A Combined Analysis of Gut and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Study
Abstract
1. Introduction
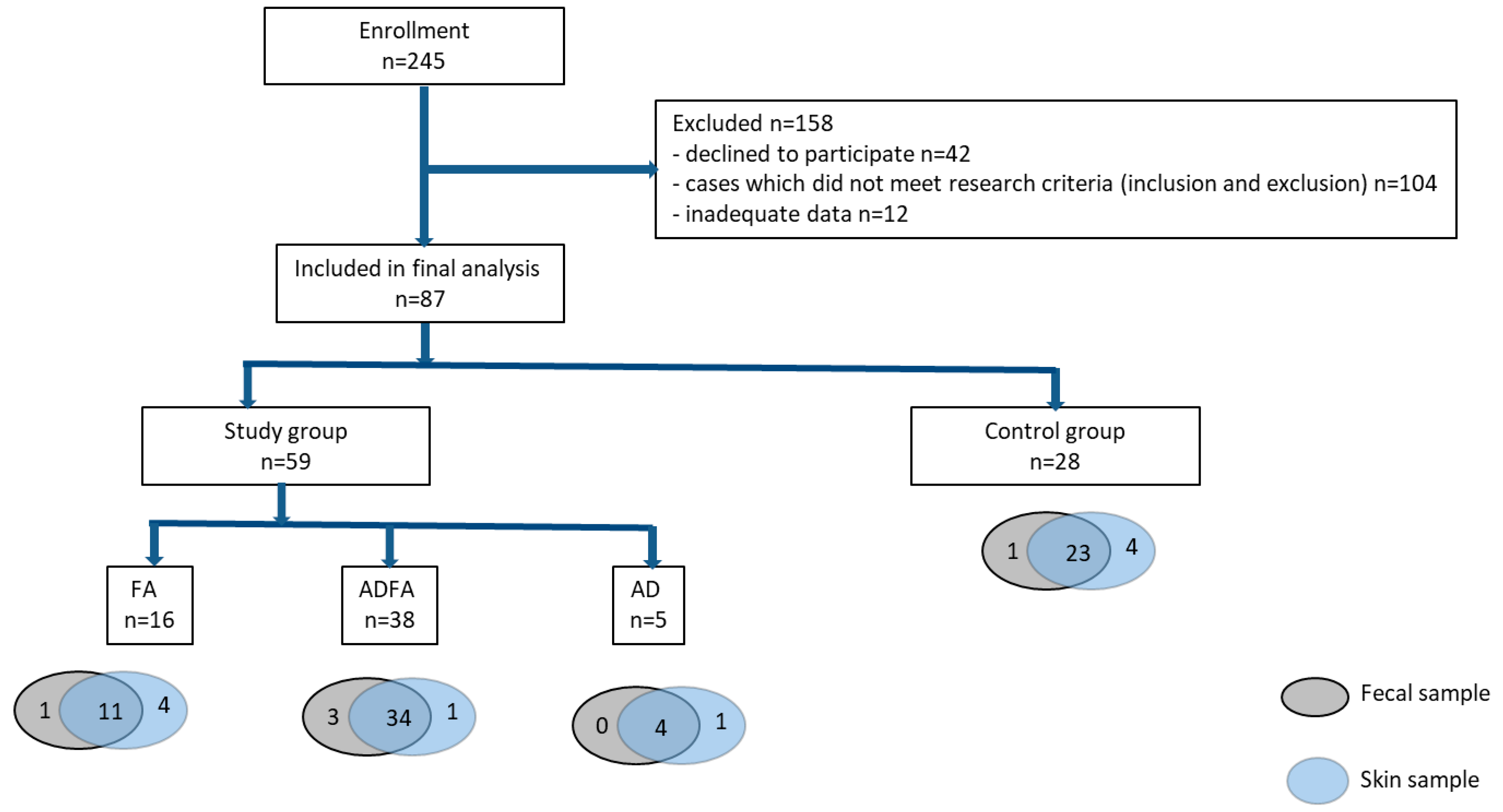
2. Methods
2.1. Study Group
2.2. Preliminary Questionnaire
2.3. FA Diagnosis
2.4. Atopic Dermatitis (AD) Diagnosis
- -
- history of skin rash affecting the flexures (folds of elbows, behind knees, fronts of ankles), cheeks, neck, around eyes or outer surfaces of limbs;
- -
- history of atopic diseases in a first-degree relative;
- -
- history of generally dry skin;
- -
- visible eczema involving cheeks, forehead, flexures and extensor/outer surfaces of limbs.
- -
- AD without FA;
- -
- AD with FA, i.e., eczema despite emollients; eczema and other typical symptoms of FA; significant improvement after elimination diet and deterioration after reintroduction of presumed allergen into the diet.
