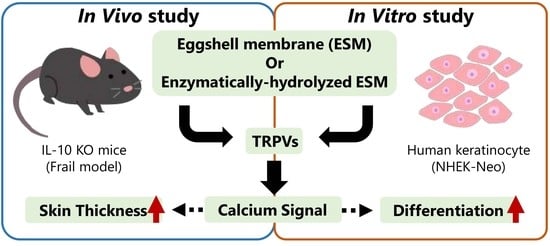
Effects of Eggshell Membrane on Keratinocyte Differentiation and Skin Aging In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
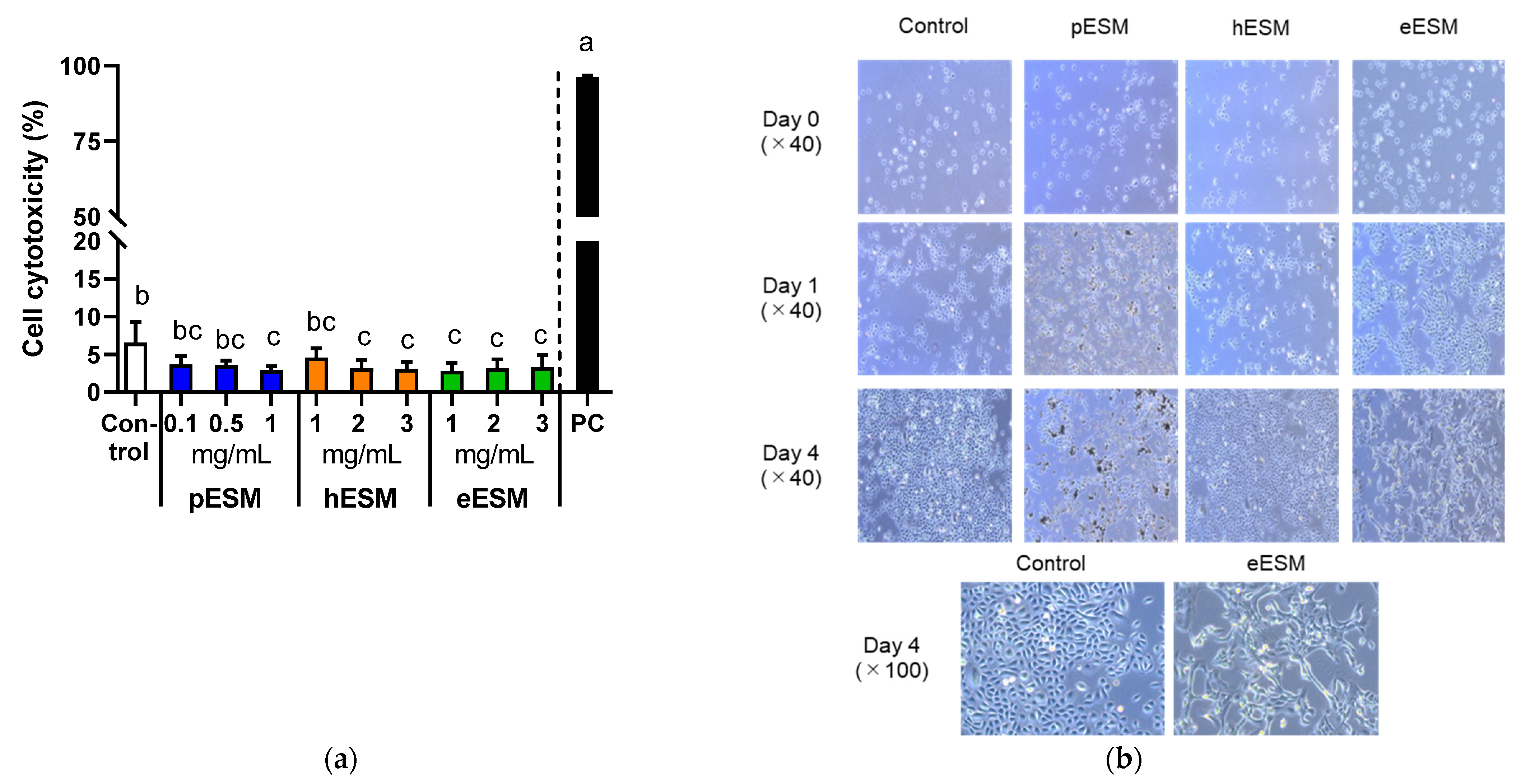
2.2. Determination of Cell Cytotoxicity
2.3. Morphological Changes
2.4. Animal Experiment
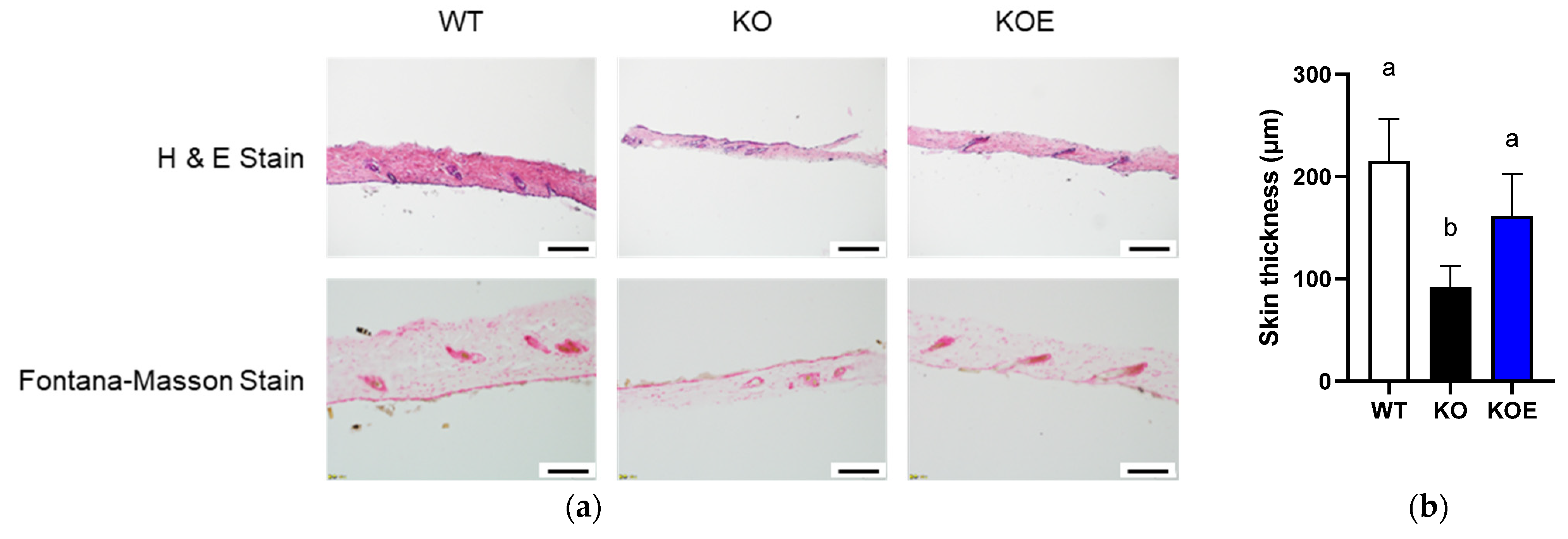
2.5. Morphological Analysis
2.6. RNA Extraction from Cell and Tissue Samples
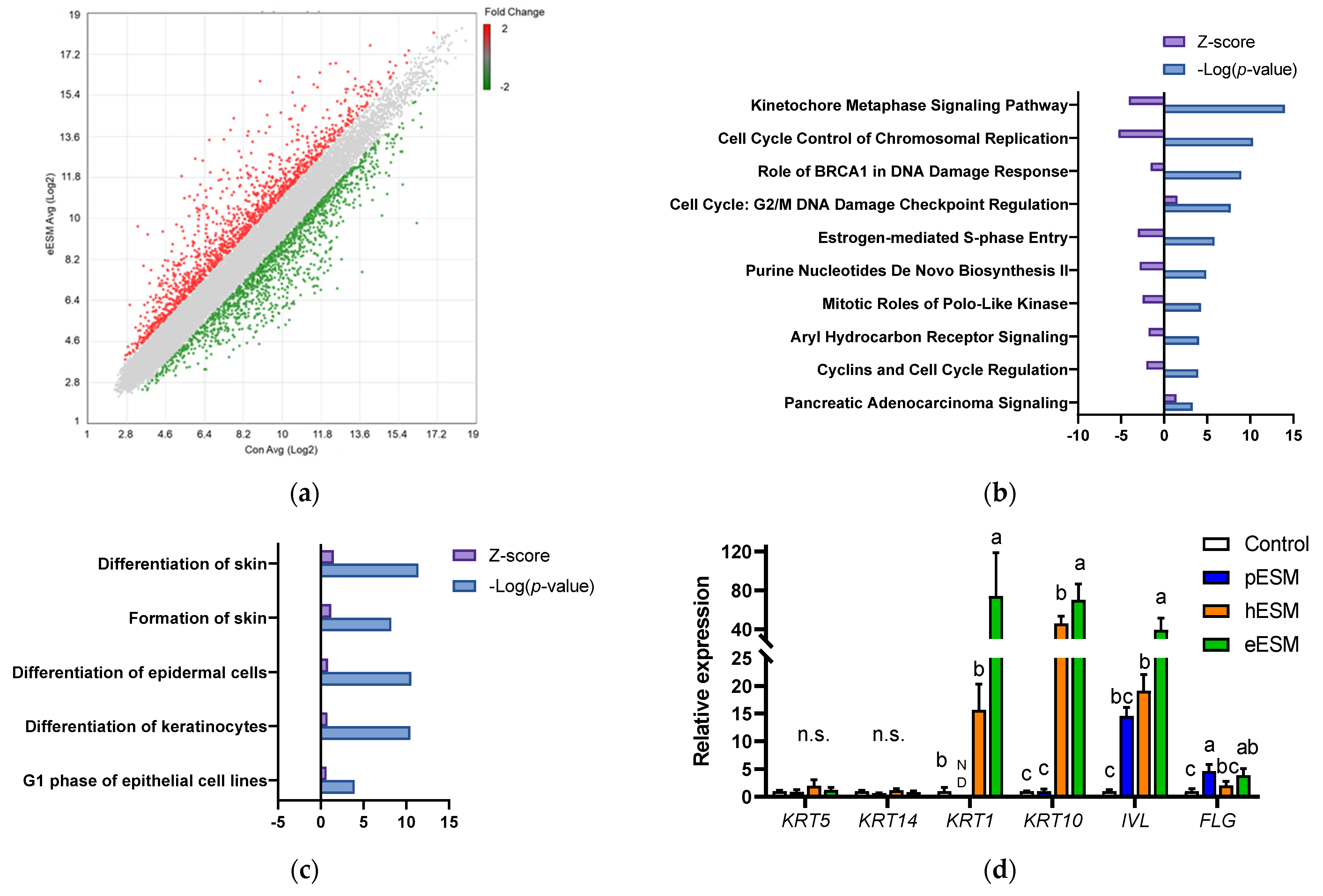
2.7. DNA Microarray Analysis
2.8. Real-Time Reverse Transcription-Polymerase Chain Reaction (Real Time RT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Effects of Different Types of ESM on Cytotoxicity and Cell Morphology
3.2. Microarray Analysis in Cell Culture Experiments
3.3. Possible Involvement of Calcium Signaling in eESM-Induced Keratinocyte Differentiation
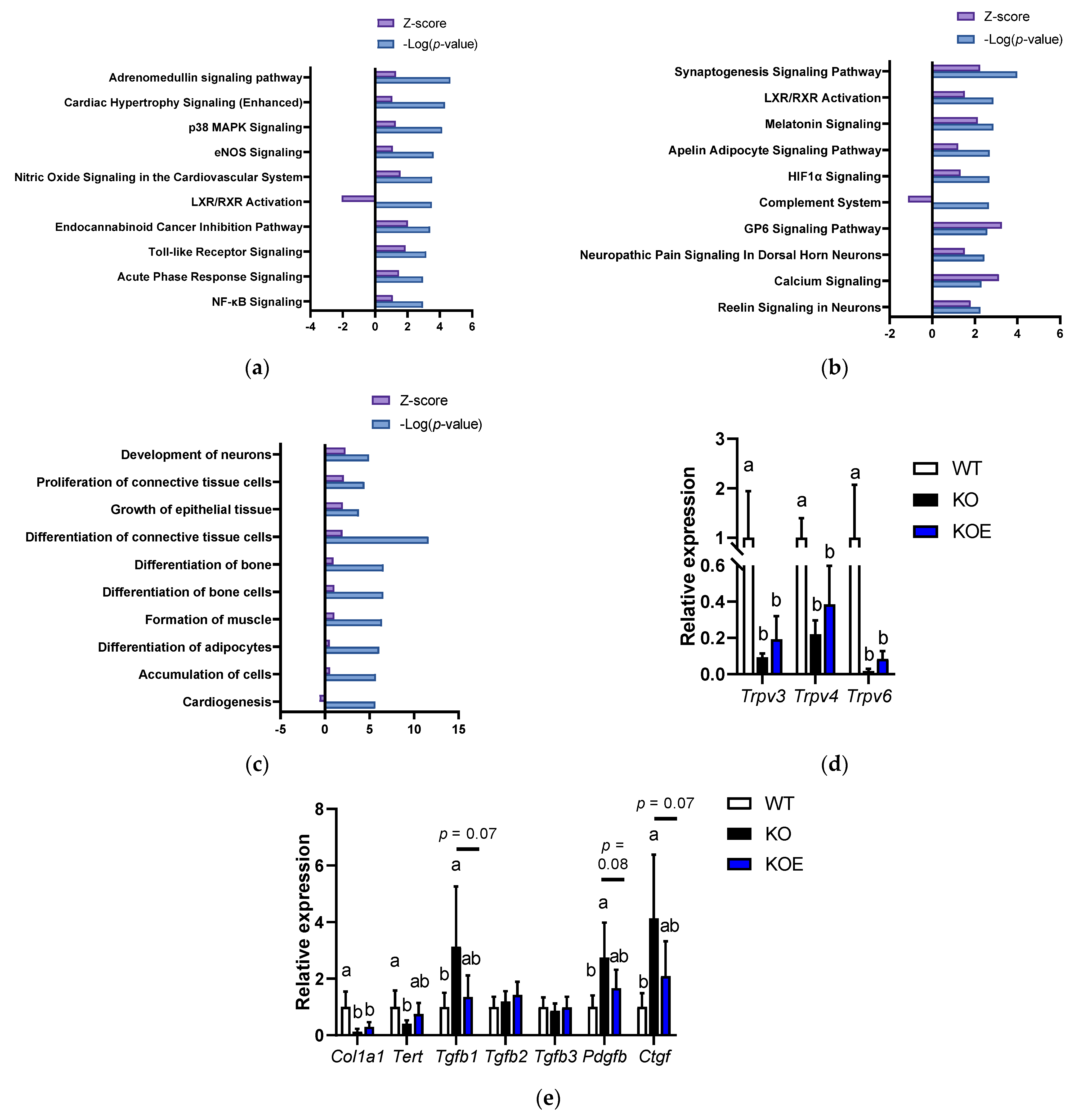
3.4. Effects of pESM Supplementation on Skin Aging in IL-10 KO Mice
3.5. Microarray Analysis in pESM-Supplemented IL-10 KO Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foroushani, A.R.; Estebsari, F.; Mostafaei, D.; Ardebili, H.E.; Shojaeizadeh, D.; Dastoorpoor, M.; Jamshidi, E.; Taghdisi, M.H. The Effect of Health Promoting Intervention on Healthy Lifestyle and Social Support in Elders: A Clinical Trial Study. Iran. Red Crescent Med. J. 2014, 16, e18399. [Google Scholar] [CrossRef] [Green Version]
- Catic, A. Cellular Metabolism and Aging. Progress Mol. Biol. Transl. Sci. 2018, 155, 85–107. [Google Scholar] [CrossRef]
