Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Analysis of Dietary Intake and Eating Habits
2.3. Anthropometric Measures
2.4. Bone Mineral Density
2.5. Blood Parameters
2.6. Physical Activity
2.7. Data Collection and Statistics
3. Results
3.1. Dietary Habits and Nutrient Intake
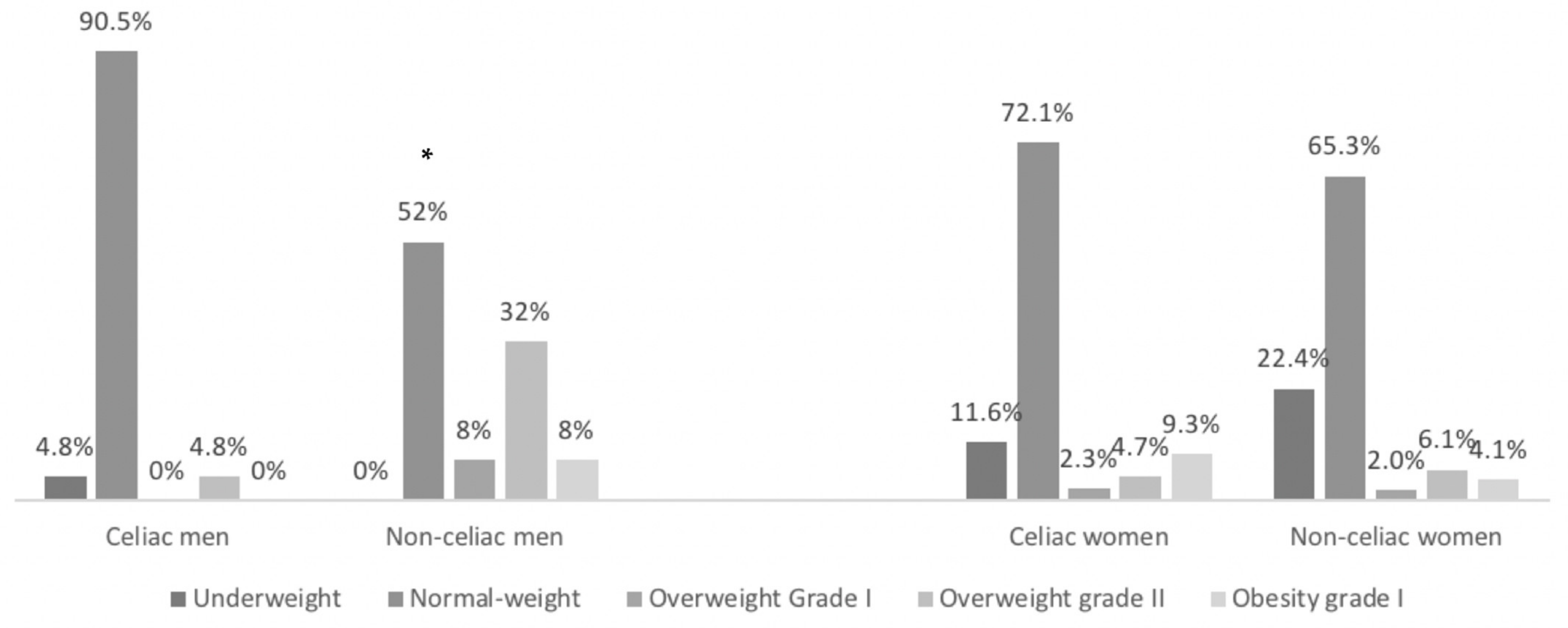
3.2. Anthropometric Measurements
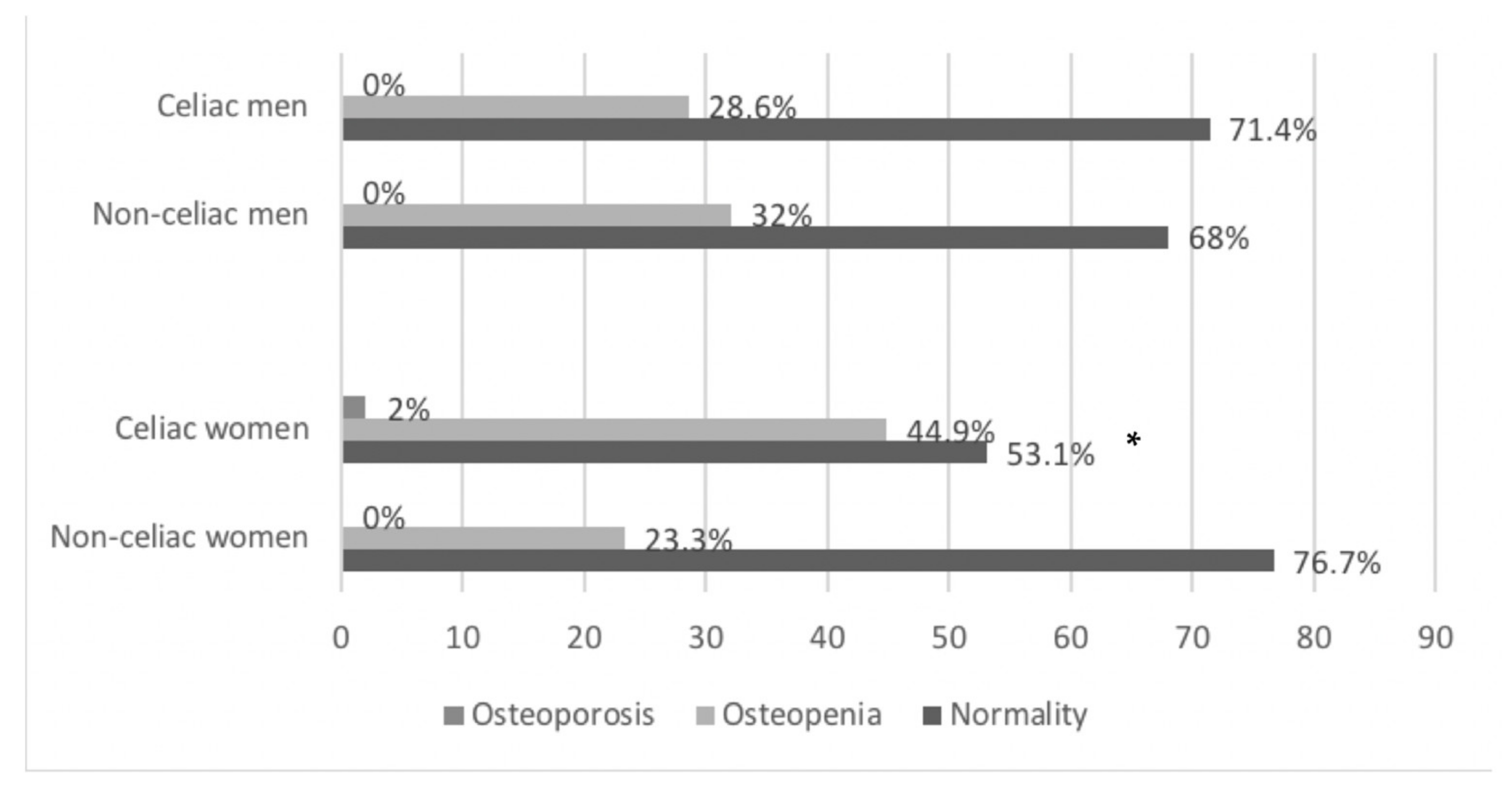
3.3. Bone Mineral Density
3.4. Blood Parameters
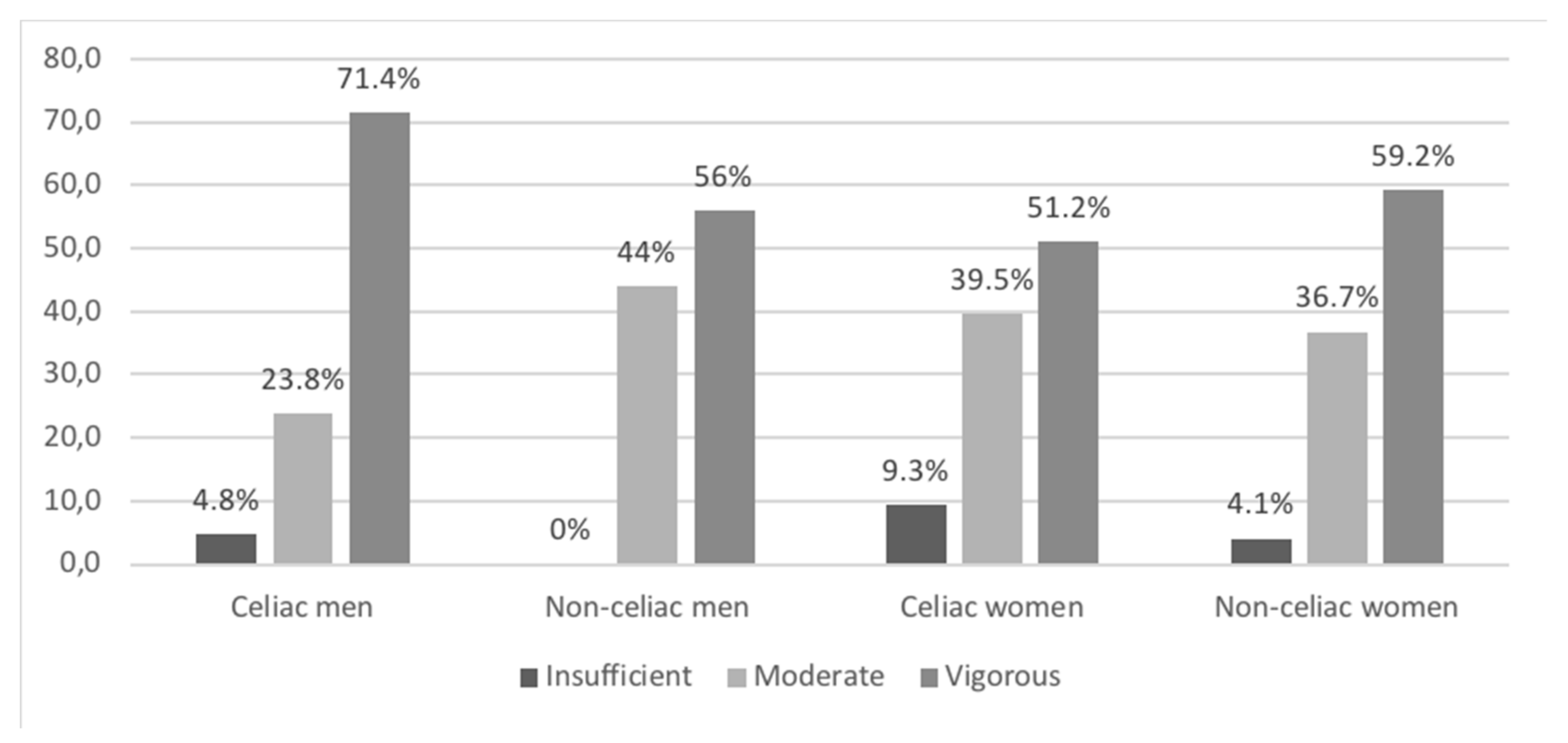
3.5. Physical Activity
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polanco, I. Libro Blanco de la Enfermedad Celiaca; Comunidad de Madrid: Madrid, Spain, 2008. [Google Scholar]
- García-Manzanares, A.; Lucendo, A.J. Nutritional and dietary aspects of celiac disease. Nutr. Clin. Pract. 2011, 26, 163–173. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; deBruyn, J.; Ronksley, P.E.; Shaheen, A.A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Khaleghi, S.; Cartee, A.K.; Marietta, E.V.; Larson, J.J.; King, K.S.; Savolainen, O.; Ross, A.B.; Rajkumar, S.V.; Camilleri, M.J.; Rubio-Tapia, A.; et al. Community-Based Study of Celiac Disease Autoimmunity Progression in Adults. Gastroenterology 2020, 158, 151–159.e3. [Google Scholar]
- Mariani, P.; Viti, M.G.; Montouri, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The gluten-free diet: A nutritional risk factor for adolescents with celiac disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Hopman, E.G.; le Cessie, S.; von Blomberg, B.M.; Mearin, M.L. Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- Churruca, I.; Miranda, J.; Lasa, A.; Bustamante, M.; Larretxi, I.; Simon, E. Analysis of Body Composition and Food Habits of Spanish Celiac Women. Nutrients 2015, 7, 5515–5531. [Google Scholar] [CrossRef]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients With Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef]
- Larretxi, I.; Simon, E.; Benjumea, L.; Miranda, J.; Bustamante, M.A.; Lasa, A.; Eizaguirre, F.J.; Churruca, I. Gluten-free-rendered products contribute to imbalanced diets in children and adolescents with celiac disease. Eur. J. Nutr. 2019, 58, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J. Hum. Nutr. Diet. 2013, 26, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Cicala, M.; Tiberi, E.; Spadaccio, C.; Marcella, L.; Gatto, A.; Calzolari, P.; Castellucci, G. High fat consumption in children with celiac disease. Acta Gastroenterol. Belg. 2009, 72, 296–300. [Google Scholar] [PubMed]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-Free Diet in Children: An Approach to a Nutritionally Adequate and Balanced Diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef] [PubMed]