2.5. Sample Collection
2.6. Determination of sIgE
2.7. Metagenomic Analysis
2.8. Bioinformatics and Statistical Analyses
2.9. Additional Information
2.10. Data Availability
3. Results
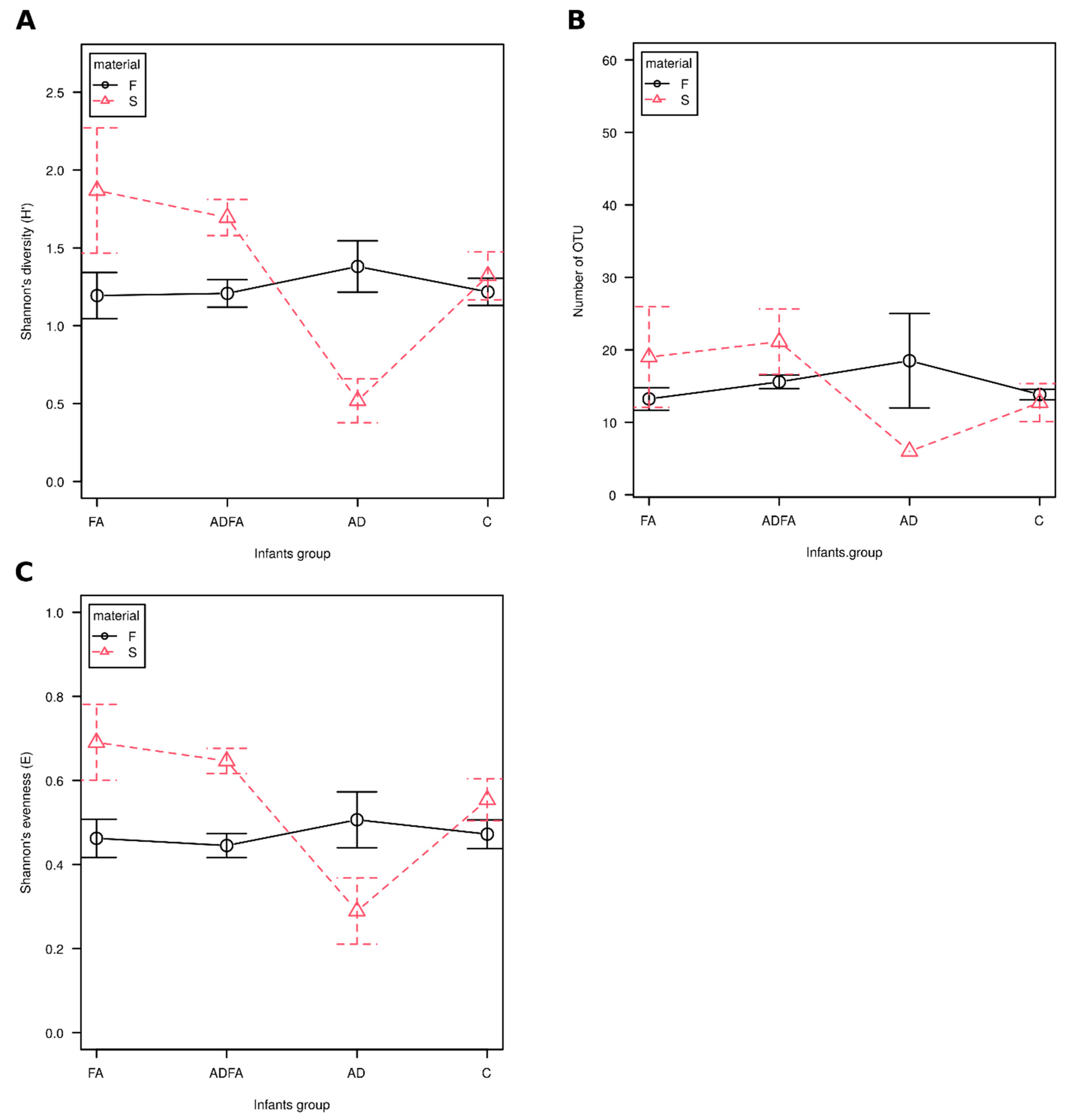
3.1. No Significant Differences in Alpha Diversity Indices
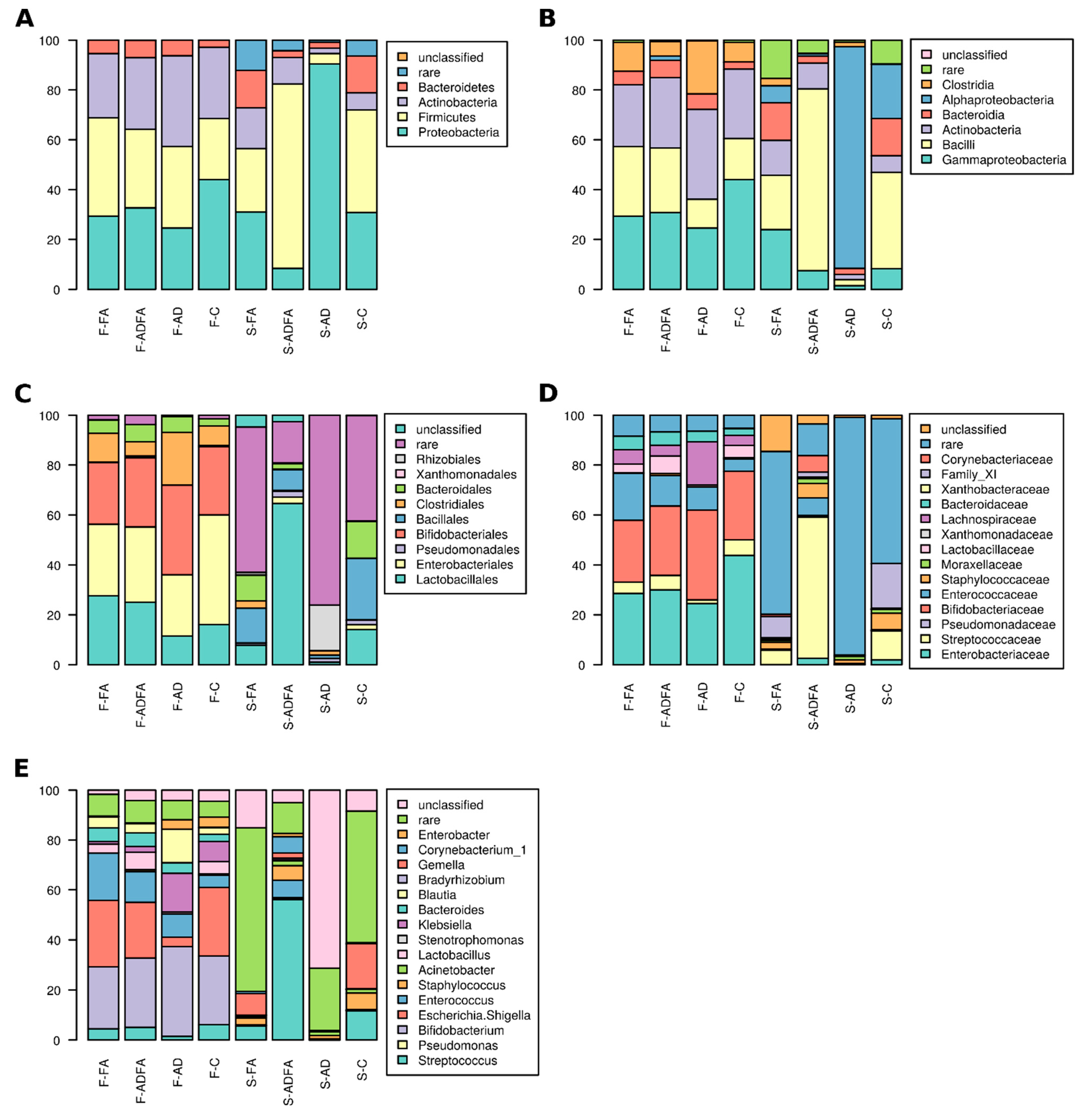
3.2. Gut and Skin Microbiota Differ According to Clinical Status
3.3. There Are Taxa Characteristic for Compartments and Clinical Status
3.4. There Are Taxa Whose Abundance was Positively Correlated in Feces and on Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | Study Group n = 59 (100%) | FA n = 16 (100%) | ADFA n = 38 (100%) | AD n = 5 (100%) |
|---|---|---|---|---|
| Clinical symptoms, n (%) | ||||
| skin | ||||
| rush, erythema, xerosis | 46 (77.96) | 3 (18.75) | 38 (100) | 5 (100) |
| itching | 0 | 0 | 17 (44.73) | 2 (40) |
| urticarial/angiooedema | 5 (8.47) | 1 (6.25) | 4 (10.25) | 0 |
| gastrointestinal | ||||
| colic/abdominal pain | 43 (72.88) | 12 (75) | 29 (76.31) | 2 (40) |
| vomiting/massive regurgitation | 26 (44.06) | 6 (37.5) | 19 (50) | 1 (20) |
| weight loss/poor weight gain | 9 (15.25) | 0 | 9 (23.68) | 0 |
| loose of apetite /flatulence/reflection | 6 (10.16) | 2 (12.5) | 4 (10.52) | 0 |
| diarrhea | 24 (40.60) | 8 (50) | 16 (42.10) | 0 |
| blood/mucus in stool | 23 (38.98) | 9 (56.25) | 13 (34.21) | 1 (20) |
| constipation | 2 (3.38) | 1 (6.25) | 0 | 1 (20) |
| others | ||||
| runny nose/snuffles | 9 (15.25) | 5 (31.25) | 4 (10.52) | 0 |
| wheezing | 4 (6.77) | 1 (6.25) | 3 (7.89) | 0 |
| recurrent cough | 2 (3.38) | 1 (6.25) | 1 (2.63) | 0 |
| pallor/cyanosis after ingestion | 6 (10.16) | 3 (18.75) | 3 (7.89) | 0 |
| sweating, weakness after eating food | 3 (5.08) | 2 (12.5) | 1 (2.63) | 0 |
| sIgE, n (%) | ||||
| at least >1 | 10 (16.94) | 2 (12.5) | 8 (21.05) | 0 |
| cow’s milk | 0 | 2 (12.5) | 6 (15.78) | 0 |
| egg | 0 | 0 | 1 (2.63) | 0 |
| wheat | 0 | 0 | 1 (2.63) | 0 |
| peanut | 0 | 0 | 1 (2.63) | 0 |
| SCORAD index, points | ||||
| mean ± SD | 23.77 ± 19 | - | 23.03 ± 17.4 | 29.4 ± 20.8 |
| Atopic dermatitis severity, n (%) | ||||
| Mild <20 pkt | 22 (37.28) | 0 | 20 (52.63) | 2 (40) |
| Moderate 20–40 pkt | 14 (23.72) | 0 | 13 (34.21) | 1 (20) |
| Severe >40 pkt | 7 (11.86) | 0 | 5 (13.15) | 2 (40) |
| AD onset (<12 weeks), n (%) | 35 (59.32) | - | 31 (81.57) | 4 (80) |
| mean ± SD | 6.4 ± 5.4 | - | 6.3 ± 5.3 | 6.6 ± 5.5 |
| Types of CMA from gastrointestinal tract | ||||
| FPIP | 19 (32.20) | 8 (50) | 11 (28.94) | 0 |
| FPIES | 6 (10.16) | 2 (12.5) | 4 (10.52) | 0 |
| FGIDs | 21 (35.59) | 5 (31.25) | 16 (42.10) | 0 |
| IgE-mediated FA | 8 (13.56) | 2 (12.5) | 6 (15.78) | 0 |
References
- Rook, G.A.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 1, 46–64. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Shen, X.; Wang, M.; Zhang, X.; He, M.; Li, M.; Cheng, G.; Wan, C.; He, F. Dynamic construction of gut microbiota may influence allergic diseases of infants in southwest China. BMC Microbiol. 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Jungles, K.N.; Jungles, K.M.; Greenfield, L.; Mahdavinia, M. The infant microbiome and its impact on development of food allergy. Immunol. Allergy Clin. N. Am. 2021, 41, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.L. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, T.; Grabnar, I.; Benedik, E.; Tušar, T.; Robič Pikel, T.; Fidler Mis, N.; Bogovič Matijašić, B.; Rogelj, I. Microbes in infant gut development: Placing abundance within environmental, clinical and growth parameters. Sci. Rep. 2017, 7, 11230. [Google Scholar] [CrossRef]
