Pre-Sleep Casein Supplementation, Metabolism, and Appetite: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Search Strategy
2.2. Eligibility Criteria
3. Results
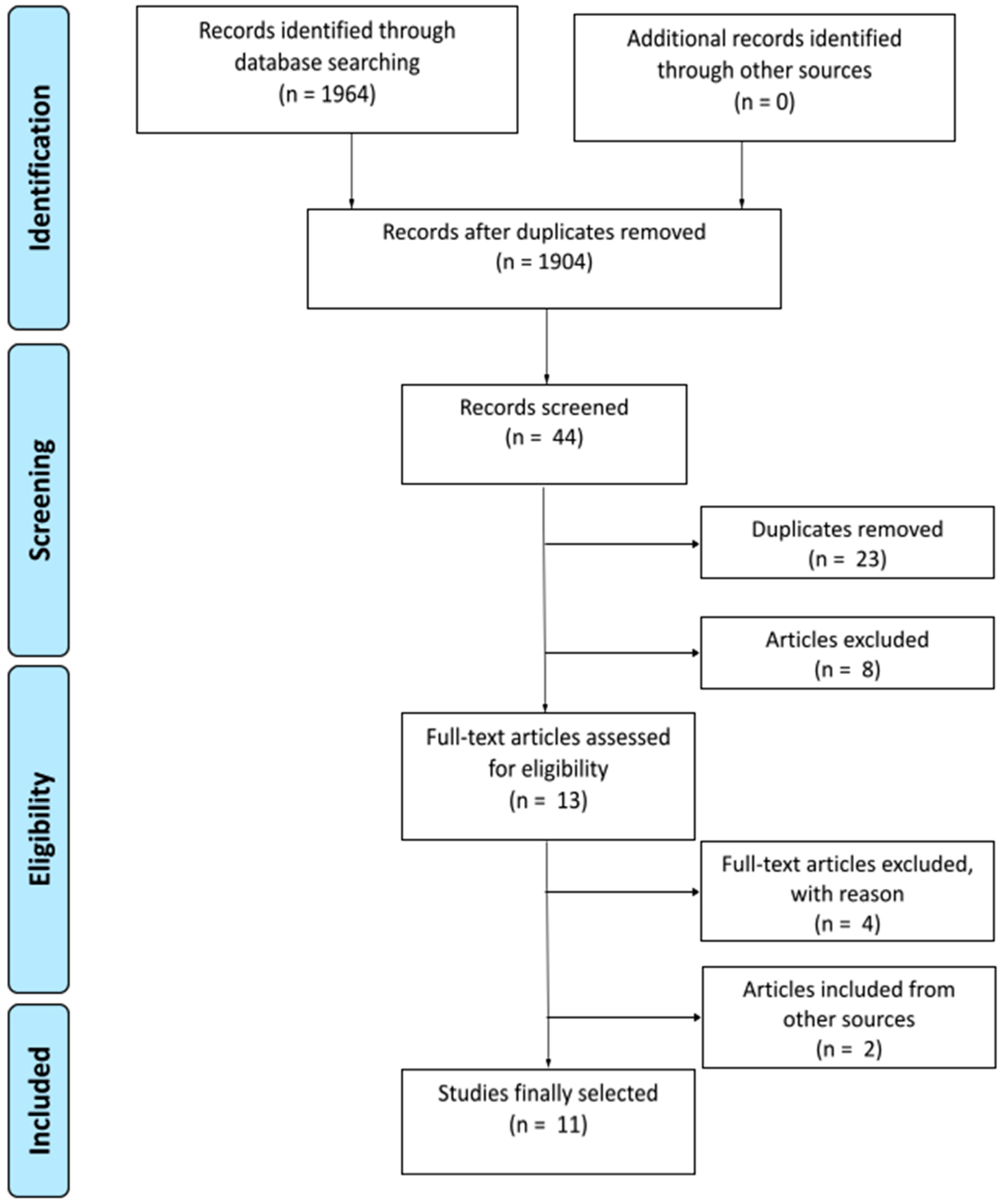
3.1. Systematic Search and Study Selection
3.2. Descriptive Data and Characteristics of Selected Studies
3.3. Effects of Pre-Sleep Casein Supplementation on Next-Day Appetite, Hunger, Satiety, and Next-Day Food Intake
3.4. Effects of Pre-Sleep Casein Supplementation on Energy Expenditure and Metabolic Rate
3.5. Effects of Pre-Sleep Casein Supplementation on Lipolysis and Fat Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Castro, J.M. The time of day of food intake influences overall intake in humans. J. Nutr. 2004, 134, 104–111. [Google Scholar] [CrossRef] [PubMed]
- de Castro, J.M. The time of day and the proportions of macronutrients eaten are related to total daily food intake. Br. J. Nutr. 2007, 98, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.S.; Stunkard, A.J.; Sørensen, T.I.A.; Petersen, L.; Heitmann, B.L. Night eating and weight change in middle-aged men and women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1338–1343. [Google Scholar] [CrossRef]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar]
- Zitting, K.-M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human resting energy expenditure varies with circadian phase. Curr. Biol. 2018, 28, 3685–3690.e3. [Google Scholar] [CrossRef]
- de Zwaan, M.; Burgard, M.A.; Schenck, C.H.; Mitchell, J.E. Night time eating: A review of the literature. Eur. Eat. Disord. Rev. 2003, 11, 7–24. [Google Scholar] [CrossRef]
- Katayose, Y.; Tasaki, M.; Ogata, H.; Nakata, Y.; Tokuyama, K.; Satoh, M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism 2009, 58, 920–926. [Google Scholar] [CrossRef]
- Gluck, M.E.; Venti, C.A.; Salbe, A.D.; Votruba, S.B.; Krakoff, J. Higher 24-h respiratory quotient and higher spontaneous physical activity in nighttime eaters. Obesity (Silver Spring) 2011, 19, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, A.W.; Ormsbee, M.J. The health impact of nighttime eating: Old and new perspectives. Nutrients 2015, 7, 2648–2662. [Google Scholar] [CrossRef]
- Koopman, R.; Wagenmakers, A.J.M.; Manders, R.J.F.; Zorenc, A.H.G.; Senden, J.M.G.; Gorselink, M.; Keizer, H.A.; van Loon, L.J.C. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E645–E653. [Google Scholar] [CrossRef]
- Res, P.T.; Groen, B.; Pennings, B.; Beelen, M.; Wallis, G.A.; Gijsen, A.P.; Senden, J.M.G.; VAN Loon, L.J.C. Protein ingestion before sleep improves postexercise overnight recovery. Med. Sci. Sports Exerc. 2012, 44, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Kouw, I.W.; Holwerda, A.M.; Trommelen, J.; Kramer, I.F.; Bastiaanse, J.; Halson, S.L.; Wodzig, W.K.; Verdijk, L.B.; van Loon, L.J. Protein ingestion before sleep increases overnight muscle protein synthesis rates in healthy older men: A randomized controlled trial. J. Nutr. 2017, 147, 2252–2261. [Google Scholar] [CrossRef]
- Trommelen, J.; Holwerda, A.M.; Senden, J.M.; Goessens, J.P.B.; VAN Kranenburg, J.; Gijsen, A.P.; Verdijk, L.B.; VAN Loon, L.J.C. Casein Ingestion Does Not Increase Muscle Connective Tissue Protein Synthesis Rates. Med. Sci. Sports Exerc. 2020, 52, 1983–1991. [Google Scholar] [CrossRef]
