Caloric Restriction Prevents Metabolic Dysfunction and the Changes in Hypothalamic Neuropeptides Associated with Obesity Independently of Dietary Fat Content in Rats
Abstract
1. Introduction
2. Materials and Methods
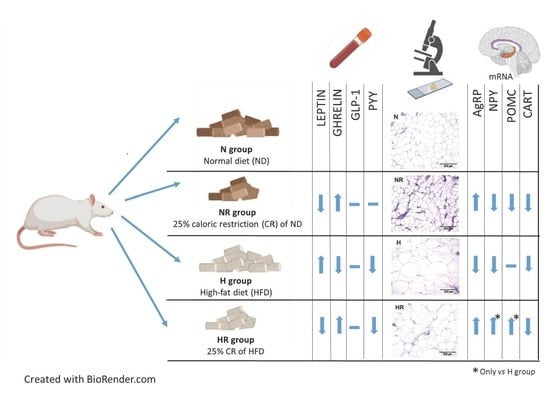
2.1. Experimental Animals and Study Design
2.2. Body Weight, Body Composition and Food Efficiency Ratio
2.3. Blood Measurements
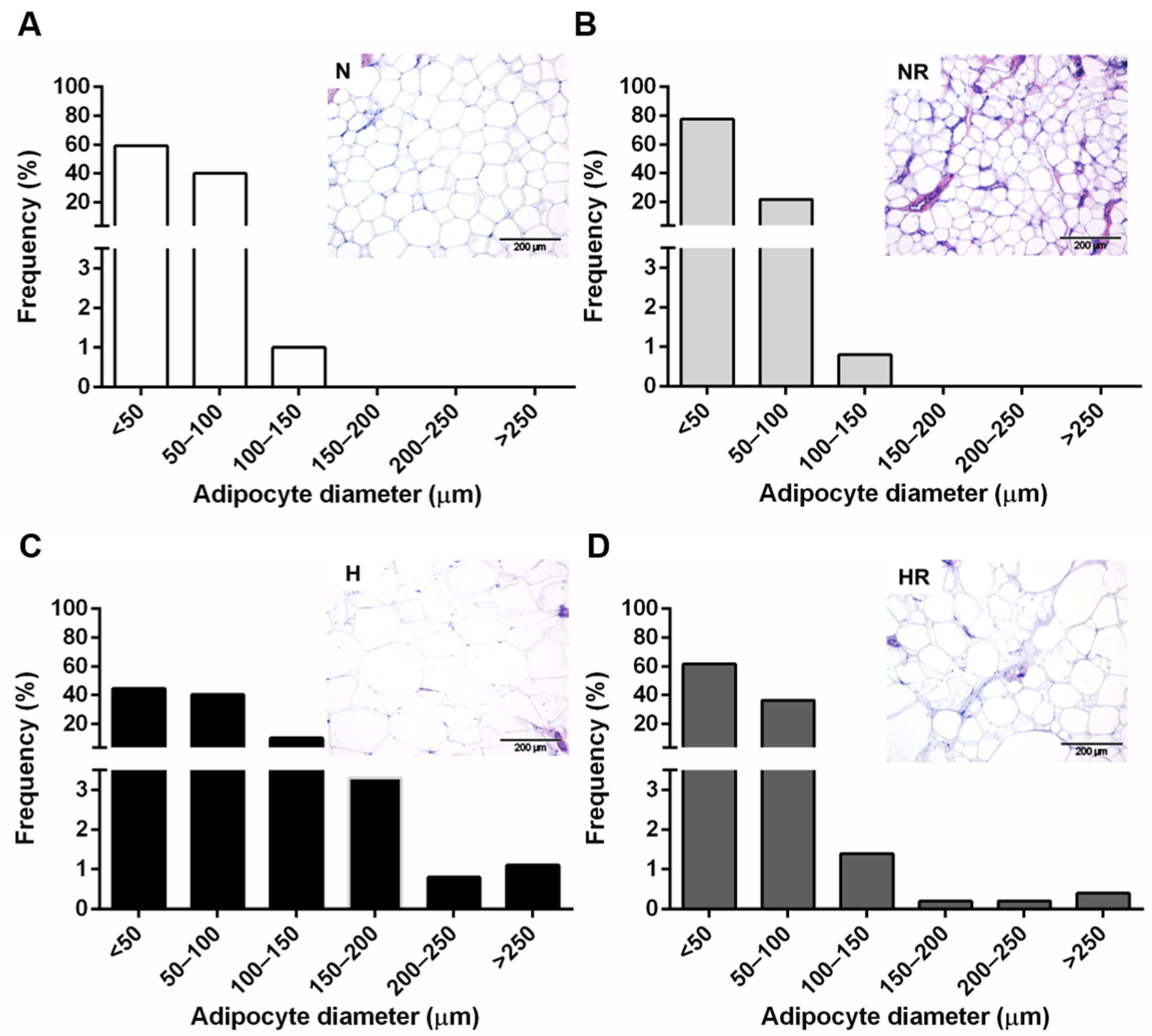
2.4. Histological Analyses
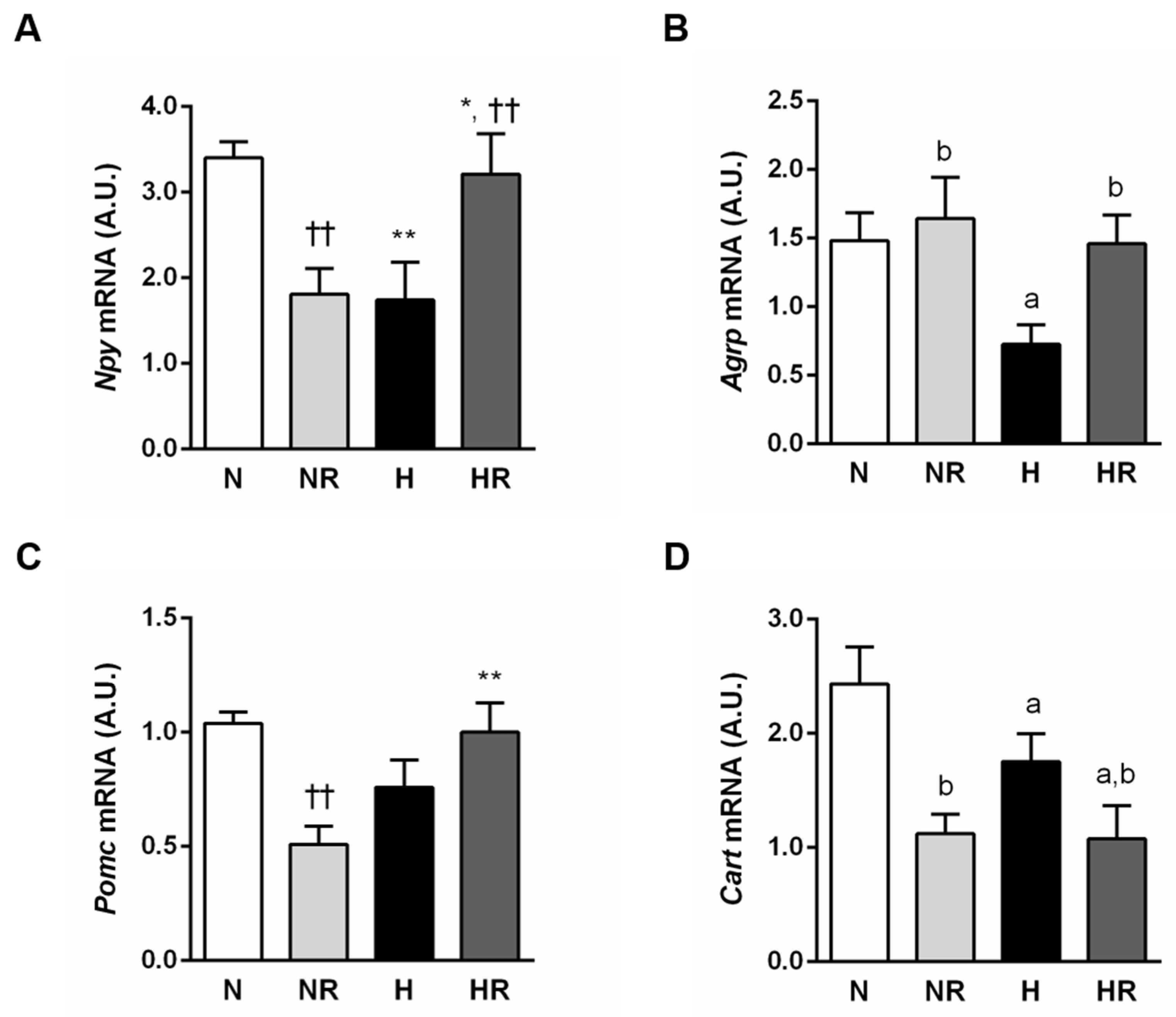
2.5. In Situ Hybridization for Hypothalamic Neuropeptides
2.6. Statistical Analysis
3. Results
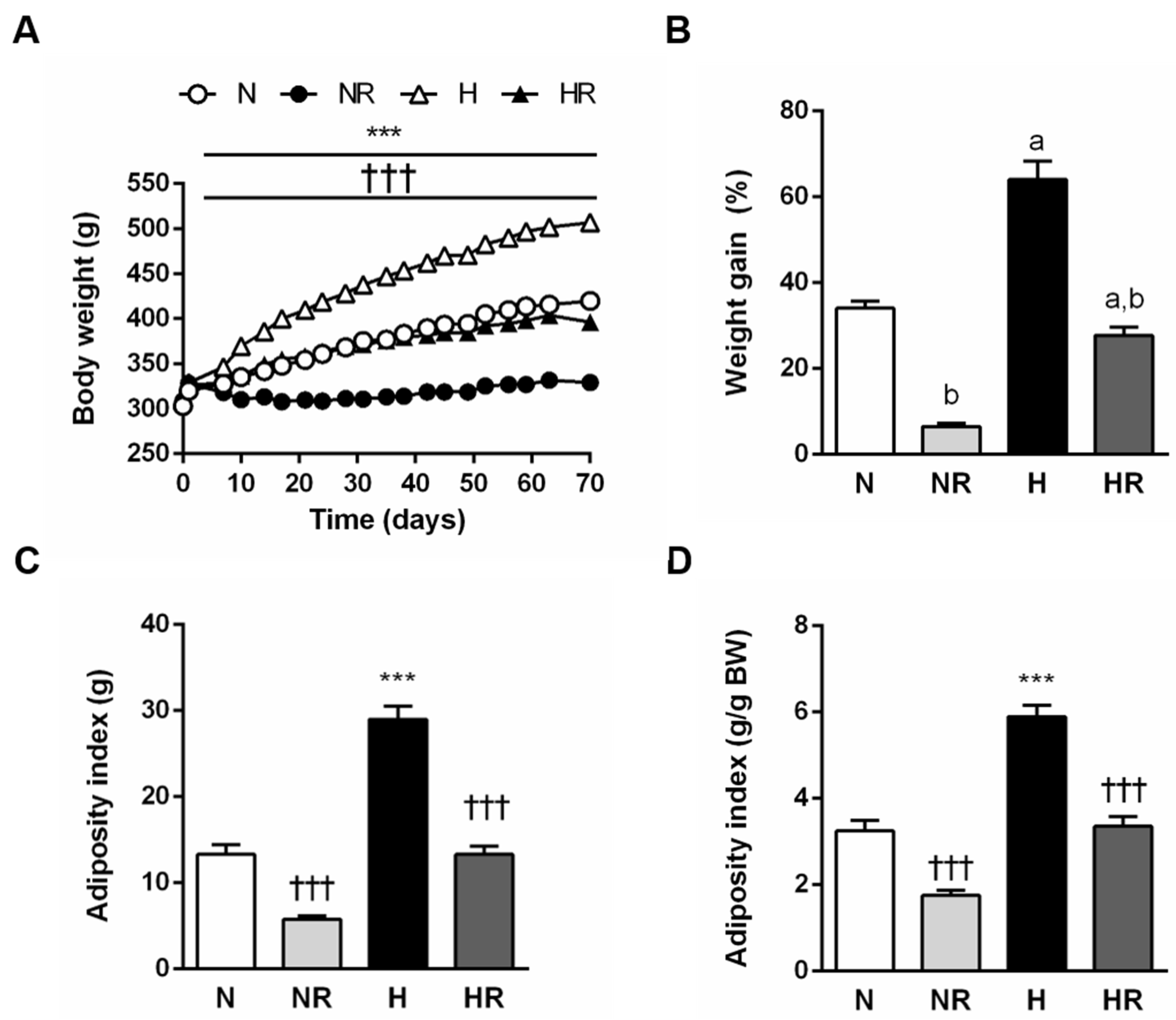
3.1. Caloric Restriction Improved the Obese Phenotype even in Rats Fed a HFD
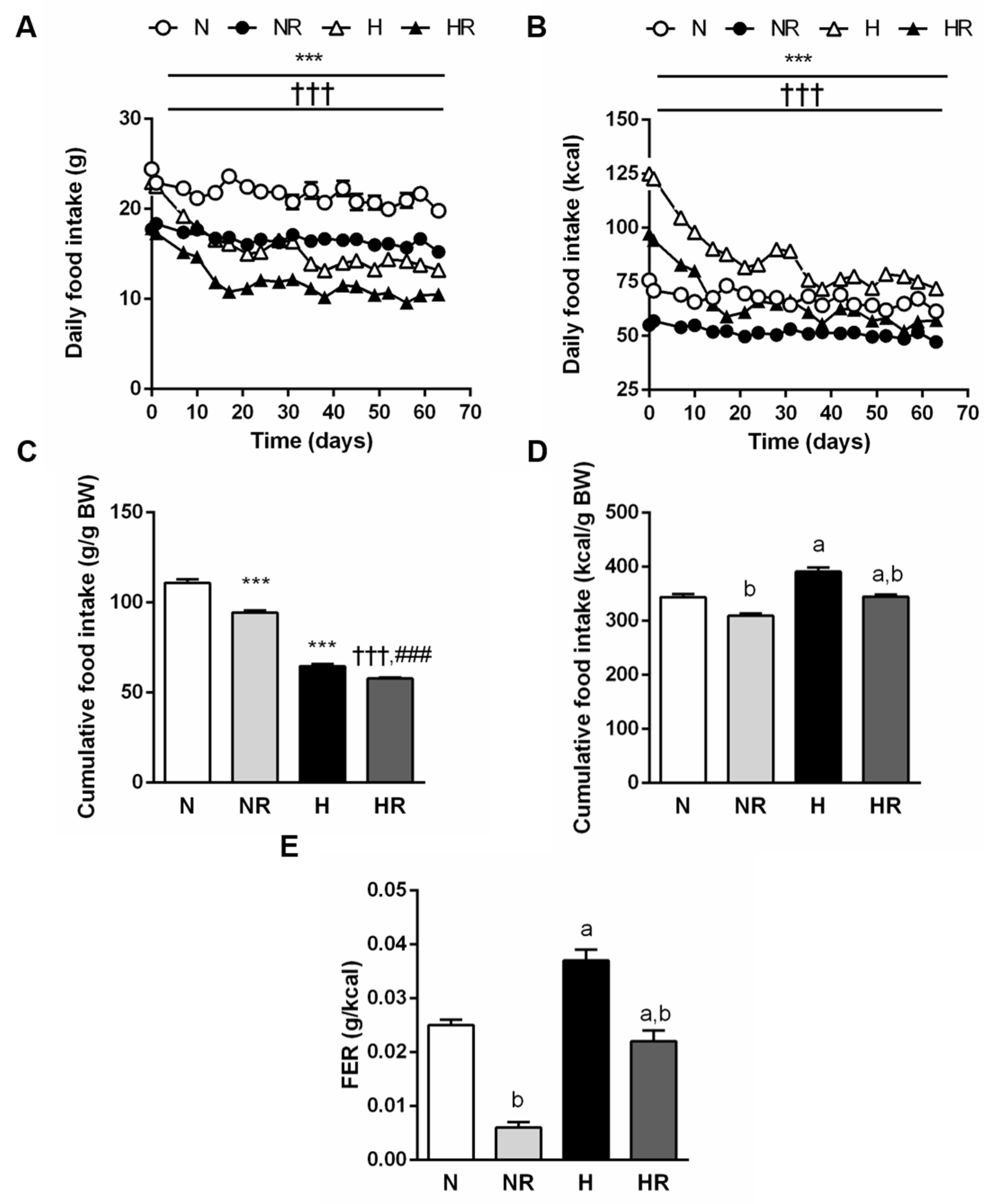
3.2. Caloric Restriction Reduced Food Efficiency Independently of the Type of Diet
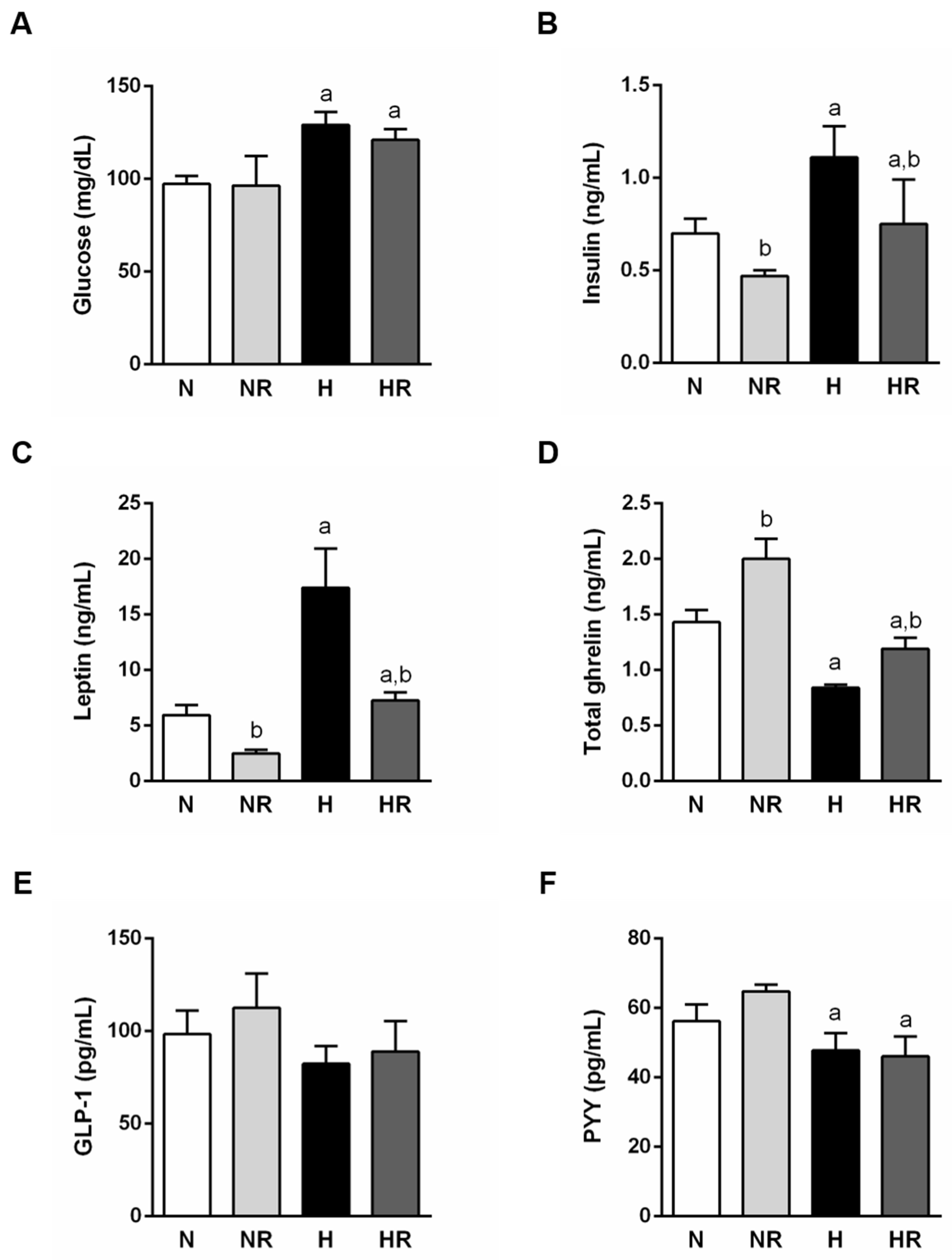
3.3. Caloric Restriction Ameliorated Metabolic Profile even in Rats Fed a HFD
3.4. Caloric Restriction Ameliorated Metabolic Profile even in Rats Fed a HFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health effects of overweight and obesity in 195 Countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Ezquerro, S.; Rodríguez, A.; Portincasa, P.; Frühbeck, G. Effects of diets on adipose tissue. Curr. Med. Chem. 2019, 26, 3593–3612. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate-high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic control of longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef]
- Theilade, S.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. An overview of obesity mechanisms in humans: Endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes. Metab. 2021, 23 (Suppl. 1), 17–35. [Google Scholar] [CrossRef] [PubMed]