- Sue, A.; Dehlsen, K.; Ooi, C.Y. Paediatric Patients with Coeliac Disease on a Gluten-Free Diet: Nutritional Adequacy and Macro- and Micronutrient Imbalances. Curr. Gastroenterol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Bianchi, M.L.; Bardella, M.T. Bone in celiac disease. Osteoporos. Int. 2008, 19, 1705–1716. [Google Scholar] [CrossRef]
- Krupa-Kozak, U. Pathologic bone alterations in celiac disease: Etiology, epidemiology, and treatment. Nutrition 2014, 30, 16–24. [Google Scholar] [CrossRef]
- Lucendo, A.J.; García-Manzanares, A. Bone mineral density in adult coeliac disease: An updated review. Rev. Esp. Enferm. Dig. 2013, 105, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Weber, P. Vitamin K and bone health. Nutrition 2001, 17, 880–887. [Google Scholar] [CrossRef]
- Kirsaçlioğlu, C.T.; Kuloğlu, Z.; Tanca, A.; Küçük, N.Ö.; Aycan, Z.; Öcal, G.; Ensari, A.; Kalayci, A.G.; Girgin, N. Bone mineral density and growth in children with coeliac disease on a gluten free-diet. Turk J. Med. Sci. 2016, 46, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Di Stefano, M.; Mauriño, E.; Bai, J.C. Bones in coeliac disease: Diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 453–465. [Google Scholar] [CrossRef]
- Kemppainen, T.; Kröger, H.; Janatuinen, E.; Arnala, I.; Lamberg-Allardt, C.; Kärkkäinen, M.; Kosma, V.M.; Julkunen, R.; Jurvelin, J.; Alhava, E.; et al. Bone recovery after a gluten-free diet: A 5-year follow-up study. Bone 1999, 25, 355–360. [Google Scholar] [CrossRef]
- Valdimarsson, T.; Toss, G.; Löfman, O.; Ström, M. Three years’ follow-up of bone density in adult coeliac disease: Significance of secondary hyperparathyroidism. Scand. J. Gastroenterol. 2000, 35, 274–280. [Google Scholar] [CrossRef]
- Capriles, V.D.; Martini, L.A.; Arêas, J.A. Metabolic osteopathy in celiac disease: Importance of a gluten-free diet. Nutr. Rev. 2009, 67, 599–606. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N. Calcium in Gluten-Free Life: Health-Related and Nutritional Implications. Foods 2016, 5, 51. [Google Scholar] [CrossRef]