- Levin, A.M.; Sitarik, A.R.; Havstad, S.L.; Fujimura, K.E.; Wegienka, G.; Cassidy-Bushrow, A.E.; Kim, H.; Zoratti, E.M.; Lukacs, N.W.; Boushey, H.A.; et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci. Rep. 2016, 6, 31775. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 74, 1823–1836. [Google Scholar] [CrossRef]
- Baviera, G.; Leoni, M.C.; Capra, L.; Cipriani, F.; Longo, G.; Maiello, N.; Ricci, G.; Galli, E. Microbiota in healthy skin and in atopic eczema. BioMed Res. Int. 2014, 2014, 436921. [Google Scholar] [CrossRef] [PubMed]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbi-ome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. CHILD Study Investigators. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef]
- Łoś-Rycharska, E.; Gołębiewski, M.; Grzybowski, T.; Rogala-Ładniak, U.; Krogulska, A. The microbiome and its impact on food allergy and atopic dermatitis in children. Adv. Dermatol. Allergol. 2020, 5, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Rachid, R.; Stephen-Victor, E.; Chatila, T.A. The microbial origins of food allergy. J. Allergy Clin. Immunol. 2021, 47, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [PubMed]
- Marrs, T.; Flohr, C. The role of skin and gut microbiota in the development of atopic eczema. Br. J. Dermatol. 2016, 175, 13–18. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Lee, M.J.; Kang, M.J.; Lee, S.Y.; Lee, E.; Kim, K.; Won, S.; Suh, D.I.; Kim, K.W.; Sheen, Y.H.; Ahn, K.; et al. Perturbations of Gut Microbiome Genes in Infants With Atopic Dermatitis According to Feeding Type. J. Allergy Clin. Immunol. 2018, 141, 1310–1319. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow’s milk protein allergy in infants and children: A practical guideline of the GI-committee of ESPGHAN. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, K.J.; Kong, M.S.; Chang, H.J.; Huang, J.L. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr. Allergy Immunol. 2016, 27, 254–262. [Google Scholar] [CrossRef]
- Aparicio, M.; Alba, C.; Rodríguez, J.M.; Fernández, L. Microbiological and immunological markers in milk and infant feces for common gastrointestinal disorders: A pilot study. Nutrients 2020, 12, 634. [Google Scholar] [CrossRef]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnotzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life gut microbiome and egg allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef]
- Fieten, K.B.; Totté, J.E.E.; Levin, E.; Reyman, M.; Meijer, Y.; Knulst, A.; Schuren, F.; Pasmans, S.G.M.A. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int. Arch. Allergy Immunol. 2018, 175, 77–84. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440. [Google Scholar] [CrossRef]