- Beutler, L.R.; Chen, Y.; Ahn, J.S.; Lin, Y.C.; Essner, R.A.; Knight, Z.A. Dynamics of gut-brain communication underlying hunger. Neuron 2017, 96, 461–475.e5. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef]
- Al Massadi, O.; López, M.; Tschöp, M.; Diéguez, C.; Nogueiras, R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends Neurosci. 2017, 40, 167–180. [Google Scholar] [CrossRef]
- López, M.; Nogueiras, R.; Tena-Sempere, M.; Diéguez, C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016, 12, 421–432. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N.; Seoane-Collazo, P.; Contreras, C.; Varela, L.; Villarroya, J.; Rial-Pensado, E.; Buque, X.; Aurrekoetxea, I.; Delgado, T.C.; Vázquez-Martínez, R.; et al. Hypothalamic AMPK-ER Stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017, 26, 212–229.e12. [Google Scholar] [CrossRef]
- Thom, G.; Lean, M. Is there an optimal diet for weight management and metabolic health? Gastroenterology 2017, 152, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Montani, J.P. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: An overview Introduction. Obes. Rev. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Briggs, D.I.; Lockie, S.H.; Wu, Q.; Lemus, M.B.; Stark, R.; Andrews, Z.B. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology 2013, 154, 709–717. [Google Scholar] [CrossRef]
- Lewis, H.B.; Ahern, A.L.; Solis-Trapala, I.; Walker, C.G.; Reimann, F.; Gribble, F.M.; Jebb, S.A. Effect of reducing portion size at a compulsory meal on later energy intake, gut hormones, and appetite in overweight adults. Obesity 2015, 23, 1362–1370. [Google Scholar] [CrossRef]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Boozer, C.N.; Schoenbach, G.; Atkinson, R.L. Dietary fat and adiposity: A dose-response relationship in adult male rats fed isocalorically. Am. J. Physiol. 1995, 268, E546–E550. [Google Scholar] [CrossRef]