- Brambilla, P.; Picca, M.; Dilillo, D.; Meneghin, F.; Cravidi, C.; Tischer, M.C.; Vivaldo, T.; Bedogni, G.; Zuccotti, G.V. Changes of body mass index in celiac children on a gluten-free diet. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 177–182. [Google Scholar] [CrossRef]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef]
- Barera, G.; Mora, S.; Brambilla, P.; Ricotti, A.; Menni, L.; Beccio, S.; Bianchi, C. Body composition in children with celiac disease and the effects of a gluten-free diet: A prospective case-control study. Am. J. Clin. Nutr. 2000, 72, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Brito, G.A. Anthropometric parameters in celiac disease: A review on the different evaluation methods and disease effects. J. Nutr. Metab. 2019, 2019, 4586963. [Google Scholar] [CrossRef] [PubMed]
- González, T.; Larretxi, I.; Vitoria, J.C.; Castaño, L.; Simón, E.; Churruca, I.; Navarro, V.; Lasa, A. Celiac Male’s Gluten-Free Diet Profile: Comparison to that of the Control Population and Celiac Women. Nutrients 2018, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar]
- Fajardo, V.; González, M.P.; Martínez, M.; Samaniego-Vaesken, M.D.L.; Achón, M.; Úbeda, N.; Alonso-Aperte, E. Updated Food Composition Database for Cereal-Based Gluten Free Products in Spain: Is Reformulation Moving on? Nutrients 2020, 12, 2369. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Ingestas diarias recomendadas de energía y nutrientes para la población española. In Tablas de Composición de Alimentos, 18th ed.; Ediciones Pirámide (Grupo Anaya, SA): Madrid, Spain, 2016. [Google Scholar]
- Bartrina, J.A.; Majem, L.S. Objetivos nutricionales para la población española. Consenso de la Sociedad Española de Nutrición Comunitaria (SENC). Rev. Esp. Nutr. Comun. 2011, 17, 178–199. [Google Scholar]
- Aguirre-Jaime, A.; Cabrera de León, A.; Domínguez Coello, S.; Borges Alamo, C.; Carrillo Fernández, L.; Gavilán Batista, J.C. Validation of a food intake frequency questionnaire adapted for the study and monitoring of the adult population of the Canary Islands, Spain. Rev. Esp. Salud Publica. 2008, 82, 509–518. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assesment (ISAK); International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Durnin, J.V.; Rahaman, M.M. The assessment of the amount of fat in the human body from measurements of skinfold thickness. 1967. Br. J. Nutr. 2003, 89, 147–155. [Google Scholar] [PubMed]