- Laborel-Préneron, E.; Bianchi, P.; Boralevi, F.; Lehours, P.; Fraysse, F.; Morice-Picard, F.; Sugai, M. Sato’o, Y.; Badiou, C.; Lina, G.; et al. Effects of the Staphylococcus aureus and Staphylococcus epidermidis Secretomes Isolated from the Skin Microbiota of Atopic Children on CD4+ T Cell Activation. PLoS ONE 2015, 10, e0141067. [Google Scholar]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, C.; Komarow, H.D.; NISC Comparative Se-quence Program; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Charbonnier, L.M.; Georgiev, P.; Oettgen, H.C.; Rachid, R.; Chatila, T.A. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 2015, 42, 512–523. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Connolly, J.; O’B Hourihane, J.; Fallon, P.G.; McLean, W.H.I.; Murray, D.; Jo, J.H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin Microbiome Before Development of Atopic Dermatitis: Early Colonization With Commensal Staphylococci at 2 Months Is Associated With a Lower Risk of Atopic Dermatitis at 1 Year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef]
- West, C.E.; Rydén, P.; Lundin, D.; Engstrand, L.; Tulic, M.K.; Prescott, S.L. Gut Microbiome and innate immune response patterns in IgE-associated eczema. Clin. Exp. Allergy 2015, 45, 1419–1429. [Google Scholar] [CrossRef]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Wen, H.; Wang, Z.; Ding, C.; Liu, X.; Gao, Y.; Su, H.; Zhang, J.; Han, Y.; et al. Inverse Association Between the Skin and Oral Microbiota in Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 1779–1787. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.W.; Pearce, N.; Sibbald, B.; Staward, A.W.; et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef]
- Meyer, R.; Chebar Lozinsky, A.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy 2020, 75, 14–32. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Hay, R.J.; Archer, C.B.; Shipley, M.J.; Hunter, J.J.; Bingham, E.A.; Finlay, A.Y.; Pembroke, A.C.; Graham-Brown, R.A.; et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br. J. Dermatol. 1994, 131, 383–396. [Google Scholar] [CrossRef]
- Williams, H.C. Clinical practice. Atopic dermatitis. N. Engl. J. Med. 2005, 352, 2314–2324. [Google Scholar] [CrossRef]
- Stalder, J.F.; Taïeb, A.; Atherton, D.J.; Bieber, P.; Bonifazi, E.; Broberg, A.; Calza, A.; Coleman, R.; De Prost, Y.; Stalder, J.F.; et al. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar]
- Thiem, D.; Gołębiewski, M.; Hulisz, P.; Piernik, A.; Hrynkiewicz, K. How does salinity shape bacterial and fungal microbiomes of Alnus glutinosa Roots? Front. Microbiol. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project:improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, M.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package v.2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 January 2020).
- Chen, J. GUniFrac: Generalized UniFrac Distances. R Package v.1.1. 2018. Available online: https://CRAN.R-project.org/package=GUniFrac (accessed on 23 January 2020).
- Chen, J.; Bittinger, K.; Charlson, E.S.; Hoffmann, C.; Lewis, J.; Wu, G.D.; Collman, R.G.; Bushman, F.D.; Li, H. Associating microbiome composition with environmental covariates using generilized UniFrac distances. Bioinformatics 2012, 28, 2106–2113. [Google Scholar] [CrossRef]
- Sheneman, L.; Evans, J.; Foster, J.A. Clearcut: A fast implementation of relaxed neighbor joining. Bioinformatics 2006, 22, 2823–2824. [Google Scholar] [CrossRef]