- Boozer, C.N.; Brasseur, A.; Atkinson, R.L. Dietary fat affects weight loss and adiposity during energy restriction in rats. Am. J. Clin. Nutr. 1993, 58, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, G.H.; Choi, H.I.; Ryu, J.; Jung, C.Y.; Lee, W. Calorie restriction improves whole-body glucose disposal and insulin resistance in association with the increased adipocyte-specific GLUT4 expression in Otsuka Long-Evans Tokushima fatty rats. Arch. Biochem. Biophys. 2005, 436, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Voy, B.H.; Saxton, A.M.; Moustaid-Moussa, N. Energy-restricted high-fat diets only partially improve markers of systemic and adipose tissue inflammation. Obesity 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Eriksson-Hogling, D.; Andersson, D.P.; Backdahl, J.; Hoffstedt, J.; Rössner, S.; Thorell, A.; Arner, E.; Arner, P.; Rydén, M. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int. J. Obes. 2015, 39, 893–898. [Google Scholar] [CrossRef]
- Heinonen, S.; Saarinen, L.; Naukkarinen, J.; Rodriguez, A.; Fruhbeck, G.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; Moilanen, E.; et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int. J. Obes. 2014, 38, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, I.; Ryden, M.; Arner, P. Family history of diabetes is associated with enhanced adipose lipolysis: Evidence for the implication of epigenetic factors. Diabetes Metab. 2018, 44, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, M.; Svensson, M.; Lindmark, S.; Renström, F.; Ruge, T.; Eriksson, J.W. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia 2007, 50, 625–633. [Google Scholar] [CrossRef]