- Holwerda, A.M.; Kouw, I.W.K.; Trommelen, J.; Halson, S.L.; Wodzig, W.K.; Verdijk, L.B.; van Loon, L.J.C. Physical activity performed in the evening increases the overnight muscle protein synthetic response to presleep protein ingestion in older men. J. Nutr. 2016, 146, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Res, P.T.; Smeets, J.S.J.; van Vliet, S.; van Kranenburg, J.; Maase, K.; Kies, A.K.; Verdijk, L.B.; van Loon, L.J.C. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J. Nutr. 2015, 145, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Peacock, C.; Silver, T. Casein protein supplementation in trained men and women: Morning versus evening. Int. J. Exerc. Sci. 2017, 10, 479–486. [Google Scholar]
- Reis, C.E.G.; Loureiro, L.M.R.; Roschel, H.; da Costa, T.H.M. Effects of pre-sleep protein consumption on muscle-related outcomes: A systematic review. J. Sci. Med. Sport 2021, 24, 177–182. [Google Scholar] [CrossRef]
- Dougkas, A.; Minihane, A.M.; Givens, D.I.; Reynolds, C.K.; Yaqoob, P. Differential effects of dairy snacks on appetite, but not overall energy intake. Br. J. Nutr. 2012, 108, 2274–2285. [Google Scholar] [CrossRef]
- de Cássia Gonçalves Alfenas, R.; Bressan, J.; de Paiva, A.C. Effects of protein quality on appetite and energy metabolism in normal weight subjects. Arq. Bras. Endocrinol. Metabol. 2010, 54, 45–51. [Google Scholar] [CrossRef]
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef]
- Kinsey, A.W.; Eddy, W.R.; Madzima, T.A.; Panton, L.B.; Arciero, P.J.; Kim, J.-S.; Ormsbee, M.J. Influence of night-time protein and carbohydrate intake on appetite and cardiometabolic risk in sedentary overweight and obese women. Br. J. Nutr. 2014, 112, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Madzima, T.A.; Panton, L.B.; Fretti, S.K.; Kinsey, A.W.; Ormsbee, M.J. Night-time consumption of protein or carbohydrate results in increased morning resting energy expenditure in active college-aged men. Br. J. Nutr. 2014, 111, 71–77. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Kinsey, A.W.; Eddy, W.R.; Madzima, T.A.; Arciero, P.J.; Figueroa, A.; Panton, L.B. The influence of nighttime feeding of carbohydrate or protein combined with exercise training on appetite and cardiometabolic risk in young obese women. Appl. Physiol. Nutr. Metab. 2015, 40, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, A.W.; Cappadona, S.R.; Panton, L.B.; Allman, B.R.; Contreras, R.J.; Hickner, R.C.; Ormsbee, M.J. The effect of casein protein prior to sleep on fat metabolism in obese men. Nutrients 2016, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Lay, A.H.H.; Crabtree, D.R.; Campbell, T.G.; Dreczkowski, G.M.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. A bedtime milk snack does not impact RMR, substrate utilisation and appetite the following morning in mildly overweight males. Br. J. Nutr. 2018, 119, 1355–1365. [Google Scholar] [CrossRef]
- Leyh, S.M.; Willingham, B.D.; Baur, D.A.; Panton, L.B.; Ormsbee, M.J. Pre-sleep protein in casein supplement or whole-food form has no impact on resting energy expenditure or hunger in women. Br. J. Nutr. 2018, 120, 988–994. [Google Scholar] [CrossRef]