- Rae, M.J.; Butler, R.N.; Campisi, J.; De Grey, A.D.N.J.; Finch, C.E.; Gough, M.; Martin, G.M.; Vijg, J.; Perrott, K.M.; Logan, B.J. The Demographic and Biomedical Case for Late-Life Interventions in Aging. Sci. Transl. Med. 2010, 2, 40cm21. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [Green Version]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.J.; DeVore, D.P.; Leu, M.D.; Robinson, M.A. Eggshell membrane: A possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin. Interv. Aging 2009, 4, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Hanate, M.; Aw, W.; Itoh, H.; Saito, K.; Kobayashi, S.; Hachimura, S.; Fukuda, S.; Tomita, M.; Hasebe, Y.; et al. Eggshell membrane powder ameliorates intestinal inflammation by facilitating the restitution of epithelial injury and alleviating microbial dysbiosis. Sci. Rep. 2017, 7, 43993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramli, N.S.; Jia, H.; Sekine, A.; Lyu, W.; Furukawa, K.; Saito, K.; Hasebe, Y.; Kato, H. Eggshell membrane powder lowers plasma triglyceride and liver total cholesterol by modulating gut microbiota and accelerating lipid metabolism in high-fat diet-fed mice. Food Sci. Nutr. 2020, 8, 2512–2523. [Google Scholar] [CrossRef] [Green Version]
- Ruff, K.J.; Endres, J.R.; Clewell, A.E.; Szabo, J.R.; Schauss, A. Safety evaluation of a natural eggshell membrane-derived product. Food Chem. Toxicol. 2012, 50, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Dresner-Pollak, R.; Gelb, N.; Rachmilewitz, D.; Karmeli, F.; Weinreb, M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 2004, 127, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Yu, Q.; Xue, Q.