 8 (16.7%) | |
| 2 | 34 (31.8%) | 12 (20.3%) | 22 (45.8%) | |
| 3 or more | 22 (20.6%) | 9 (15.3%) | 13 (27.1%) | |
| Cytoreduction | 0.92 | |||
| Complete (CC0) | 94 (87.9%) | 52 (88.1%) | 42 (87.5%) | |
| Optimal (CC1) | 13 (12.1%) | 7 (11.9%) | 6 (12.5%) | |
| HIPEC | 0.006 | |||
| No | 21 (20%) | 6 (10.2%) | 15 (31.3%) | |
| Yes | 86 (80%) | 53 (89.8%) | 33 (68.8%) |
| Overall | No Immunonutrition | Immunonutrition | p | |
|---|---|---|---|---|
| n = 107 | n = 59 | n = 48 | ||
| Clavien-Dindo classification | 0.514 | |||
| Grade 0–1 | 38 (35.5%) | 22 (37.3%) | 16 (33.3%) | |
| Grade II | 41 (38.3%) | 19 (32.2%) | 22 (45.8%) | |
| Grade IIIa | 12 (11.2%) | 9 (15.3%) | 4 (8.3%) | |
| Grade IIIb | 7 (6.5%) | 2 (3.4%) | 4 (8.3%) | |
| Grade IVa | 6 (5.6%) | 5 (8.5%) | 2 (4.2%) | |
| Grade V | 2 (1.9%) | 2 (3.4%) | 0 | |
| Clavien-Dindo categories | 0.311 | |||
| No morbidity | 23 (21.5%) | 14 (23.7%) | 9 (18.8%) | |
| Minor morbidity | 56 (52.3%) | 27 (45.8%) | 29 (60.4%) | |
| Major morbidity | 28 (26.2%) | 18 (30.5%) | 10 (20.8%) | |
| Digestive leak | 0.319 | |||
| No | 95 (88.8%) | 54 (91.5%) | 41 (85.4%) | |
| Yes | 12 (11.2%) | 5 (8.5%) | 7 (14.6%) | |
| Colorectal | 1 (1.7%) | 5 (10.7%) | ||
| Small bowel | 2 (3.4%) | 1 (2.1%) | ||
| Gastric | 1 (1.7%) | |||
| Biliary | 1 (1.7%) | |||
| Pancreatic | 1 (2.1%) | |||
| Reoperation | 0.746 | |||
| No | 97 (90.7%) | 53 (89.8%) | 44 (91.7%) | |
| Yes | 10 (9.3%) | 6 (10.2%) | 4 (8.3%) | |
| Digestive leak | 3 (5.1%) | 3 (6.3%) | ||
| Hemoperitoneum | 1 (1.7%) | 1 (2.1%) | ||
| Evisceration | 2 (3.4%) | |||
| In-hospital stay | 14 (10–19) | 15 (10–19) | 14 (10–20) | 0.65 |
| No Major Morbidity | Major Morbidity | p | |
|---|---|---|---|
| n = 79 | n = 28 | ||
| Age | 59 (53–66) | 65 (59–72) | 0.119 |
| Sex | 0.736 | ||
| Men | 20 (25.3%) | 8 (28.6%) | |