- Gołębiewski, M.; Tretyn, A. Generating amplicon reads for microbial community assessment with next-generation sequencing. J. Appl. Microbiol. 2020, 128, 330–354. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A Prospective Microbiome-Wide Association Study of Food Sensitization and Food Allergy in Early Childhood. Allergy 2018, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut Microbiota Composition and Butyrate Production in Children Affected by non-IgE-mediated Cow’s Milk Allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, S.Y.; Kang, M.J.; Kim, B.S.; Lee, M.J.; Jung, S.S.; Yoon, J.S.; Cho, H.J.; Lee, E.; Yang, S.I.; et al. Imbalance of Gut Streptococcus; Clostridium, and Akkermansia Determines the Natural Course of Atopic Dermatitis in Infant. Allergy Asthma Immunol. Res. 2020, 12, 322–337. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, J.Y.; Shin, J.W.; Huh, C.H.; Park, K.C.; Du, M.H.; Yoon, S.; Na, J.I. Changes in Lesional and Non-lesional Skin Microbiome During Treatment of Atopic Dermatitis. Acta Derm. Venereol. 2019, 99, 284–290. [Google Scholar] [CrossRef]
- Dong, P.; Feng, J.J.; Yan, D.Y.; Lyu, Y.J.; Xu, X. Early-life gut microbiome and cow’s milk allergy- a prospective case—Control 6-month follow-up study. Saudi. J. Biol. Sci. 2018, 25, 875–880. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Goedert, J.J.; Pu, A.; Yu, G.; Shi, J.S. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. EBioMedicine 2016, 3, 172–179. [Google Scholar] [CrossRef]
- Ruohtula, T.; de Goffau, M.C.; Nieminen, J.K.; Honkanen, J.; Siljander, H.; Hämäläinen, A.M.; Peet, A.; Tillmann, V.; Ilonen, J.; Niemelä, O.; et al. Maturation of Gut Microbiota and Circulating Regulatory T Cells and Development of IgE Sensitization in Early Life. Front. Immunol. 2019, 10, 2494. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.E.; Bhatia, N.D.; Friedman, A.; Eng, R.M.; Seite, S. The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier. J. Drugs Dermatol. 2017, 16, 12–18. [Google Scholar] [CrossRef]
- Seité, S.; Zelenkova, H.; Martin, R. Clinical efficacy of emollients in atopic dermatitis patients—Relationship with the skin microbiota modification. Clin. Cosmet. Investig. Dermatol. 2017, 10, 25–33. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci. Transl. Med. 2019, 11, eaav2685. [Google Scholar] [CrossRef] [PubMed]
- Bosman, E.S.; Albert, A.Y.; Lui, H.; Dutz, J.P.; Vallance, B.A. Skin exposure to narrow band ultraviolet (UVB) light modulates the human intestinal microbiome. Front. Microbiol. 2019, 10, 2410. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Lee, R.P.; Huang, J.; Hsu, M.; Thames, G.; Gilbuena, I.; Long, J.; Xu, Y.; Park, E.H.; et al. Pomegranate Juice and Extract Consumption Increases the Resistance to UVB-induced Erythema and Changes the Skin Microbiome in Healthy Women: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 14528. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Nam, B.; Kim, S.A.; Park, S.D.; Kim, H.J.; Kim, J.S.; Bae, C.H.; Kim, J.Y.; Nam, W.; Lee, J.L.; Sim, J.H. Regulatory effects of Lactobacillus plantarum HY7714 on skin health by improving intestinal condition. PLoS ONE 2020, 15, e0231268. [Google Scholar] [CrossRef]
- Nagino, T.; Kaga, C.; Kano, M.; Masuoka, N.; Anbe, M.; Moriyama, K.; Maruyama, K.; Nakamura, S.; Shida, K.; Miyazaki, K. Effects of fermented soymilk with Lactobacillus casei Shirota on skin condition and the gut microbiota: A randomised clinical pilot trial. Benef. Microbes 2018, 9, 209–218. [Google Scholar] [CrossRef]