-L.; Yao, W.; Brayton, C.; Yang, H.; Fedarko, N.; Walston, J. Inflammation and mortality in a frail mouse model. AGE 2011, 34, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Fedarko, N.; Yang, H.; Leng, S.; Beamer, B.; Espinoza, S.; Lipton, A.; Zheng, H.; Becker, K. The Physical and Biological Characterization of a Frail Mouse Model. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, R.M.; Le Yang, H.; Langdon, J.M.; Roy, C.N.; Kim, J.A.; Choudhury, P.P.; Xue, Q.-L.; Di Francesco, A.; De Cabo, R.; Walston, J. Aged interleukin-10tm1Cgn chronically inflamed mice have substantially reduced fat mass, metabolic rate, and adipokines. PLoS ONE 2017, 12, e0186811. [Google Scholar] [CrossRef] [PubMed]
- Elsholz, F.; Harteneck, C.; Muller, W.; Friedland, K. Calcium—A central regulator of keratinocyte differentiation in health and disease. Eur. J. Dermatol. EJD 2014, 24, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D.D.; Xie, Z.; Tu, C.-L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N.D.P. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Ski. Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef]
- Bikkavilli, R.K.; Avasarala, S.; Van Scoyk, M.; Arcaroli, J.; Brzezinski, C.; Zhang, W.; Edwards, M.G.; Rathinam, M.K.K.; Zhou, T.; Tauler, J.; et al. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene 2015, 34, 5317–5328. [Google Scholar] [CrossRef] [Green Version]
- Makrantonaki, E.; Brink, T.C.; Zampeli, V.; Elewa, R.M.; Mlody, B.; Hossini, A.M.; Hermes, B.; Krause, U.; Knolle, J.; Abdallah, M.; et al. Identification of Biomarkers of Human Skin Ageing in Both Genders. Wnt Signalling—A Label of Skin Ageing? PLoS ONE 2012, 7, e50393. [Google Scholar] [CrossRef]
