Perspective: Practical Approach to Preventing Subclinical B12 Deficiency in Elderly Population
Abstract
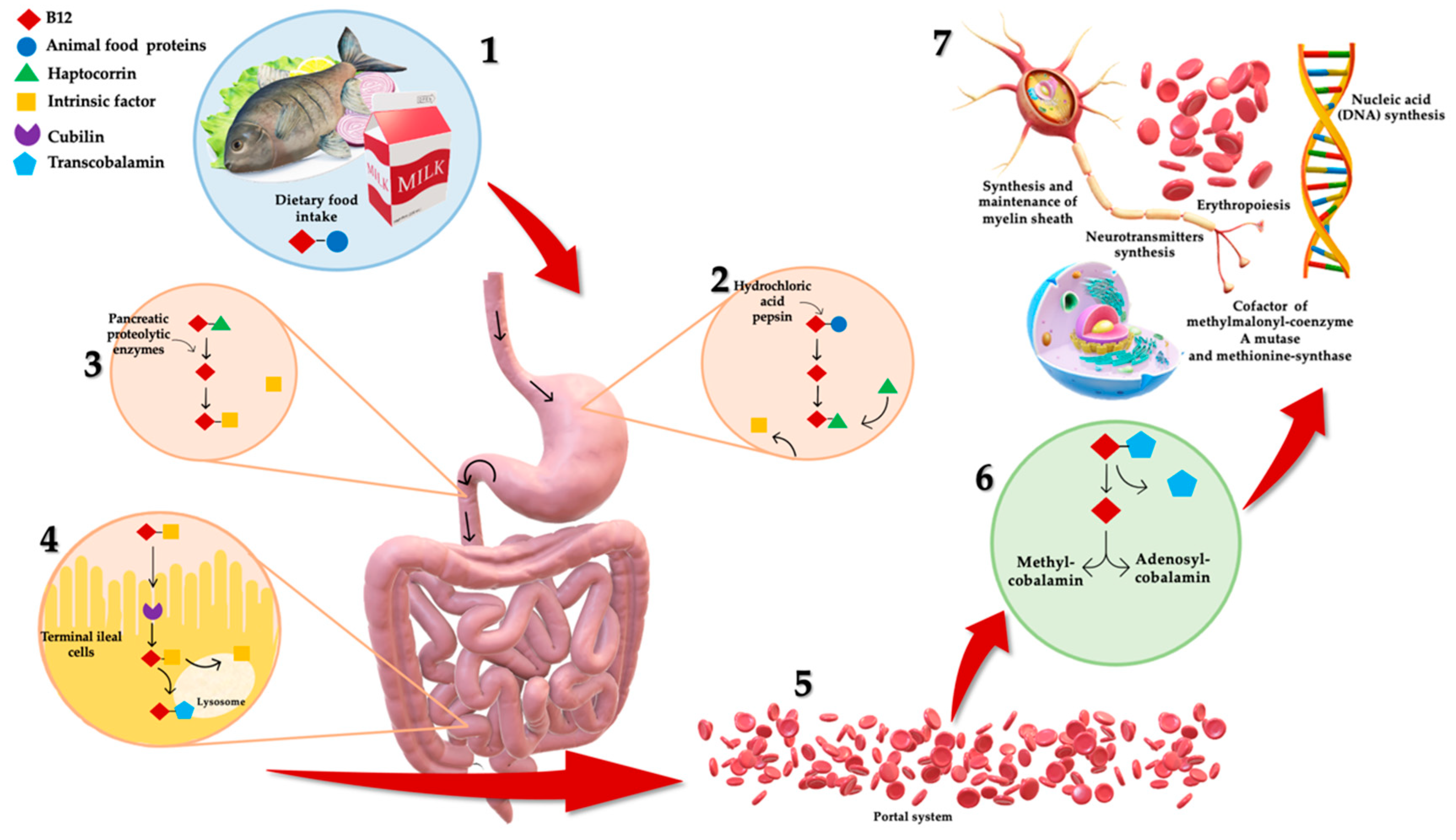
1. Introduction
2. Prevalence of Deficiency
3. B12 Deficiency: Dietary Intake and Bioavailability
4. Sustainable Strategies to Prevent Cbl Deficiency
4.1. Food Fortification
4.2. Novel Bacteria and In Situ Fortification
4.3. Biofortification
4.4. Vegetable Sources of Vitamin B12
4.5. Supplementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Marsman, D.; Belsky, D.W.; Gregori, D.; Johnson, M.A.; Low Dog, T.; Meydani, S.; Pigat, S.; Sadana, R.; Shao, A.; Griffiths, J.C. Healthy ageing: The natural consequences of good nutrition-a conference report. Eur. J. Nutr. 2018, 57, 15–34. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Bird, J.K.; Murphy, R.A.; Ciappio, E.D.; McBurney, M.I. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients 2017, 9, 655. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar]
- Nohr, D.; Biesalski, H.K. Vitamin B12. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Herbert, V. Vitamin B-12: Plant sources, requirements, and assay. Am. J. Clin. Nutr. 1988, 48, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279. [Google Scholar] [PubMed]
- De Giuseppe, R.; Venturelli, G.; Guez, S.; Salera, S.; De Vita, C.; Consonni, D.; Dellanoce, C.; Bamonti, F.; Chiarelli, G.; Manzoni, F.; et al. Homocysteine metabolism in children and adolescents with epidermolysis bullosa. BMC Pediatr. 2016, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.; Lominadze, D.; Tyagi, S.C. Hyperhomocysteinemia inhibits satellite cell regenerative capacity through p38 alpha/beta MAPK signaling. Am. J. Physiol. Heart Circ. Physiol. 2015, 15, H325–H334. [Google Scholar] [CrossRef]
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; West, K.P.; Black, R.E., Jr. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 22–33. [Google Scholar] [CrossRef]
- Nawaz, A.; Khattak, N.N.; Khan, M.S.; Nangyal, H.; Sabri, S.; Shakir, M. Deficiency of vitamin B12 and its relation with neurological disorders: A critical review. J. Basic Appl. Zool. 2020, 81, 1–9. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Apostolopoulos, V. B Vitamins and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science. Subcellular Biochemistry; Harris, J., Korolchuk, V., Eds.; Springer: Singapore, 2018; Volume 90, pp. 451–470. [Google Scholar]
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health. 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef]
- Keflie, T.S.; Biesalski, H.K. Micronutrients and bioactive substances: Their potential roles in combating COVID-19. Nutrition 2021, 84, 111103. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Hoey, L.; Hughes, C.F.; Ward, M.; McNulty, H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021. In Press. [Google Scholar] [CrossRef]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.P.G.M.; Eussen, S.J.P.M. Vitamin B12 Intake from Animal Foods, Biomarkers, and Health Aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Galimberti, A.; Cena, H.; Campone, L.; Ferri, E.; Dell’Agli, M.; Sangiovanni, E.; Belingheri, M.; Riva, M.A.; Casiraghi, M.; Labra, M. Rethinking Urban and Food Policies to Improve Citizens Safety After COVID-19 Pandemic. Front. Nutr. 2020, 7, 569542. [Google Scholar] [CrossRef] [PubMed]