- Madzima, T.A.; Melanson, J.T.; Black, J.R.; Nepocatych, S. Pre-sleep consumption of casein and whey protein: Effects on morning metabolism and resistance exercise performance in active women. Nutrients 2018, 10, 1273. [Google Scholar] [CrossRef]
- Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Snijders, T.; Halson, S.L.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Presleep dietary protein-derived amino acids are incorporated in myofibrillar protein during postexercise overnight recovery. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E457–E467. [Google Scholar] [CrossRef]
- Allman, B.R.; Morrissey, M.C.; Kim, J.-S.; Panton, L.B.; Contreras, R.J.; Hickner, R.C.; Ormsbee, M.J. Lipolysis and fat oxidation are not altered with presleep compared with daytime casein protein intake in resistance-trained women. J. Nutr. 2020, 150, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Morehen, S.; Smeuninx, B.; Perkins, M.; Morgan, P.; Breen, L. Pre-sleep casein protein ingestion does not impact next-day appetite, energy intake and metabolism in older individuals. Nutrients 2019, 12, 90. [Google Scholar] [CrossRef]
- Nelson, H.; Valladão, S.P.; Schwarz, N.; Valliant, M.; Thomas, L.A. Effect of pre-sleep casein and tryptophan supplementation on energy expenditure before, during, and after exercise in active females. J. Ex. Nutr. 2021, 4. [Google Scholar]
- Jespersen, S.E.; Agergaard, J. Evenness of dietary protein distribution is associated with higher muscle mass but not muscle strength or protein turnover in healthy adults: A systematic review. Eur. J. Nutr. 2021, 1–18. [Google Scholar]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef]
- Hudson, J.L.; Iii, R.E.B.; Campbell, W.W. Protein distribution and muscle-related outcomes: Does the evidence support the concept? Nutrients 2020, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.M.; van Loon, L.J.C. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Lund, P.; Kristensen, N.B.; Frystyk, J.; Flyvbjerg, A.; Schjerling, P.; van Hall, G.; et al. Whey and casein labeled with l-[1-13c]leucine and muscle protein synthesis: Effect of resistance exercise and protein ingestion. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E231–E242. [Google Scholar] [CrossRef] [PubMed]
- Marsset-Baglieri, A.; Fromentin, G.; Airinei, G.; Pedersen, C.; Léonil, J.; Piedcoq, J.; Rémond, D.; Benamouzig, R.; Tomé, D.; Gaudichon, C. Milk protein fractions moderately extend the duration of satiety compared with carbohydrates independently of their digestive kinetics in overweight subjects. Br. J. Nutr. 2014, 112, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Masumoto, A.; Naito, Y.; Kiuchi, K.; Yoshimoto, Y.; Matsumoto, M.; Katashima, M.; Oka, J.; Ikemoto, S. Nighttime snacking reduces whole body fat oxidation and increases ldl cholesterol in healthy young women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R94–R101. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.G.M.; Westerterp, K.R.; Adam, T.C.M.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.J.; Soenen, S.; Luscombe-Marsh, N.D.; Ueland, Ø.; Westerterp-Plantenga, M.S. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J. Nutr. 2008, 138, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Acheson, K.J.; Blondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Emady-Azar, S.; Ammon-Zufferey, C.; Monnard, I.; Pinaud, S.; Nielsen-Moennoz, C.; Bovetto, L. Protein choices targeting thermogenesis and metabolism. Am. J. Clin. Nutr. 2011, 93, 525–534. [Google Scholar] [CrossRef]