| Women | 59 (74.7%) | 20 (71.4%) | |
| ASA score | 0.223 | ||
| I | 9 (11.4%) | 1 (3.6%) | |
| II | 48 (60.8%) | 15 (53.6%) | |
| III | 22 (27.8%) | 12 (42.9%) | |
| Neoadjuvant chemotherapy | 0.816 | ||
| No | 37 (47.4%) | 14 (50%) | |
| Yes | 41 (52.6%) | 14 (50%) | |
| Immunonutrition | 0.181 | ||
| No | 41 (51.9%) | 18 (64.3%) | |
| Yes | 38 (48.1%) | 10 (35.7%) | |
| Carcinomatosis | 0.487 | ||
| Ovarian | 43 (54.4%) | 10 (35.7%) | |
| Colorectal | 22 (27.8%) | 10 (35.7%) | |
| Pseudomyxoma | 9 (11.4%) | 4 (14.3%) | |
| Gastric | 1 (1.3%) | 1 (3.6) | |
| Endometrial | 1 (1.3%) | 1 (3.6%) | |
| Mesothelioma | 0 | 1(3.6%) | |
| Primary | 1 (1.3%) | 0 | |
| Others | 2 (2.5%) | 1 (3.6%) | |
| PCI median | 8 (5–13) | 11 (7–18) | 0.012 |
| PCI | 0.083 | ||
| 1–5 | 20 (25.3%) | 4 (14.3%) | |
| 6–15 | 48 (60.8%) | 15 (53.6%) | |
| >15 | 11 (13.9%) | 9 (32.1%) | |
| Visceral resections (median) | 1 (1–2) | 2 (1–3) | 0.007 |
| Number visceral resections | 0.006 | ||
| 0 | 18 (22.8%) | 2 (7.1%) | |
| 1 | 24 (30.4%) | 7 (25%) | |
| 2 | 27 (34.2%) | 7 (25%) | |
| 3 or more | 10 (12.7%) | 12 (42.9%) | |
| HIPEC | 0.165 | ||
| No | 13 (16.5%) | 8 (28.6%) | |
| Yes | 66 (83.5%) | 20 (71.4%) | |
| Preoperative CRP | 3 (1–7) | 3 (2–9) | 0.071 |
| OR (CI 95%) | p-Value | |
|---|---|---|
| Age | 1.016 (0.958–1.078) | 0.597 |
| ASA | 2.192 (0.649–7.395) | 0.206 |
| Neoadjuvant chemotherapy | 0.802 (0.256–2.513) | 0.705 |
| Immunonutrition | 0.247 (0.071–0.859) | 0.028 |
| PCI | 1.007 (0.904–1.122) | 0.897 |
| Visceral resections | 1.947 (1.086–3.488) | 0.025 |
| HIPEC | 1.044 (0.250–4.356) | 0.953 |
| Preoperative CRP | 1.025 (0.996–1.056) | 0.095 |
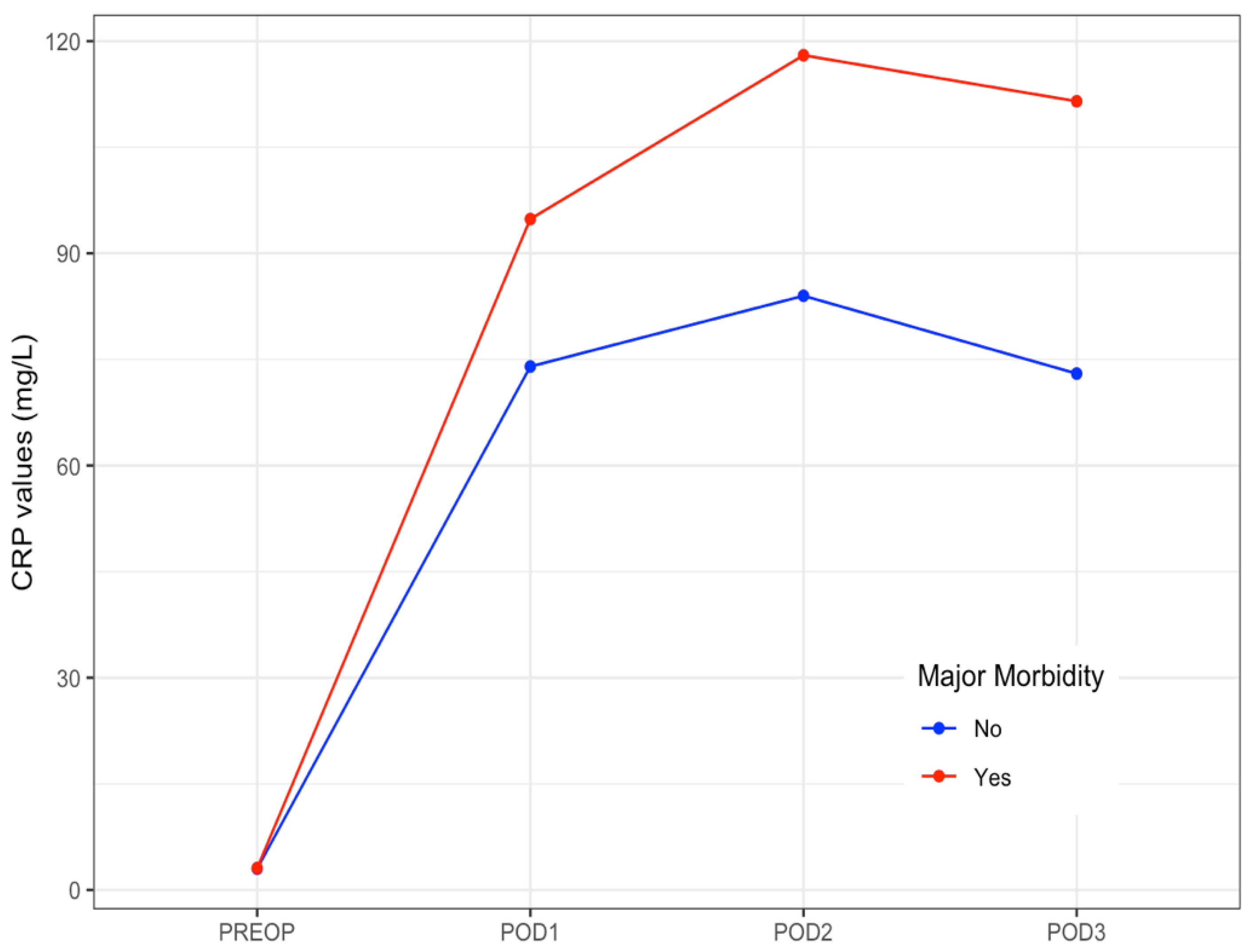
| Day | Patients | Major Morbidity | |
|---|---|---|---|
| Overall n = 107 | No n = 79 | Yes n = 28 | |