- Kubo, C.; Ogawa, M.; Uehara, N.; Katakura, Y. Fisetin Promotes Hair Growth by Augmenting TERT Expression. Front. Cell Dev. Biol. 2020, 8, 566617. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.; Friman, T.; Kowanetz, M.; van Wieringen, T.; Gustafsson, R.; Sundberg, C. Phenotypical Differences in Connective Tissue Cells Emerging from Microvascular Pericytes in Response to Overexpression of PDGF-B and TGF-β1 in Normal Skin in Vivo. Am. J. Pathol. 2013, 182, 2132–2146. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Takigawa, M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin. Sci. 2015, 128, 181–196. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Streubel, M.K.; Bischof, J.; Richter, K. Skin aging, gene expression and calcium. Exp. Gerontol. 2015, 68, 59–65. [Google Scholar] [CrossRef]
- Streubel, M.K.; Neuhofer, C.; Bischof, J.; Steinbacher, P.; Russe, E.; Wechselberger, G.; Richter, K.; Rinnerthaler, M. From Mice to Men: An Evolutionary Conserved Breakdown of the Epidermal Calcium Gradient and Its Impact on the Cornified Envelope. Cosmetics 2018, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef]
- Pillai, S.; Bikle, D.D.; Hincenbergs, M.; Elias, P.M. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J. Cell. Physiol. 1988, 134, 229–237. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Wu, J. Dietary peptides in aging: Evidence and prospects. Food Sci. Hum. Wellness 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; De Cabo, R. Measuring biological aging in humans: A quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef] [PubMed]