- Uribe, C.C. Evaluación de la osteoporosis mediante el ultrasonido cuantitativo de calcáneo. Rev. Esp. Enferm. Metab. Oseas 2001, 10, 65–69. [Google Scholar]
- Hallal, P.C.; Victora, C.G. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [Google Scholar] [CrossRef]
- Bartrina, J.A.; Val, M.V.A.; Aldalur, E.M.; de Victoria Muñoz, E.M.; Anta, R.M.O.; Rodrigo, C.P.; i Izquierdo, J.Q.; Martín, A.R.; Viñas, B.R.; Castell, G.S.; et al. Guías alimentarias para la población española. Nutr. Hosp. 2016, 33, 1–48. [Google Scholar]
- Fernández, C.L.H.; Vrotsou, K.; Aresti, U.; Rica, I.; Sánchez, E. Estudio de Crecimiento de Bilbao. Curvas y tablas de crecimiento. Estudio transversal. Instituto de investigación sobre crecimiento y desarrollo. Fundación Faustino Orbegozo Eizaguirre, Edición 2011.
- Segura, M.E.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.S.C.; Clodoveo, M.L.; Pasqualone, A. Evaluation of the nutritional quality of the lipid fraction of gluten-free biscuits. Eur. Food Res. Technol. 2008, 227, 135–139. [Google Scholar] [CrossRef]
- Öhlund, K.; Olsson, C.; Hernell, O.; Öhlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet. 2010, 23, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kautto, E.; Ivarsson, A.; Norstrom, F.; Hogberg, L.; Carlsson, A.; Hornell, A. Nutrient intake in adolescent girls and boys diagnosed with coeliac disease at an early age is mostly comparable to their non-coeliac contemporaries. J. Hum. Nutr. Diet. 2014, 27, 41–53. [Google Scholar] [CrossRef]
- Balamtekin, N.; Aksoy, Ç.; Baysoy, G.; Uslu, N.; Demir, H.; Saltık-Temizel, İ.N.; Özen, H.; Gürakan, F.; Yüce, A. Is compliance with gluten-free diet sufficient? Diet composition of celiac patients. Turk J. Pediatr. 2015, 57, 374–379. [Google Scholar]
- Ruiz, E.; Ávila, J.M.; Valero, T.; Del Pozo, S.; Rodriguez, P.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; et al. Macronutrient Distribution and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2016, 8, 177. [Google Scholar] [CrossRef]
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). 2011. Encuesta Nacional de Ingesta Dietética Española (ENIDE). 2011. [Google Scholar]
- Ballestero Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Children and Adolescents with Celiac Disease on a Gluten Free Diet Compared to Non-Celiac Disease Controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Vincentini, O.; Quaranta, M.G.; Viora, M.; Agostoni, C.; Silano, M. Docosahexaenoic acid modulates in vitro the inflammation of celiac disease in intestinal epithelial cells via the inhibition of cPLA2. Clin. Nutr. 2011, 30, 541–546. [Google Scholar] [CrossRef]