- Arner, P.; Bernard, S.; Appelsved, L.; Fu, K.Y.; Andersson, D.P.; Salehpour, M.; Thorell, A.; Ryden, M.; Spalding, K.L. Adipose lipid turnover and long-term changes in body weight. Nat. Med. 2019, 25, 1385–1389. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Ferno, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Parker, J.A.; McCullough, K.A.; Field, B.C.T.; Minnion, J.S.; Martin, N.M.; Ghatei, M.A.; Bloom, S.R. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int. J. Obes. 2013, 37, 1391–1398. [Google Scholar] [CrossRef]
- Méndez-Giménez, L.; Becerril, S.; Camões, S.P.; da Silva, I.V.; Rodrigues, C.; Moncada, R.; Valentí, V.; Catalán, V.; Gómez-Ambrosi, J.; Miranda, J.P.; et al. Role of aquaporin-7 in ghrelin- and GLP-1-induced improvement of pancreatic beta-cell function after sleeve gastrectomy in obese rats. Int. J. Obes. 2017, 41, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, K.; Gelegen, C.; Karra, E.; Choudhury, A.I.; Drew, M.E.; Fauveau, V.; Viollet, B.; Andreelli, F.; Withers, D.J.; Batterham, R.L. Diet and gastrointestinal bypass-induced weight loss: The roles of ghrelin and peptide YY. Diabetes 2011, 60, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Batterham, R.L.; Aylwin, S.J.; Patterson, M.; Borg, C.M.; Wynne, K.J.; Kent, A.; Vincent, R.P.; Gardiner, J.; Ghatei, M.A.; et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006, 147, 3–8. [Google Scholar] [CrossRef]
- Sam, A.H.; Gunner, D.J.; King, A.; Persaud, S.J.; Brooks, L.; Hostomska, K.; Ford, H.E.; Liu, B.; Ghatei, M.A.; Bloom, S.R.; et al. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology 2012, 143, 459–468. [Google Scholar] [CrossRef]
- Shen, W.J.; Yao, T.; Kong, X.; Williams, K.W.; Liu, T. Melanocortin neurons: Multiple routes to regulation of metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2477–2485. [Google Scholar] [CrossRef]