- Bamonti, F.; Moscato, G.A.; Novembrino, C.; Gregori, D.; Novi, C.; De Giuseppe, R.; Galli, C.; Uva, V.; Lonati, S.; Maiavacca, R. Determination of serum holotranscobalamin concentrations with the AxSYM active B(12) assay: Cut-off point evaluation in the clinical laboratory. Clin. Chem. Lab. Med. 2010, 48, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2018, 3, CD004655. [Google Scholar] [CrossRef]
- Marchi, G.; Busti, F.; Zidanes, A.L.; Vianello, A.; Girelli, D. Cobalamin Deficiency in the Elderly. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020043. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Allen, R.H. Vitamin B12 deficiency as a worldwide problem. Annu. Rev. Nutr. 2004, 24, 299–326. [Google Scholar] [CrossRef]
- Evatt, M.L.; Terry, P.D.; Ziegler, T.R.; Oakley, G.P. Association between vitamin B12-containing supplement consumption and prevalence of biochemically defined B12 deficiency in adults in NHANES III (third national health and nutrition examination survey). Public Health Nutr. 2010, 13, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Bialostosky, K.; Gunter, E.W.; Carroll, M.D.; Najjar, M.F.; Bowman, B.A.; Johnson, C.L. Blood folate and vitamin B12: United States, 1988–1994. Vital Health Stat. 11 1998, 243, 1–78. [Google Scholar]
- van Asselt, D.Z.; de Groot, L.C.; van Staveren, W.A.; Blom, H.J.; Wevers, R.A.; Biemond, I.; Hoefnagels, W.H. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am. J. Clin. Nutr. 1998, 68, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Nygaard, H.A.; Engedal, K.; Tell, G.S.; Refsum, H. Cognitive function in an elderly population: Interaction between vitamin B12 status, depression, and apolipoprotein E ε4: The Hordaland Homocysteine Study. Psychosom. Med. 2013, 1, 20–29. [Google Scholar] [CrossRef]
- Allen, L.H. Folate and vitamin B12 status in the Americas. Nutr. Rev. 2004, 62, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Pan, W.H.; Yang, F.L.; Wei, I.L.; Shaw, N.S.; Lin, B.F. Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pac. J. Clin. Nutr. 2005, 3, 250–255. [Google Scholar]
- Refsum, H.; Yajnik, C.S.; Gadkari, M.; Schneede, J.; Vollset, S.E.; Orning, L.; Guttormsen, A.B.; Joglekar, A.; Sayyad, M.G.; Ulvik, A.; et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am. J. Clin. Nutr. 2001, 2, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lindenbaum, J.; Rosenberg, I.H.; Wilson, P.W.; Stabler, S.P.; Allen, R.H. Prevalence of cobalamin deficiency in the Framingham elderly population. Am. J. Clin. Nutr. 1994, 60, 2–11. [Google Scholar] [CrossRef]
- Andrès, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Noblet-Dick, M.; Maloisel, F.; Schlienger, J.L.; Blicklé, J.F. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mézière, A.; Audureau, E.; Vairelles, S.; Krypciak, S.; Dicko, M.; Monié, M.; Giraudier, S. B12 deficiency increases with age in hospitalized patients: A study on 14,904 samples. J. Gerontol. A Biol Sci. Med. Sci. 2014, 69, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Chatthanawaree, W. Biomarkers of cobalamin (vitamin B12) deficiency and its application. J. Nutr. Health Aging 2011, 15, 227–231. [Google Scholar] [CrossRef]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Institute of Medicine of the National Academies. Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Food and Nutrition Board; National Academies Press: Atlanta, GA, USA, 2005. [Google Scholar]
- Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Vitamin B12 and ageing: Current issues and interaction with folate. Ann. Clin. Biochem. 2013, 50, 315–329. [Google Scholar] [CrossRef]
- Viñas, B.R.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.; van’t Veer, P.; Matthys, C.; Serra Majem, L. Projected prevalence of inadequate nutrient intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148 (Suppl. 4), 1995S–2027S. [Google Scholar] [CrossRef] [PubMed]
- Strand, T.A.; Taneja, S.; Ueland, P.M.; Refsum, H.; Bahl, R.; Schneede, J.; Sommerfelt, H.; Bhandari, N. Cobalamin and folate status predicts mental development scores in North Indian children 12–18 mo of age. Am. J. Clin. Nutr. 2013, 97, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Lubree, H.G.; Naik, S.S.; Bhat, D.S.; Uradey, B.S.; Deshpande, J.A.; Rege, S.S.; Refsum, H.; Yudkin, J.S. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J. Assoc. Physicians India 2006, 54, 775–782. [Google Scholar]
- Ulak, M.; Chandyo, R.K.; Adhikari, R.K.; Sharma, P.R.; Sommerfelt, H.; Refsum, H.; Strand, T.A. Cobalamin and folate status in 6 to 35 months old children presenting with acute diarrhea in Bhaktapur, Nepal. PLoS ONE 2014, 9, e90079. [Google Scholar] [CrossRef] [PubMed]
- Mark, H.E.; Houghton, L.A.; Gibson, R.S.; Monterrosa, E.; Kraemer, K. Estimating dietary micronutrient supply and the prevalence of inadequate intakes from national Food Balance Sheets in the South Asia regiona. Asia Pac. J. Clin. Nutr. 2016, 2, 368–376. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.; La Ferrera, G.M.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Andrès, E.; Kaltenbach, G.; Perrin, A.E.; Kurtz, J.E.; Schlienger, J.L. Food-cobalamin malabsorption in the elderly. Am. J. Med. 2002, 113, 351–352. [Google Scholar] [CrossRef]
- Davison, K.M.; Gondara, L.; Kaplan, B.J. Food Insecurity, Poor Diet Quality, and Suboptimal Intakes of Folate and Iron Are Independently Associated with Perceived Mental Health in Canadian Adults. Nutrients 2017, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Specter, S.E. Poverty and obesity: The role of energy density and energy costs. Am. J. Clin. Nutr. 2004, 79, 6–16. [Google Scholar] [CrossRef]
- Dixon, L.B.; Winkleby, M.A.; Radimer, K.L. Dietary intake and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2001, 131, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Begley, A.; Paynter, E.; Butcher, L.M.; Dhaliwal, S.S. Examining the Association between Food Literacy and Food Insecurity. Nutrients 2019, 11, 445. [Google Scholar] [CrossRef]
- Hanson, A.D.; Beaudoin, G.A.; McCarty, D.R.; Gregory, J.F., 3rd. Does Abiotic Stress Cause Functional B Vitamin Deficiency in Plants? Plant. Physiol. 2016, 172, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Garriguet, D. Diet quality in Canada. Health Rep. 2009, 20, 41–52. [Google Scholar] [PubMed]
- Ford, D.W.; Hartman, T.J.; Still, C.; Wood, C.; Mitchell, D.; Hsiao, P.Y.; Bailey, R.; Smiciklas-Wright, H.; Coffman, D.L.; Jensen, G.L. Diet-related practices and BMI are associated with diet quality in older adults. Public Health Nutr. 2014, 17, 1565–1569. [Google Scholar] [CrossRef]