| Component (%) | Control Diet | 8% pESM Supplemented Diet |
|---|---|---|
| Casein | 20.0 | 16.3 |
| β-corn starch | 39.7 | 35.4 |
| α-corn starch | 13.2 | 13.2 |
| Soybean oil | 7.0 | 7.0 |
| Sucrose | 10.3 | 10.3 |
| Cellulose | 5.0 | 5.0 |
| Vitamin mixture * | 1.0 | 1.0 |
| Mineral mixture * | 3.5 | 3.5 |
| L-cystine | 0.3 | 0.3 |
| ESM powder | 0.0 | 8.0 |
| Gene Symbol | Gene Name | Fold Change (eESM vs. Control) |
|---|---|---|
| ALOX15B | Arachidonate 15-lipoxygenase type b | 2.01 |
| CDH1 | Cadherin 1 | 2.17 |
| CDSN | Corneodesmosin | 48.29 |
| CST6 | Cystatin e/m | 5.15 |
| CTSV | Cathepsin V | 6.69 |
| CYLD | Cyld lysine 63 deubiquitinase | 2.65 |
| DLX3 | Distal-less homeobox 3 | 2.08 |
| DSG1 | Desmoglein 1 | 15.85 |
| FLG | Filaggrin | 2.77 |
| HBP1 | HMG-box transcription factor 1 | 8.34 |
| IVL | Involucrin | 24.62 |
| KLF4 | Kruppel-like factor 4 | 3.90 |
| KLK5 | Kallikrein-related peptidase 5 | 22.74 |
| KLK7 | Kallikrein-related peptidase 7 | 39.73 |
| KRT1 | Keratin 1 | 2.37 |
| KRT13 | Keratin 13 | 78.42 |
| NCOA3 | Nuclear receptor coactivator 3 | 3.28 |
| PPL | Periplakin | 18.86 |
| SPINK5 | Serine protease inhibitor kazal type-5 | 6.77 |
| TGM1 | Transglutaminase 1 | 5.14 |
| TMEM79 | Transmembrane protein 79 | 3.11 |
| Gene Symbol | Gene Name | Fold Change (eESM vs. Control) |
|---|---|---|
| Cacna1a | Calcium voltage-gated channel subunit alpha1 A | 1.87 |
| Cacna1d | Calcium voltage-gated channel subunit alpha1 D | 1.59 |
| Cacna1g | Calcium voltage-gated channel subunit alpha1 G | 1.66 |
| Camk1d | Calcium calmodulin-dependent protein kinase ID | 1.75 |