- Younes, M.; Safer, L.; Fadoua, H.; Zrour, S.; Bejia, I.; Touzi, M.; Najjar, M.F.; Saffar, H.; Bergaoui, N. Prevalence of bone loss in adult celiac disease and associated factors: A control case study. Tunis Med. 2012, 90, 129–135. [Google Scholar]
- García-Manzanares, A.L.A. Metabolismo óseo y osteoporosis en la enfermedad celíaca. In Enfermedad Celíaca y Sensibilidad al Gluten no Celíaca; Rodrigo, L., Peña, A.S., Eds.; OmniaScience: Barcelona, España, 2013; pp. 325–344. [Google Scholar]
- Martín-Masot, R.; Nestares, M.T.; Diaz-Castro, J.; López-Aliaga, I.; Alférez, M.J.M.; Moreno-Fernandez, J.; Maldonado, J. Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients 2019, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Varela-Moreiras, G.; Avila, J.M.; Cuadrado, C.; del Pozo, S.; Ruiz, E.; Moreiras, O. Evaluation of food consumption and dietary patterns in Spain by the Food Consumption Survey: Updated information. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S37–S43. [Google Scholar] [CrossRef]
- Grupo de trabajo del Protocolo para el diagnóstico precoz de la enfermedad celíaca. Protocolo para el diagnóstico precoz de la enfermedad celiaca; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain; Servicio de Evaluación del Servicio Canario de Salud (SESCS): El Chorrillo, Panama, 2018. [Google Scholar]
- Vici, G.; Camilletti, D.; Polzonetti, V. Possible Role of Vitamin D in Celiac Disease Onset. Nutrients 2020, 12, 1051. [Google Scholar] [CrossRef]
- Tavakkoli, A.; DiGiacomo, D.; Green, P.H.; Lebwohl, B. Vitamin D status and concomitant autoimmunity in celiac disease. J. Clin. Gastroenterol. 2013, 47, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C. Vreugdenhil ACE. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac disease and obesity: Need for nutritional follow-up after diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef]
- Norsa, L.; Shamir, R.; Zevit, N.; Verduci, E.; Hartman, C.; Ghisleni, D.; Riva, E.; Giovannini, M. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J. Gastroenterol. 2013, 19, 5658–5664. [Google Scholar] [CrossRef]
- Reilly, N.R.; Aguilar, K.; Hassid, B.G.; Cheng, J.; DeFelice, A.R.; Kazlow, P.; Bhagat, G.; Green, P.H. Celiac disease in normal-weight and overweight children: Clinical features and growth outcomes following a gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 528–531. [Google Scholar] [CrossRef]