- Morales, M.E.; Berkowitz, S.A. The Relationship between Food Insecurity, Dietary Patterns, and Obesity. Curr. Nutr. Rep. 2016, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.P.; Zwaluw, N.L.; van der Velde, N.; de Groot, L.C. Dietary Sources of Vitamin B-12 and Their Association with Vitamin B-12 Status Markers in Healthy Older Adults in the B-PROOF Study. Nutrients 2015, 7, 7781–7797. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Kanosue, F.; Yabuta, Y.; Watanabe, F. Loss of vitamin B(12) in fish (round herring) meats during various cooking treatments. J. Nutr. Sci. Vitaminol. 2011, 6, 432–436. [Google Scholar] [CrossRef]
- Ball, G.F.M. Vitamin B12. In Bioavailability and Analysis of Vitamins in Foods; Chapman & Hall: London, UK, 1998; pp. 497–515. [Google Scholar]
- Watanabe, F.; Abe, K.; Fujita, T.; Goto, M.; Hiemori, M.; Nakano, Y. Effects of Microwave Heating on the Loss of Vitamin B(12) in Foods. J. Agric. Food Chem. 1998, 1, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Kwan, L.L.; Bermudez, O.I.; Tucker, K.L. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J. Nutr. 2002, 132, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W. Vitamin B12 deficiency in the elderly: Is it worth screening? Hong Kong Med. J. 2015, 21, 155–164. [Google Scholar] [CrossRef]
- Thompson, B.; Amoroso, L. Combating Micronutrient Deficiencies: Food-Based Approaches; Food and Agricultural Organisation of the United Nations: Oxfordshire, UK, 2011. [Google Scholar]
- Dwyer, J.T.; Woteki, C.; Bailey, R.; Britten, P.; Carriquiry, A.; Gaine, P.C.; Miller, D.; Moshfegh, A.; Murphy, M.M.; Smith Edge, M. Fortification: New findings and implications. Nutr. Rev. 2014, 72, 127–141. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Mahmood, S.B.; Moin, A.; Kumar, R.; Mukhtar, K.; Lassi, Z.S.; Bhutta, Z.A. Food fortification with multiple micronutrients: Impact on health outcomes in general population. Cochrane Database Syst. Rev. 2019, 12, CD011400. [Google Scholar] [CrossRef]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients 2021, 4, 1118. [Google Scholar] [CrossRef]
- Mkambula, P.; Mbuya, M.N.N.; Rowe, L.A.; Sablah, M.; Friesen, V.M.; Chadha, M.; Osei, A.K.; Ringholz, C.; Vasta, F.C.; Gorstein, J. The Unfinished Agenda for Food Fortification in Low- and Middle-Income Countries: Quantifying Progress, Gaps and Potential Opportunities. Nutrients 2020, 2, 354. [Google Scholar] [CrossRef]
- Shahab-Ferdows, S.; Engle-Stone, R.; Hampel, D.; Ndjebayi, A.O.; Nankap, M.; Brown, K.H.; Allen, L.H. Regional, Socioeconomic, and Dietary Risk Factors for Vitamin B-12 Deficiency Differ from Those for Folate Deficiency in Cameroonian Women and Children. J. Nutr. 2015, 11, 2587–2595. [Google Scholar] [CrossRef]
- Allen, L.H.; De Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Winkels, R.M.; Brouwer, I.A.; Clarke, R.; Katan, M.B.; Verhoef, P. Bread cofortified with folic acid and vitamin B-12 improves the folate and vitamin B-12 status of healthy older people: A randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe-Rutten, R.A.; van Zutphen, M.; de Groot, L.C.; Eussen, S.J.; Blom, H.J.; van Staveren, W.A. Effect of supplementation with cobalamin carried either by a milk product or a capsule in mildly cobalamin-deficient elderly Dutch persons. Am. J. Clin. Nutr. 2005, 82, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I. Public Health Aspects in the Prevention and Control of Vitamin Deficiencies. Curr. Dev. Nutr. 2019, 3, nzz075. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.J.M.; Martinez, H.; Garrett, G.S.; Neufeld, L.M.; De-Regil, L.M.; Vossenaar, M.; Darnton-Hill, I. Large-Scale Food Fortification and Biofortification in Low- and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps. Food Nutr. Bull. 2018, 39, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Tapola, N.S.; Karvonen, H.M.; Niskanen, L.K.; Sarkkinen, E.S. Mineral water fortified with folic acid, vitamins B6, B12, D and calcium improves folate status and decreases plasma homocysteine concentration in men and women. Eur J. Clin. Nutr. 2004, 58, 376–385. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.J.; Jahn, D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B12: A review and future perspectives. Microb. Cell Fact. 2017, 16, 15. [Google Scholar] [CrossRef]
- Perlman, D. Microbial synthesis of cobamides. Adv. Appl. Microbiol. 1959, 1, 122. [Google Scholar] [CrossRef]
- Burgess, C.M.; Smid, E.J.; van Sinderen, D. Bacterial vitamin B2, B11 and B12 overproduction: An overview. Int. J. Food Microbiol. 2009, 133, 1–7. [Google Scholar] [CrossRef]
- Pereira, J.; Simões, M.; Silva, J.L. Microalgal assimilation of vitamin B12 toward the production of a superfood. J. Food Biochem. 2019, 43, e12911. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.-source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus reuteri CRL1098 produces cobalamin. J. Bacteriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef] [PubMed]
- Taranto, M.P.; Medici, M.; Perdigon, G.; Ruiz Holgado, A.P.; Valdez, G.F. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in mice. J. Dairy Sci. 2000, 83, 401–403. [Google Scholar] [CrossRef]
- Santos, F.; Vera, J.L.; Lamosa, P.; de Valdez, G.F.; de Vos, W.M.; Santos, H.; Sesma, F.; Hugenholtz, J. Pseudovitamin B(12) is the corrinoid produced by Lactobacillus reuteri CRL1098 under anaerobic conditions. FEBS Lett. 2007, 581, 4865–4870. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Olivares, M.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Characterization of a reuterin-producing Lactobacillus coryniformis strain isolated from a goat’s milk cheese. Int. J. Food Microbiol. 2005, 104, 267–277. [Google Scholar] [CrossRef]
- Madhu, A.N.; Giribhattanavar, P.; Narayan, M.S.; Prapulla, S.G. Probiotic lactic acid bacterium from kanjika as a potential source of vitamin B12: Evidence from LC-MS, immunological and microbiological techniques. Biotechnol. Lett. 2010, 32, 503–506. [Google Scholar] [CrossRef]
- Masuda, M.; Ide, M.; Utsumi, H.; Niiro, T.; Shimamura, Y.; Murata, M. Production potency of folate, vitamin B(12), and thiamine by lactic acid bacteria isolated from Japanese pickles. Biosci. Biotechnol. Biochem. 2012, 76, 2061–2067. [Google Scholar] [CrossRef]
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; van Sinderen, D.; Gobbetti, M. Lactobacillus rossiae, a vitamin B12 producer, represents a metabolically versatile species within the Genus Lactobacillus. PLoS ONE 2014, 9, e107232. [Google Scholar] [CrossRef]