| Camk2a | Calcium calmodulin-dependent protein kinase II alpha | 1.61 |
| Casq1 | calsequestrin 1 | 5.29 |
| Creb3l4 | cAMP responsive element binding protein 3-like 4 | 2.10 |
| Micu1 | Mitochondrial calcium uptake 1 | 1.57 |
| Prkacb | protein kinase cAMP-activated catalytic subunit beta | 1.53 |
| Prkar1b | Protein kinase cAMP-dependent type I regulatory subunit beta | 1.55 |
| Prkar2b | Protein kinase cAMP-dependent type II regulatory subunit beta | 1.59 |
| Trpc3 | Transient receptor potential cation channel subfamily C member 3 | 1.84 |
| Trpv6 | Transient receptor potential cation channel subfamily V member 6 | 1.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, K.; Kono, M.; Kataoka, T.; Hasebe, Y.; Jia, H.; Kato, H. Effects of Eggshell Membrane on Keratinocyte Differentiation and Skin Aging In Vitro and In Vivo. Nutrients 2021, 13, 2144. https://doi.org/10.3390/nu13072144
Furukawa K, Kono M, Kataoka T, Hasebe Y, Jia H, Kato H. Effects of Eggshell Membrane on Keratinocyte Differentiation and Skin Aging In Vitro and In Vivo. Nutrients. 2021; 13(7):2144. https://doi.org/10.3390/nu13072144
Chicago/Turabian StyleFurukawa, Kyohei, Masaya Kono, Tetsuro Kataoka, Yukio Hasebe, Huijuan Jia, and Hisanori Kato. 2021. "Effects of Eggshell Membrane on Keratinocyte Differentiation and Skin Aging In Vitro and In Vivo" Nutrients 13, no. 7: 2144. https://doi.org/10.3390/nu13072144
APA StyleFurukawa, K., Kono, M., Kataoka, T., Hasebe, Y., Jia, H., & Kato, H. (2021). Effects of Eggshell Membrane on Keratinocyte Differentiation and Skin Aging In Vitro and In Vivo. Nutrients, 13(7), 2144. https://doi.org/10.3390/nu13072144