- Plum, L.; Ahmed, L.; Febres, G.; Bessler, M.; Inabnet, W.; Kunreuther, E.; McMahon, D.J.; Korner, J. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity 2011, 19, 2149–2157. [Google Scholar] [CrossRef]
- Foster-Schubert, K.E.; Overduin, J.; Prudom, C.E.; Liu, J.; Callahan, H.S.; Gaylinn, B.D.; Thorner, M.O.; Cummings, D.E. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 2008, 93, 1971–1979. [Google Scholar] [CrossRef]
- Orr, J.; Davy, B. Dietary influences on peripheral hormones regulating energy intake: Potential applications for weight management. J. Am. Diet. Assoc. 2005, 105, 1115–1124. [Google Scholar] [CrossRef]
- Little, T.J.; Feinle-Bisset, C. Effects of dietary fat on appetite and energy intake in health and obesity—Oral and gastrointestinal sensory contributions. Physiol. Behav. 2011, 104, 613–620. [Google Scholar] [CrossRef]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Unamuno, X.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G. Functional relationship between leptin and nitric oxide in metabolism. Nutrients 2019, 11, 2129. [Google Scholar] [CrossRef]
- Ellis, A.C.; Chandler-Laney, P.; Casazza, K.; Goree, L.L.; McGwin, G.; Gower, B.A. Circulating ghrelin and GLP-1 are not affected by habitual diet. Regul. Pept. 2012, 176, 1–5. [Google Scholar] [CrossRef]
- Al Massadi, O.; Pardo, M.; Roca-Rivada, A.; Castelao, C.; Casanueva, F.F.; Seoane, L.M. Macronutrients act directly on the stomach to regulate gastric ghrelin release. J. Endocrinol. Investig. 2010, 33, 599–602. [Google Scholar] [CrossRef]