- Basavanna, G.; Prapulla, S.G. Evaluation of functional aspects of Lactobacillus fermentum CFR 2195 isolated from breast fed healthy infants’ fecal matter. J. Food Sci. Technol. 2013, 50, 360–366. [Google Scholar] [CrossRef]
- Ale, E.C.; Binetti, A.G. Role of Probiotics, Prebiotics, and Synbiotics in the Elderly: Insights into Their Applications. Front. Microbiol. 2021, 12, 631254. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gu, Q.; Yang, L.; Yu, Y.; Wang, Y. Characterization of extracellular vitamin B12 producing Lactobacillus plantarum strains and assessment of the probiotic potentials. Food Chem. 2017, 234, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, N.L.; Manyong, J.V.M.; DeGroote, H.; Javelosa, J.; Yanggen, D.R.; Naher, F.; Gonzalez, C.; García, J.; Meng, E. How Cost-Effective is Biofortification in Combating Micronutrient Malnutrition? An Ex ante Assessment. World Dev. 2010, 38, 64–65. [Google Scholar] [CrossRef]
- Meenakshi, J.V. Copenhagen Consensus. Best Practice Paper. Cost-Effectiveness of Biofortification; Copenhagen Consensus Center: Copenhagen, Denmark, 2008. [Google Scholar]
- Titcomb, T.J.; Tanumihardjo, S.A. Global Concerns with B Vitamin Statuses: Biofortification, Fortification, Hidden Hunger, Interactions, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1968–1984. [Google Scholar] [CrossRef]
- Mozafar, A. Enrichment of some B-vitamins in plants with application of organic fertilizers. Plant. Soil 1994, 167, 305–311. [Google Scholar] [CrossRef]
- Mozafar, A.; Oertli, J.J. Uptake of a microbially-produced vitamin (B12) by soybean roots. Plant. Soil 1992, 139, 23–30. [Google Scholar] [CrossRef]
- Sato, K.; Kudo, Y.; Muramatsu, K. Incorporation of a high level of vitamin B12 into a vegetable, kaiware daikon (Japanese radish sprout), by the absorption from its seeds. Biochim. Biophys. Acta 2004, 1672, 135–137. [Google Scholar] [CrossRef]
- Garcia-Casal, M.N.; Peña-Rosas, J.; Pachón, H.; De-Regil, L.; Centeno Tablante, E.; Flores-Urrutia, M.C. Staple crops biofortified with increased micronutrient content: Effects on vitamin and mineral status, as well as health and cognitive function in the general population. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B₁₂-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef]
- La Guardia, M.; Venturella, G.; Venturella, F. On the chemical composition and nutritional value of pleurotus taxa growing on umbelliferous plants (Apiaceae). J. Agric. Food Chem. 2005, 53, 5997–6002. [Google Scholar] [CrossRef]
- Watanabe, F.; Schwarz, J.; Takenaka, S.; Miyamoto, E.; Ohishi, N.; Nelle, E.; Hochstrasser, R.; Yabuta, Y. Characterization of vitamin B₁₂compounds in the wild edible mushrooms black trumpet (Craterellus cornucopioides) and golden chanterelle (Cantharellus cibarius). J. Nutr. Sci. Vitaminol. 2012, 58, 438–441. [Google Scholar] [CrossRef]
- Bito, T.; Teng, F.; Ohishi, N.; Takenaka, S.; Miyamoto, E.; Sakuno, E.; Terashima, K.; Yabuta, Y.; Watanabe, F. Characterization of vitamin B12 compounds in the fruiting bodies of shiitake mushroom (Lentinula edodes) and bed logs after fruiting of the mushroom. Mycoscience 2014, 55, 462–468. [Google Scholar] [CrossRef]
- Report of the Subdivision on Resources. In Standard Tables of Food Composition in Japan, 5th ed.; The Council for Science and Technology, Ministry of Education, Culture, Sports, Science, and Technology: Tokyo, Japan, 2005; pp. 150–151.