- García-Manzanares Vázquez, A. Prevalencia, Etiología y Evolución de la Osteoporosis y Osteopenia en la Enfermedad Celíaca de Diagnóstico en la Edad Adulta; Universidad Autónoma de Madrid: Madrid, Spain, 2012. [Google Scholar]
- Hartman, C.; Hino, B.; Lerner, A.; Eshach-Adiv, O.; Berkowitz, D.; Shaoul, R.; Pacht, A.; Rozenthal, E.; Tamir, A.; Shamaly, H.; et al. Bone quantitative ultrasound and bone mineral density in children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 504–510. [Google Scholar] [CrossRef]
- Krzesiek, E.; Iwańczak, B. Assessment of bone mineral density in children with celiac disease. Pol. Merkur. Lekarski 2008, 24, 219–226. [Google Scholar] [PubMed]
| Celiac | Non-Celiac | Total | Age (Years) | |
|---|---|---|---|---|
| Women | 43 (67.2%) | 49 (66.2%) | 92 (66.7%) | 39.17 ± 10.62 |
| Men | 21 (32.8%) | 25 (33.8%) | 46 (33.3%) | 38.58 ± 9.61 |
| Total | 64 | 74 | 138 |
| Total Sample | Men | Women | SENC 1 Recommendations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | ||
| Dairy (servings/day) | 1.6 (0.9–2.2) | 1.6 (1.0–2.3) | n.s. | 1.6 (0.9–2.3) | 1.7 (1.0–2.5) | n.s. | 1.6 (0.9–2.3) | 1.6 (0.9–2.3) | n.s. | 2–3 servings/day |
| Fruits (servings/day) | 1.7 (1.1–2.8) | 1.7 (1.0–2.3) | n.s. | 1.8 (1.2–3.2) | 1.2 (0.8–2.3) | n.s. | 1.7 (1.1–2.6) | 1.7 (1.0–2.4) | n.s. | 3–4 servings/day |
| Vegetables (servings/day) | 1.1 * (1.1–1.5) | 1.1 (0.8–1.5) | 0.047 | 1.1 * (0.9–1.3) | 0.8 (0.8–1.1) | 0.044 | 1.3 (1.1–1.7) | 1.1 (1.1–1.5) | n.s. | 2–3 servings/day |
| Legumes (servings/week) | 1.7 * (1.5–3.7) | 1.5 (1.5–2.2) | 0.009 | 2.00 * (1.5–4.0) | 1.5 (1.5–1.7) | 0.042 | 1.5 (1.5–3.0) | 1.5 (1.5–3.0) | n.s. | 2–4 servings/day |
| Meat and eggs (servings/week) | 8.5 (4.0–11.0) | 8.5 (4.0–11.0) | n.s. | 11.0 * (8.2–12.5) | 7.0 (6.0–11.0) | 0.014 | 8.0 (6.0–9.5) | 8.5 (6.7–11.0) | n.s. | White meats, 3 servings/week Eggs, 3 servings/week |
| Fish and seafood (servings/week) | 4.0 (3.0–6.0) | 3.2 (3.0–6.0) | n.s. | 5.5 (3.0–6.7) | 3.0 (3.0–5.5) | n.s. | 4.0 (3.0–5.5) | 4.0 (3.0–5.5) | n.s. | 3–4 servings/week |
| Bread/paste/cereals (servings/day) | 2.00 * (1.4–2.8) | 2.7 (1.7–3.3) | 0.005 | 2.1 (1.4–3.6) | 2.9 (2.4–3.3) | n.s. | 1.9 * (1.1–2.7) | 2.3 (1.6–3.3) | 0.022 | 4–6 servings/day |