- Koliaki, C.; Kokkinos, A.; Tentolouris, N.; Katsilambros, N. The effect of ingested macronutrients on postprandial ghrelin response: A critical review of existing literature data. Int. J. Pept. 2010. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, M.W.; Spanswick, D.C. Starving for ghrelin. Cell Metab. 2014, 20, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McFarlane, M.R.; Brown, M.S.; Goldstein, J.L.; Zhao, T.J. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Zhao, T.J.; Li, R.L.; Sherbet, D.P.; Liang, G.; Brown, M.S. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, P.S.; Upton, R.; Henderson, L.; Buckingham, R.; Wilson, S.; Williams, G. Reciprocal regional changes in brain NPY receptor density during dietary restriction and dietary-induced obesity in the rat. Brain Res. 1997, 774, 1–10. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, C.; Huang, X.F. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav. Brain Res. 2009, 205, 50–56. [Google Scholar] [CrossRef]
- Kristensen, P.; Judge, M.E.; Thim, L.; Ribel, U.; Christjansen, K.N.; Wulff, B.S.; Clausen, J.T.; Jensen, P.B.; Madsen, O.D.; Vrang, N.; et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998, 393, 72–76. [Google Scholar] [CrossRef]
- Alen, F.; Crespo, I.; Ramirez-Lopez, M.T.; Jagerovic, N.; Goya, P.; de Fonseca, F.R.; de Heras, R.G.; Orio, L. Ghrelin-induced orexigenic effect in rats depends on the metabolic status and is counteracted by peripheral CB1 receptor antagonism. PLoS ONE 2013, 8, e60918. [Google Scholar] [CrossRef]
- Banke, E.; Riva, M.; Shcherbina, L.; Wierup, N.; Degerman, E. Cocaine- and amphetamine-regulated transcript is expressed in adipocytes and regulate lipid- and glucose homeostasis. Regul. Pept. 2013, 182, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, K.T.; Subhedar, N.K.; Kokare, D.M. Involvement of neuropeptide CART in the central effects of insulin on feeding and body weight. Pharmacol. Biochem. Behav. 