- Miyamoto, E.; Yabuta, Y.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Characterization of vitamin B12 compounds from Korean purple laver (Porphyra sp.) products. J. Agric. Food Chem. 2009, 57, 2793–2796. [Google Scholar] [CrossRef]
- Yamada, S.; Shibata, Y.; Takayama, M.; Narita, Y.; Sugawara, K.; Fukuda, M. Content and characteristics of vitamin B12 in some seaweeds. J. Nutr. Sci. Vitaminol 1996, 42, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Takenaka, S.; Katsura, H.; Miyamoto, E.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Characterization of a vitamin B12 compound in the edible purple laver, Porphyra yezoensis. Biosci. Biotechnol. Biochem. 2000, 64, 2712–2715. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Tanioka, Y.; Miyamoto, E.; Fujita, T.; Takenaka, H.; Nakano, Y. Purification and characterization of corrinoid-compounds from the dried powder of an edible cyanobacterium, Nostoc commune (Ishikurage). J. Nutr. Sci. Vitaminol. 2007, 53, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- American Dietetic Association. Position of the American Dietetic Association: Food fortification and dietary supplements. J. Am. Diet. Assoc. 2001, 101, 115–125. [Google Scholar] [CrossRef]
- Sebastian, R.S.; Cleveland, L.E.; Goldman, J.D.; Moshfegh, A.J. Older adults who use vitamin/mineral supplements differ from nonusers in nutrient intake adequacy and dietary attitudes. J. Am. Diet. Assoc. 2007, 107, 1322–1332. [Google Scholar] [CrossRef]
- Donaldson, M.S. Metabolic vitamin B12 status on a mostly raw vegan diet with follow-up using tablets, nutritional yeast, or probiotic supplements. Ann. Nutr. Metab. 2000, 44, 229–234. [Google Scholar] [CrossRef]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef]
- Lachner, C.; Steinle, N.I.; Regenold, W.T. The neuropsychiatry of vitamin B12 deficiency in elderly patients. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 5–15. [Google Scholar] [CrossRef]
- Zendehdel, A.; Roham, M. Role of Helicobacter pylori infection in the manifestation of old age-related diseases. Mol. Genet. Genomic Med. 2020, 8, e1157. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S. A review of vitamin B12. Mol. Nutr. 2020, 105–129. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; American Dietetic Association. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Verdugo, R.; Hertrampf, E.; Miller, J.W.; Green, R.; Fedosov, S.N.; Shahab-Ferdows, S.; Sanchez, H.; Albala, C.; Castillo, J.L.; et al. Vitamin B-12 treatment of asymptomatic, deficient, elderly Chileans improves conductivity in myelinated peripheral nerves, but high serum folate impairs vitamin B-12 status response assessed by the combined indicator of vitamin B-12 status. Am. J. Clin. Nutr. 2016, 103, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Dangour, A.D.; Allen, E.; Clarke, R.; Elbourne, D.; Fletcher, A.E.; Letley, L.; Richards, M.; Whyte, K.; Uauy, R.; Mills, K. Effects of vitamin B-12 supplementation on neurologic and cognitive function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bulut, E.; Soysal, P.; Aydin, A.E.; Dokuzlar, O.; Kocyigit, S.E.; Isik, A.T. Vitamin B12 deficiency might be related to sarcopenia in older adults. Exp. Gerontol. 2017, 95, 136–140. [Google Scholar] [CrossRef]
- Swart, K.M.A.; Ham, A.C.; van Wijngaarden, J.P.; Enneman, A.W.; van Dijk, S.C.; Sohl, E.; Brouwer-Brolsma, E.M.; van der Zwaluw, N.L.; Zillikens, M.C.; Dhonukshe-Rutten, R.A.M.; et al. A Randomized Controlled Trial to Examine the Effect of 2-Year Vitamin B12 and Folic Acid Supplementation on Physical Performance, Strength, and Falling: Additional Findings from the B-PROOF Study. Calcif. Tissue Int. 2016, 98, 18–27. [Google Scholar] [CrossRef][Green Version]