- Veldhorst, M.A.B.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; Westerterp, K.R.; Engelen, M.P.K.J.; Brummer, R.-J.M.; Deutz, N.E.P.; Westerterp-Plantenga, M.S. Comparison of the effects of a high- and normal-casein breakfast on satiety, “satiety” hormones, plasma amino acids and subsequent energy intake. Br. J. Nutr. 2009, 101, 295–303. [Google Scholar] [CrossRef]
- Dufour, A.B.; Hannan, M.T.; Murabito, J.M.; Kiel, D.P.; McLean, R.R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 168–174. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Tosato, M.; Bernabei, R.; Onder, G. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Dir. Assoc. 2012, 13, 121–126. [Google Scholar] [CrossRef]
- Witard, O.C.; Wardle, S.L.; Macnaughton, L.S.; Hodgson, A.B.; Tipton, K.D. Protein considerations for optimising skeletal muscle mass in healthy young and older adults. Nutrients 2016, 8, 181. [Google Scholar] [CrossRef]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing is associated with decreases in appetite and energy intake—A meta-analysis in healthy adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Verdijk, L.B.; van Loon, L.J.C. The impact of pre-sleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: An update. Front. Nutr. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.; Frederiksen, R.; Hoppe, C.; Hvid, R.; Astrup, A. The effect of milk proteins on appetite regulation and diet-induced thermogenesis. Eur. J. Clin. Nutr. 2012, 66, 622–627. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description |
|---|---|
| Population | Human trials in adults or elderly (≥18 years old) |
| Intervention | Casein supplementation before sleep |
| Comparison | Casein vs. control (placebo, carbohydrate, or water) |
| Outcome | Metabolism or appetite |
| Setting (Study Design) | Randomized control trial |
| Study (Author, Year) | Subjects | Sample Size (Mean Age) | Study Design | Metabolic Parameters Measured | Protein Source | Exercise Trial Included? | Standardized Meal? | |
|---|---|---|---|---|---|---|---|---|
| Kinsey et al., 2014 [21] | Obese and overweight women | 44(29) | DRCT | -Appetite -Metabolism (RMR) | Casein | No | No | |
| Madzima et al., 2014 [22] | Active men | 11(24) | DRCT, CD | -Hunger, satiety, desire to eat -Metabolism (REE) | Casein | No | No | |
| Ormsbee et al., 2015 [23] | Obese women | 37(29) | DRCT | -Appetite -Metabolism | Casein | Yes | No | |
| Kinsey et al., 2016 [24] | Obese men | 12(27) | DRCT | -Appetite -Metabolism -SCAAT lipolysis | Casein | No | Yes | |
| Lay et al., 2018 [25] | Overweight men | 8(24) | DRCT, CD | -Hunger, fullness, desire to eat -Next day ad libitum breakfast | Casein mixture with whey and carbohydrate | No | Yes | |