| Pastries/desserts (servings/week) | 4.2 (0.4–7.7) | 4.0 (0.0–8.0) | n.s. | 6.5 (2.2–8.5) | 4.5 (1.5–8.0) | n.s. | 4.0 (0.0–7.0) | 3.0 (0.0–8.5) | n.s. | Optional, occasional, and moderate consumption |
| Nuts (servings/week) | 1.5 (0.0–5.5) | 0.0 (0.0–3.0) | n.s. | 1.5 (0.0–6.0) | 0.0(0.0–1.7) | n.s. | 1.5 (0.0–5.5) | 0.0 (0.0–4.0) | n.s. | 3–7 servings/week |
| Beer/wine (servings/week) | 1.5 (0.0–4.0) | 1.5 (0.0–5.1) | n.s. | 1.5 (0.2–5.5) | 1.5 (0.0–5.5) | n.s. | 1.5 (0.0–4.0) | 1.5 (0.0–3.0) | n.s. | Optional, occasional, and moderate consumption |
| Total Sample | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | |
| Energy (kcal/d) | 1856.5 (1629.8–2134.5) | 1799.0 (1570.0–2103.5) | n.s. | 2082.0 (1773.5–2365.0) | 1932.0 (1739.0–2154.0) | n.s. | 1838.0 (1609.0–2031.0) | 1691.0 (1433.0–2007.0) | n.s. |
| Energy (% of RI) | 78.9 (67.5–87.5) | 71.3 (62.2–86.1) | n.s. | 75.3 (59.1–81.7) | 64.7 (60.4–76.5) | n.s. | 81.1 (73.2–89.7) | 73.5 (64.5–88.4) | n.s. |
| Proteins(g/d) | 80.8 (68.0–94.4) | 79.4 (69.3–89.1) | n.s. | 95.0 (80.1–114.0) | 87.9 (79.5–96.0) | n.s. | 78.3 (61.3–86.4) | 73.1 (66.1–86.3) | n.s. |
| Proteins (% of RI) | 182.2 (160.1–210.6) | 174.9 (150.2–195.7) | n.s. | 175.9 (148.3–211.1) | 162.8 (147.1–177.7) | n.s. | 187.1 (161.2–210.7) | 178.3 (161.2–210.3) | n.s. |
| Proteins (% of TE) | 16.7 (14.5–20.5) | 17.5 (16.0–19.3) | n.s. | 17.0 (14.5–21.4) | 17.3 (16.2–19.20) | n.s. | 16.7 (14.5–19.9) | 17.6 (15.7–19.4) | n.s. |
| Total carbohydrates (% of TE) | 38.9 (33.0–43.4) | 38.2 (34.3–43.1) | n.s. | 39.7 (33.4–44.5) | 37.4 (33.9–41.25) | n.s. | 38.8 (32.7–43.1) | 39.0 (34.5–43.3) | n.s. |
| Simple sugars (% of TE) | 17.1 (13.8–21.6) | 16.4 (13.1–20.1) | n.s. | 16.9 (12.6–22.0) | 14.1 (11.9–18.0) | n.s. | 18.1 (14.1–21.6) | 17.3 (13.9–20.8) | n.s. |
| Fiber (g/day) | 22.4 (15.6–26.9) | 19.9 (15.7–26.3) | n.s. | 25.7 (16.2–30.9) | 19.4 (16.6–26.4) | n.s. | 21.1 (14.9–25.5) | 20.3 (14.7–26.3) | n.s. |
| Total lipids (% of TE) | 39.3 (34.8–4.4) | 40.3 (36.0–44.4) | n.s. | 35.3 (34.1–46.1) | 40.6 (38.0–44.3) | n.s. | 40.1 (35.5–45.3) | 39.8 (35.4–44.5) | n.s. |
| SFA (% of TE) | 12.2 (10.5–14.2) | 12.3 (9.9–14.0) | n.s. | 11.2* (10.0–14.1) | 13.3 (11.6–15.1) | 0.046 | 12.6 (10.0–14.8) | 11.4 (9.6–13.6) | n.s. |
| MUFA (% of TE) | 15.6 (12.6–19-6) | 17.4 (14.6–19.6) | n.s. | 15.7 (12.8–19.2) | 17.6 (14.9–19.2) | n.s. | 15.5 (12.6–19.7) | 16.6 (14.4–20.2) | n.s. |
| PUFA (% of TE) | 4.9 * (3.7–6.4) | 5.4 (4.4–6.7) | 0.032 | 4.6 (3.7–6.7) | 5.8 (5.2–6.8) | n.s. | 4.9 (3.7–6.3) | 5.1 (4.4–6.7) | n.s. |