- Zheng, H.; Liang, H.; Wang, Y.; Miao, M.; Shi, T.; Yang, F.; Liu, E.; Yuan, W.; Ji, Z.S.; Li, D.K. Altered Gut Microbiota Composition Associated With Eczema in Infants. PLoS ONE 2016, 11, e0166026. [Google Scholar] [CrossRef]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut Microbiota Profile in Children Affected by Atopic Dermatitis and Evaluation of Intestinal Persistence of a Probiotic Mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Clavel, T.; Gomes Neto, J.C.; Lagkouvardos, I.; RamerTait, A.T. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomesd. Immunol. Rev. 2017, 279, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Leong, L.E.X.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J. Allergy Clin. Immunol. 2018, 41, 94–103. [Google Scholar] [CrossRef]
- Mackenzie, B.W.; Waite, D.W.; Hoggard, M.; Douglas, R.G.; Taylor, M.W.; Biswas, K. Bacterial community collapse: A meta-analysis of the sinonasal microbiota in chronic rhinosinusitis. Environ. Microbiol. 2017, 19, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Retuerto, M.; Seité, S.; Martin, R.; Kus, M.; Ghannoum, M.A.; Baron, E.; Mukherjee, P.K. Effect of an Emollient on the Mycobiome of Atopic Dermatitis Patients. J. Drugs Dermatol. 2018, 17, 1039–1048. [Google Scholar] [PubMed]
- Hoyles, L.; Inganas, E.; Falsen, E.; Drancourt, M.; Weiss, N.; McCartney, A.L.; Collins, M.D. Bifidobacterium scardovii sp. nov., from human sources. Int. J. Syst. Evol. Microbiol. 2002, 52, 995–999. [Google Scholar] [PubMed]
- Man, W.H.; Scheltema, N.M.; Clerc, M.; van Houten, M.A.; Nibbelke, E.E.; Achten, N.B.; Arp, K.; Sanders, E.A.M.; Bont, L.J.; Bogaert, D. Infant respiratory syncytial virus prophylaxis and nasopharyngeal microbiota until 6 years of life: A subanalysis of the MAKI randomised controlled trial. Lancet Respir. Med. 2020, 8, 1022–1031. [Google Scholar] [CrossRef]
- Johnson, R.C.; Ellis, M.W.; Lanier, J.B.; Schlett, C.D.; Cui, T.; Merrell, D.S. Correlation between nasal microbiome composition and remote purulent skin and soft tissue infections. Infect. Immun. 2015, 83, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Fernando, J.R.; Butler, C.A.; Adams, G.G.; Mitchell, H.L.; Dashper, S.G.; Escobar, K.; Hoffmann, B.; Shen, P.; Walker, G.D.; Yuan, Y.; et al. The prebiotic effect of CPP-ACP sugar-free chewing gum. J. Dent. 2019, 91, 103225. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiotaand Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef]
- Meisel, J.S.; Hannigan, G.D.; Tyldsley, A.S.; SanMiguel, A.J.; Hodkinson, B.P.; Zheng, Q.; Grice, E.A. Skin Microbiome Surveys Are Strongly Influenced by Experimental Design. J. Investig. Dermatol. 2016, 136, 947–956. [Google Scholar] [CrossRef]
- Stehlikova, Z.; Kostovcik, M.; Kostovcikova, K.; Kverka, M.; Juzlova, K.; Rob, F.; Hercogova, J.; Bohac, P.; Pinto, Y.; Uzan, A.; et al. Dysbiosis of Skin Microbiota in Psoriatic Patients: Co-occurrence of Fungal and Bacterial Communities. Front. Microbiol. 2019, 10, 438. [Google Scholar] [CrossRef]

| Group | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study group |
|
|
| Control group |
|