| Preoperative * | 3 (2–7) | 3 (1–7) | 3 (2–9) |
| POD 1 | 79 (58–101) | 74 (57–95) | 95 (77–121) |
| POD 2 | 89 (63–147) | 84 (59–127) | 118 (92–182) |
| POD 3 | 88 (48–141) | 73 (43–140) | 112 (61–168) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Candela, A.; Calero, A.; Sánchez-Guillén, L.; Escrig-Sos, J.; Barreras, J.A.; López-Rodríguez-Arias, F.; Armañanzas, L.; Murcia, A.; Arroyo, A.; Lacueva, F.J. Effect of Preoperative Immunonutrition on Postoperative Major Morbidity after Cytoreductive Surgery and HIPEC in Patients with Peritoneal Metastasis. Nutrients 2021, 13, 2147. https://doi.org/10.3390/nu13072147
Fernández-Candela A, Calero A, Sánchez-Guillén L, Escrig-Sos J, Barreras JA, López-Rodríguez-Arias F, Armañanzas L, Murcia A, Arroyo A, Lacueva FJ. Effect of Preoperative Immunonutrition on Postoperative Major Morbidity after Cytoreductive Surgery and HIPEC in Patients with Peritoneal Metastasis. Nutrients. 2021; 13(7):2147. https://doi.org/10.3390/nu13072147
Chicago/Turabian StyleFernández-Candela, Alba, Alicia Calero, Luís Sánchez-Guillén, Javier Escrig-Sos, José A. Barreras, Francisco López-Rodríguez-Arias, Laura Armañanzas, Ana Murcia, Antonio Arroyo, and Francisco Javier Lacueva. 2021. "Effect of Preoperative Immunonutrition on Postoperative Major Morbidity after Cytoreductive Surgery and HIPEC in Patients with Peritoneal Metastasis" Nutrients 13, no. 7: 2147. https://doi.org/10.3390/nu13072147
APA StyleFernández-Candela, A., Calero, A., Sánchez-Guillén, L., Escrig-Sos, J., Barreras, J. A., López-Rodríguez-Arias, F., Armañanzas, L., Murcia, A., Arroyo, A., & Lacueva, F. J. (2021). Effect of Preoperative Immunonutrition on Postoperative Major Morbidity after Cytoreductive Surgery and HIPEC in Patients with Peritoneal Metastasis. Nutrients, 13(7), 2147. https://doi.org/10.3390/nu13072147