2019, 181, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Mariano, I.R.; Yamada, L.A.; Soares Rabassi, R.; Rissi Sabino, V.L.; Bataglini, C.; Azevedo, S.; Garcia, R.F.; Pedrosa, M.M.D. Differential responses of liver and hypothalamus to the nutritional condition during lactation and adult life. Front. Physiol. 2020, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Patkar, P.P.; Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Berthoud, H.R.; Shin, A.C. Unlike calorie restriction, Roux-en-Y gastric bypass surgery does not increase hypothalamic AgRP and NPY in mice on a high-fat diet. Int. J. Obes. 2019, 43, 2143–2150. [Google Scholar] [CrossRef]
- Adriaenssens, A.E.; Biggs, E.K.; Darwish, T.; Tadross, J.; Sukthankar, T.; Girish, M.; Polex-Wolf, J.; Lam, B.Y.; Zvetkova, I.; Pan, W.; et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food Intake. Cell Metab. 2019, 30, 987–996.e6. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Morselli, L.L.; Wagner, V.A.; Balapattabi, K.; Sapouckey, S.A.; Knudtson, K.L.; Rahmouni, K.; Cui, H.; Sigmund, C.D.; Kwitek, A.E.; et al. Single-nucleus RNA sequencing of the hypothalamic arcuate nucleus of C57BL/6J mice after prolonged diet-induced obesity. Hypertension 2020, 76, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.Y.H.; Cimino, I.; Polex-Wolf, J.; Nicole Kohnke, S.; Rimmington, D.; Iyemere, V.; Heeley, N.; Cossetti, C.; Schulte, R.; Saraiva, L.R.; et al. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol. Metab. 2017, 6, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschop, M.H.; Diano, S.; Bruning, J.C.; Cota, D. POMC neuronal heterogeneity in energy balance and beyond: An integrated view. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef] [PubMed]
| Gene | GenBank ID | Sequence (5′-3′) |
|---|---|---|