| Parameter | Study Group n = 59 (100%) | FA n = 16 (100%) | ADFA n = 38 (100%) | AD n = 5 (100%) | Control Group n = 28 (100%) | p |
|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||
| female | 24 (40.7) | 6 (37.5) | 16 (42.1) | 2 (40) | 12 (42.9) | >0.05 |
| male | 35 (59.3) | 10 (62.5) | 22 (57.9) | 3 (60) | 16 (57.1) | |
| Age at specimen collection (weeks) mean ± SD | 15.56 ± 7.00 | 16.63 ± 6.72 | 15.92 ± 7.43 | 16.6 ± 8.39 | 14.29 ± 6.52 | >0.05 |
| Weight at birth (g), mean ± SD | 3495 ± 548 | 3518 ± 493 | 3571 ± 480 | 3588 ± 228 | 3360 ± 686 | >0.05 |
| Mode of delivery, n (%) | ||||||
| Vaginal | 43 (72.9) | 12 (75) | 27 (71.1) | 4 (80) | 16 (57.1) | >0.05 |
| C-section | 16 (27.1) | 4 (25) | 11 (28.9) | 1 (20) | 12 (42.9) | |
| Time of birth, Hbd, mean ± SD | 39.3 ± 1.9 | 39.6 ± 1.7 | 39.5 ± 1.2 | 40 ± 0.7 | 38.7 ± 2.6 | >0.05 |
| Apg scale, points, mean ± SD | 9.6 ± 1.2 | 9.6 ± 0.8 | 9.7 ± 0.6 | 10 ± 0 | 9.2 ± 1.9 | >0.05 |
| Mode of feeding at entry, n (%) | ||||||
| Exclusively breastfeeding | 28 (47.5) | 9 (56.3) | 18 (47.4) | 1 (20) | 13 (46.4) | >0.05 |
| Mixed | 7 (11.0) | 2 (12.5) | 4 (10.5) | 1 (20) | 3 (10.7) | |
| Milk formula | 24 (40.7) | 5 (31.3) | 16 (42.1) | 3 (60) | 12 (42.9) | |
| Breast-feeding, n (%) | ||||||
| No, never | 8 (13.6) | 1 (6.3) | 1 (2.6) | 1 (20) | 5 (17.9) | >0.05 |
| Yes, currently | 35 (59.3) | 11 (68.8) | 12 (57.9) | 2 (40) | 16 (57.1) | |
| Solid food intake, Yes, n (%) | 11 (18.6) | 2 (12.5) | 8 (21.1) | 1 (20) | 8 (28.6) | >0.05 |
| Place of living n (%) | ||||||
| non-rural | 56 (94.9) | 15 (93.8) | 36 (94.7) | 5 (100) | 28 (100) | >0.05 |
| rural | 3 (5.1) | 1 (6.3) | 2 (5.3) | 0 | 0 | |
| Average living space per person, (m2) mean ± SD | 24.6 ± 14.1 | 27.4 ± 17.9 | 24.4 ± 13.1 | 29.2 ± 19.3 | 22.4 ± 12.3 | >0.05 |
| Comiss score, points mean ± SD | 12.71 ± 3.2 | 12.73 ± 1.54 | 13.62 ± 1.96 | 6 ± 0.65 | 5.63 ± 2.74 | <0.00001 |
| Parent’s age, years | ||||||
| Mother | 28.9 ± 4.6 | 29 ± 4.7 | 30.1 ± 3.9 | 29 ± 5.1 | 27.29 ± 4.9 | >0.05 |
| Father | 31.7 ± 5.2 | 32 ± 5.4 | 32.37 ± 4.7 | 31.2 ± 4.1 | 30.6 ± 5.9 | |
| Parent’s education, n (%) | ||||||
| Mother Basic | 6 (10.2) | 4 (25) | 1 (2.6) | 1 (20) | 6 (21.4) | 0.03 |
| Secondary | 19 (32.2) | 4 (25) | 12 (31.6) | 3 (60) | 12 (42.9) | |
| Higher | 34 (57.6) | 8 (50) | 25 (65.8) | 1 (20) | 10 (35.7) | |
| Father Basic | 10 (16.9) | 5 (31.3) | 5 (13.2) | 0 | 7 (25) | >0.05 |
| Secondary | 23 (39) | 5 (31.3) | 15 (39.5) | 3 (60) | 14 (50) | |
| Higher | 26 (44.1) | 6 (37.5) | 18 (47.4) | 2 (40) | 7 (25) | |
| Atopy in family, n (%) | ||||||
| Yes | 43 (72.9) | 13 (81.3) | 28 (73.7) | 2 (40) | 15 (53.6) | >0.05 |
| Mother | 29 (49.2) | 10 (62.5) | 18 (47.4) | 1 (20) | 6 (21.4) | 0.025 |
| Father | 19 (32.2) | 4 (25) | 14 (36.8) | 1 (20) | 6 (21.4) | >0.05 |
| Siblings | 14 (56.0) | 4 (57.1) | 10 (62.5) | 0 | 4 (40) | >0.05 |
| Siblings, n (%) | 25 (42.4) | 7 (43.8) | 16 (42.1) | 2 (40) | 10 (35.7) | >0.05 |
| Pets at home, n (%) | ||||||
| Yes | 25 (42.4) | 8 (50) | 15 (39.5) | 2 (40) | 13 (46.4) | >0.05 |
| Dog | 20 (33.9) | 6 (37.5) | 12 (31.6) | 2 (40) | 9 (32.1) | >0.05 |
| Cat | 5 (8.5) | 4 (25) | 1 (2.6) | 0 | 3 (10.7) | >0.05 |
| Tobaco smoke exposure, n (%) | ||||||
| Mother active during pregnancy | 2 (3.4) | 1 (6.3) | 1 (2.6) | 0 | 3 (10.7) | >0.05 |