| Leyh et al., 2018 [26] | Active women | 10(23) | DRCT, CD | -Appetite -Metabolism (REE) | Casein and cottage cheese | No | Yes | |
| Madzima et al., 2018 [27] | Active women | 9(25) | DRCT, CD | -Metabolism | Casein | Yes | No | |
| Trommelen et al., 2018 [28] | Active men | 36(23) | DRCT | -Next morning hunger and satiety | Casein | Yes | Yes | |
| Allman et al., 2020 [29] | Active men | 13(22) | DRCT, CD | -Metabolism (REE) -SCAAT lipolysis | Casein | Yes | Yes | |
| Morehen et al., 2020 [30] | Older men and women | 12(71) | SRCT | -Appetite -Metabolism -Next day ad libitum breakfast | Casein | No | Yes | |
| Nelson et al., 2021 [31] | Active women | 13(23) | RCT | -Appetite -Metabolism | Casein mixture with tryptophan | No | No | |
| Study (Author, Year) | Exercise Trial | Meal Standardization | |||
|---|---|---|---|---|---|
| Exercise Modality | Exercise Trial Length | Exercise Protocol | Single Meal or Entire Day | Energy Expenditure Estimate | |
| Ormsbee et al., 2015 [23] | Resistance training and HIIT | 4 week (3 days/week nonconsecutively) 2 days of resistance training and 1 day of HIIT | Resistance training -Total of 3 sets per exercise (first 2 sets for 10 repetitions and last set performed to muscular exhaustion). -Exercises performed included: chest press, seated row, leg press, shoulder press, leg extension, and leg curl. -Chest press and leg press at 70–85% of 1RM and the remaining exercises at a weight that could be lifted for 10–12 repetitions. -Total of 90–120 s rest periods. HIIT -Based on individual RPE scale (1–10). -Self-selected machine (cycle ergometer, treadmill, or elliptical trainer). -Total of 4 HIIT cycles performed for a total of 20 min. -HIIT cycle: warm up at RPE 5 (2 min), increased by 1 RPE every minute until reaching RPE 9, RPE reduced to 6 for 1 min, repeated ramping cycle up to RPE 9. | ||
| Lay et al., 2018 [25] | Entire day | -Distribution of 50% CHO, 32% FAT, 18% PRO -Energy matched to the participants’ average evening meal intake from their food record. | |||
| Leyh et al., 2018 [26] | Single meal | Energy expenditure estimate and macronutrient distribution details not stated. | |||
| Madzima et al., 2018 [27] | Resistance training | Single day session | -Exercises performed: chest press, leg press, lat pull-down, shoulder press, leg extension, and leg curl. -Performed at metronome cadence of 30 beats/minute (ratio of 2:2 s concentric and eccentric). -Total of 3 sets per exercise (first two sets for 10 repetitions and last set performed to muscular exhaustion). -Exercises performed at 60% of 1RM. | ||
| Allman et al., 2020 [29] | Resistance training | Single day session | -Exercises performed: back squat, bench press, Romanian deadlift, bent-over row, shoulder press, and reverse lunges. -Total of 4 sets of 10 repetitions (Set 1: 40% of 1RM, Set 2–4: 65% at 65% 1RM). | Entire day | -Distribution of 40% CHO, 30% FAT, %30 PRO -Energy matched to the participants’ Cunningham equation calculation. |
| Morehen et al., 2020 [30] | Single meal | -Distribution of 50% CHO, 32% FAT,18% PRO -Energy matched to the participants’ habitual intake record. | |||