| Agrp | NM_033650.1 | CGACGCGGAGAACGAGACTCGCGGTTCTGTGGATCTAGCACCTCTGCC |
| Cart | NM_017110 | CCGAAGGAGGCTGTCACCCCTTCACA |
| Npy | NM_012614.2 | AGATGAGATGTGGGGGGAAACTAGGAAAAGTCAGGAGAGCAAGTTTCATT |
| Pomc | NM_139326 | TCCATAGACGTGTGGAGCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, M.; Rodríguez, A.; Gómez-Ambrosi, J.; Ramírez, B.; Becerril, S.; Catalán, V.; López, M.; Diéguez, C.; Frühbeck, G.; Burrell, M.A. Caloric Restriction Prevents Metabolic Dysfunction and the Changes in Hypothalamic Neuropeptides Associated with Obesity Independently of Dietary Fat Content in Rats. Nutrients 2021, 13, 2128. https://doi.org/10.3390/nu13072128
Martín M, Rodríguez A, Gómez-Ambrosi J, Ramírez B, Becerril S, Catalán V, López M, Diéguez C, Frühbeck G, Burrell MA. Caloric Restriction Prevents Metabolic Dysfunction and the Changes in Hypothalamic Neuropeptides Associated with Obesity Independently of Dietary Fat Content in Rats. Nutrients. 2021; 13(7):2128. https://doi.org/10.3390/nu13072128
Chicago/Turabian StyleMartín, Marina, Amaia Rodríguez, Javier Gómez-Ambrosi, Beatriz Ramírez, Sara Becerril, Victoria Catalán, Miguel López, Carlos Diéguez, Gema Frühbeck, and María A. Burrell. 2021. "Caloric Restriction Prevents Metabolic Dysfunction and the Changes in Hypothalamic Neuropeptides Associated with Obesity Independently of Dietary Fat Content in Rats" Nutrients 13, no. 7: 2128. https://doi.org/10.3390/nu13072128
APA StyleMartín, M., Rodríguez, A., Gómez-Ambrosi, J., Ramírez, B., Becerril, S., Catalán, V., López, M., Diéguez, C., Frühbeck, G., & Burrell, M. A. (2021). Caloric Restriction Prevents Metabolic Dysfunction and the Changes in Hypothalamic Neuropeptides Associated with Obesity Independently of Dietary Fat Content in Rats. Nutrients, 13(7), 2128. https://doi.org/10.3390/nu13072128