| Mother during pregnancy passive | 12 (20.3) | 5 (31.3) | 7 (18.4) | 0 | 8 (28.6) | >0.05 |
| Mother during lactation | 1 (1.7) | 0 | 1 (2.6) | 0 | 3 (10.7) | >0.05 |
| Child passive | 14 (23.7) | 5 (31.3) | 7 (18.4) | 2 (40) | 10 (35.7) | >0.05 |
| Antibiotics during pregnancy (3rd tr), n (%) | 4 (6.8) | 0 | 4 (10.5) | 0 | 4 (14.3) | >0.05 |
| OTU | Abundance in Different Groups | p | |
|---|---|---|---|
| 11 | Stenotrophomonas maltophilia | AD > ADFA > FA, C | 0.030 |
| 21 | Bacteroides | FA, ADFA > AD > C | 0.030 |
| 35 | Parabacteroides | AD, ADFA > FA, C | 0.010 |
| 62 | Rhizobium (Agrobacterium fabrum) | C > AD > FA, ADFA | 0.023 |
| 85 | Veilonella dispar | FA > ADFA > AD, C | 0.015 |
| Group | Skin | Feces | ||||||
|---|---|---|---|---|---|---|---|---|
| OTU | p | More Abundant in | OTU | p | More Abundant in | |||
| AD + ADFA vs C | 87 | Acinetobacter variabilis | 0.012 | AD + ADFA | 11 | Stenotrophomonas maltophilia | 0.012 | AD + ADFA |
| 35 | Parabacteroides | 0.006 | ||||||
| 2 | Streptococcus sp. | 0.044 | C | 41 | Bacteroides dorei | 0.043 | ||
| FA + ADFA vs C | 13 | Bradyrhizobium sp | 0.027 | C | 21 | Bacteroides | 0.008 | FA + ADFA |
| 30 | Serratia marcescens | 0.042 | C | |||||
| 68 | Lactococcus lactis | 0.042 | ||||||
| Infants with Allergy Symptoms | Healthy Infants | ||||||
|---|---|---|---|---|---|---|---|
| OTU | rho | p | OTU | rho | p | ||
| 2 | Streptococcus sp. | 0.34 | 0.030 | 2 | Streptococcus sp. | 0.17 | 0.030 |
| 14 | Gemella sp. | 0.45 | 0.002 | 25 | Acinetobacter sp | 0.72 | 0.0002 |
| 16 | Bifidobacterium scardovii | 0.35 | 0.020 | 31 | Lactobacillus gasseri | 0.64 | 0.0002 |
| 17 | Corynebacterium nuruki | 0.72 | 0 | 32 | Haemophilus haemolyticus | 0.55 | 0.010 |
| 20 | Rothia mucillaginosa | 0.45 | 0.002 | 40 | Bacteroides ovatus | 0.61 | 0.004 |
| 31 | Lactobacillus gasseri | 0.35 | 0.020 | 53 | Schaalia odontolytica | 0.46 | 0.030 |
| 38 | Lactobacillus sp. | 0.68 | 0 | 74 | Actinomyces graevenitzi | 0.72 | 0.0002 |
| 92 | Lactobacillus salivarius | 1 | 0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łoś-Rycharska, E.; Gołębiewski, M.; Sikora, M.; Grzybowski, T.; Gorzkiewicz, M.; Popielarz, M.; Gawryjołek, J.; Krogulska, A. A Combined Analysis of Gut and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Study. Nutrients 2021, 13, 1682. https://doi.org/10.3390/nu13051682
Łoś-Rycharska E, Gołębiewski M, Sikora M, Grzybowski T, Gorzkiewicz M, Popielarz M, Gawryjołek J, Krogulska A. A Combined Analysis of Gut and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Study. Nutrients. 2021; 13(5):1682. https://doi.org/10.3390/nu13051682
Chicago/Turabian StyleŁoś-Rycharska, Ewa, Marcin Gołębiewski, Marcin Sikora, Tomasz Grzybowski, Marta Gorzkiewicz, Maria Popielarz, Julia Gawryjołek, and Aneta Krogulska. 2021. "A Combined Analysis of Gut and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Study" Nutrients 13, no. 5: 1682. https://doi.org/10.3390/nu13051682
APA StyleŁoś-Rycharska, E., Gołębiewski, M., Sikora, M., Grzybowski, T., Gorzkiewicz, M., Popielarz, M., Gawryjołek, J., & Krogulska, A. (2021). A Combined Analysis of Gut and Skin Microbiota in Infants with Food Allergy and Atopic Dermatitis: A Pilot Study. Nutrients, 13(5), 1682. https://doi.org/10.3390/nu13051682