| Study (Author, Year) | Next-Morning Appetite, Hunger, and Satiety | Metabolism | Lipolysis | Next-Morning Food Intake |
|---|---|---|---|---|
| Healthy young adult | ||||
| Madzima et al., 2014 [22] | No effect on appetite sensations (p > 0.05). | No group x time interaction for RMR (p > 0.05). Mean VO2 significantly greater in casein than in control (p < 0.0001). | ||
| Leyh et al., 2018 [26] | No effect on appetite sensations (p > 0.05). | No effect on metabolism (p > 0.05). | ||
| Madzima et al., 2018 [27] | No effect on metabolism (p > 0.05) | 24 g casein had significantly lower (p = 0.04) fat oxidation compared to 24 g whey when measured indirectly by RER. | ||
| Trommelen et al., 2018 [28] | No effect on appetite sensations (p > 0.05). | No effect on next-morning food intake (p > 0.05). | ||
| Allman et al., 2020 [29] | No effect on metabolism (p > 0.05). | No effect on lipolysis (p > 0.05). | ||
| Nelson et al., 2021 [31] | No effect on appetite sensations (p > 0.05). | No effect on metabolism (p > 0.05). | ||
| Overweight/obese young adult | ||||
| Kinsey et al., 2014 [21] | No group x time interaction for any appetite sensations (p > 0.05). Significant main effect of time interaction: Increased satiety (p = 0.03) Reduced desire to eat (p = 0.006) | No time or group x time interaction for RMR (p > 0.05). | ||
| Ormsbee et al., 2015 [23] | No effect on hunger or desire to eat (p > 0.05). Significant group x time interaction: Casein increased morning satiety compared to controls after 4 weeks (p = 0.02). | No group x time interaction for RMR (p > 0.05). Not statistically significant (p = 0.07) but casein had greater increases on RMR than in control. | ||
| Kinsey et al., 2016 [24] | No effect on hunger or satiety (p > 0.05). Significant group effect: Casein increased desire to eat compared to control (p = 0.03). | No effect on metabolism (p > 0.05). | No effect on lipolysis (p > 0.05). | |
| Overweight/obese young adult | ||||
| Lay et al., 2018 [25] | No effect on hunger or desire to eat (p > 0.05). Not statistically significant (p = 0.07) but casein had greater increases on next-morning fullness. | No effect on metabolism (p > 0.05). | No effect on next-morning food intake (p > 0.05). | |
| Healthy elderly adult | ||||
| Morehen et al., 2020 [30] | No effect on appetite sensations (p > 0.05). | No effect on metabolism (p > 0.05). | No effect on next-morning food intake (p > 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dela Cruz, J.; Kahan, D. Pre-Sleep Casein Supplementation, Metabolism, and Appetite: A Systematic Review. Nutrients 2021, 13, 1872. https://doi.org/10.3390/nu13061872
Dela Cruz J, Kahan D. Pre-Sleep Casein Supplementation, Metabolism, and Appetite: A Systematic Review. Nutrients. 2021; 13(6):1872. https://doi.org/10.3390/nu13061872
Chicago/Turabian StyleDela Cruz, Justin, and David Kahan. 2021. "Pre-Sleep Casein Supplementation, Metabolism, and Appetite: A Systematic Review" Nutrients 13, no. 6: 1872. https://doi.org/10.3390/nu13061872
APA StyleDela Cruz, J., & Kahan, D. (2021). Pre-Sleep Casein Supplementation, Metabolism, and Appetite: A Systematic Review. Nutrients, 13(6), 1872. https://doi.org/10.3390/nu13061872