- Buhr, G.; Bales, C.W. Nutritional supplements for older adults: Review and recommendations–Part II. J. Nutr. Elder. 2010, 29, 42–71. [Google Scholar] [CrossRef]
- Andrès, E.; Zulfiqar, A.A.; Vogel, T. State of the art review: Oral and nasal vitamin B12 therapy in the elderly. QJM 2020, 113, 5–15. [Google Scholar] [CrossRef]
- van Walraven, C.; Austin, P.; Naylor, C.D. Vitamin B12 injections versus oral supplements. How much money could be saved by switching from injections to pills? Can. Fam. Physician. 2001, 47, 79–86. [Google Scholar] [PubMed]
- Andrès, E.; Fothergill, H.; Mecili, M. Efficacy of oral cobalamin (vitamin B12) therapy. Expert Opin. Pharmacother. 2010, 2, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Fedosov, S.N.; Nexo, E. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol. Nutr. Food Res. 2015, 7, 1364–1372. [Google Scholar] [CrossRef]
- British Medical Association, Royal Pharmaceutical Society of Great Britain. British National Formulary, 3rd ed.; British Medical Association: London, UK, 2004; Volume 48, pp. 456–457. [Google Scholar]
- Blumberg, J.B.; Bailey, R.L.; Sesso, H.D.; Ulrich, C.M. The Evolving Role of Multivitamin/Multimineral Supplement Use among Adults in the Age of Personalized Nutrition. Nutrients 2018, 10, 248. [Google Scholar] [CrossRef]
- Takenaka, S.; Sugiyama, S.; Watanabe, F.; Abe, K.; Tamura, Y.; Nakano, Y. Effects of Carnosine and Anserine on the Destruction of Vitamin B12 with Vitamin C in the Presence of Copper. Biosci. Biotechnol. Biochem. 1997, 61, 2137–2139. [Google Scholar] [CrossRef]
- Reizenstein, P.; Ek, G.; Matthews, C.M. Vitamin B-12 kinetics in man. Implications on total-body-B-12-determinations, human requriements, and normal and pathological cellular B12 uptake. Phys. Med. Biol. 1966, 2, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Gräsbeck, R. Imerslund-Gräsbeck syndrome (selective vitamin B12 malabsorption with proteinuria). Orphanet J. Rare Dis. 2006, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, B.; Wouters, H.; Heiner-Fokkema, M.R.; van der Klauw, M.M. The Many Faces of Cobalamin (Vitamin B12) Deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 2, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Gravel, R.A. Genetic disorders of vitamin B₁₂ metabolism: Eight complementation groups—Eight genes. Expert Rev. Mol. Med. 2010, 12, e37. [Google Scholar] [CrossRef] [PubMed]
- De Giuseppe, R.; Tomasinelli, C.E.; Vincenti, A.; Di Napoli, I.; Negro, M.; Cena, H. Sarcopenia and homocysteine: Is there a possible association in the elderly? A narrative review. Nutr. Res. Rev. 2021, 1–36. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenti, A.; Bertuzzo, L.; Limitone, A.; D’Antona, G.; Cena, H. Perspective: Practical Approach to Preventing Subclinical B12 Deficiency in Elderly Population. Nutrients 2021, 13, 1913. https://doi.org/10.3390/nu13061913
Vincenti A, Bertuzzo L, Limitone A, D’Antona G, Cena H. Perspective: Practical Approach to Preventing Subclinical B12 Deficiency in Elderly Population. Nutrients. 2021; 13(6):1913. https://doi.org/10.3390/nu13061913
Chicago/Turabian StyleVincenti, Alessandra, Laura Bertuzzo, Antonio Limitone, Giuseppe D’Antona, and Hellas Cena. 2021. "Perspective: Practical Approach to Preventing Subclinical B12 Deficiency in Elderly Population" Nutrients 13, no. 6: 1913. https://doi.org/10.3390/nu13061913
APA StyleVincenti, A., Bertuzzo, L., Limitone, A., D’Antona, G., & Cena, H. (2021). Perspective: Practical Approach to Preventing Subclinical B12 Deficiency in Elderly Population. Nutrients, 13(6), 1913. https://doi.org/10.3390/nu13061913