| Cholesterol (mg/day) | 316.0 (231.7–438.0) | 312.5 (222.5–414.2) | n.s. | 355.0 (251.0–509.5) | 364.0 (279.5–440.5) | n.s. | 308.0 (226.0–386.0) | 292.0 (189.0–390.5) | n.s. |
| Trans fatty acids (mg/day) | 87 (0.0–300) | 140 (46–250) | n.s. | 90 (46–355) | 220 (120–290) | n.s. | 84 (0.0–270) | 92 (0.0–180) | n.s. |
| ω6 fatty acids (g/day) | 2.0 (1.5–3.0) | 2.3 (1.7–4.0) | n.s. | 2.3 (1.7–4.5) | 3.3 (2.0–5.3) | n.s. | 1.9 (1.4–2.5) | 1.9 (1.3–3.3) | n.s. |
| ω3 fatty acids (mg/day) | 180 (102–240) | 195 (140–292) | n.s. | 210 (125–365) | 250 (180–350) | n.s. | 160 (0.100–0.210) | 180 (130–265) | n.s. |
| EPA (mg/day) | 103 (6–367) | 145 (38–312) | n.s. | 110 (7–435) | 140 (39–295) | n.s. | 200 (38–370) | 200 (38–335) | n.s. |
| DHA (mg/day) | 220 (88–712) | 320 (92–600) | n.s. | 240 (98–900) | 260 (86–580) | n.s. | 360 (89–640) | 360 (89–625) | n.s. |
| Total Sample | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | |
| Calcium (% of RI) | 71.0 (52.9–93.0) | 72.7 (60.0–86.4) | n.s. | 75.7 (55.1–107.9) | 79.6 (63.7–90.5) | n.s. | 68.9 (52.7–92.1) | 69.6 (58.5–85.5) | n.s. |
| Phosphorus (% of RI) | 164.8 * (136.1–194.6) | 181.1 (156.2–203.4) | 0.043 | 191.6 (165.4–232.6) | 195.6 (181.8–214.5) | n.s. | 148.7 * (130.9–177.1) | 167.4 (141.1–197.8) | 0.030 |
| Iron (% of RI) | 78.0 (53.3–130.5) | 79.4 (67.8–132.0) | n.s. | 133.0 (110.0–179.0) | 148.0 (129.5–165.0) | n.s. | 58.9 (49.4–78.3) | 72.2 (61.9–80.8) | n.s. |
| Zinc (% of RI) | 52.0 (46.2–66.5) | 57.0 (47.1–65.5) | n.s. | 67.3 (52.3–83.3) | 60.7 (51.6–75.3) | n.s. | 48.0 (42.7–56.7) | 54.7 (46.0–63.6) | n.s. |
| Iodine (% of RI) | 68.9 (51.7–89.5) | 59.9 (49.7–83.8) | n.s. | 64.4 (43.0–79.3) | 56.5 (48.1–69.9) | n.s. | 70.5 (55.9–90.9) | 61.7 (51.7–85.5) | n.s. |
| Total Sample | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | |
| Thiamine (% of RI) | 114.6 (98.1–140.6) | 126.1 (100.8–146.6) | n.s. | 109.1 (89.55–148.10) | 127.3 (100.0–145.5) | n.s. | 120.0 (98.9–137.5) | 122.2 (102.2–158.3) | n.s. |
| Riboflavin (% of RI) | 100.0 (91.8–128.6) | 107.1 (85.7–128.6) | n.s. | 100.0 (76.50–126.05) | 100.0 (76.5–117.1) | n.s. | 107.1 (92.3–128.6) | 107.7 (92.3–135.7) | n.s. |
| Pyridoxine (% of RI) | 125.0 (100–187.0) | 116.7 (100.0–131.3) | n.s. | 138.9* (119.45–161.15) | 116.7 (97.2–127.8) | 0.015 | 118.8 (93.8–143.8) | 112.5 (100.0–134.4) | n.s. |
| Vitamin B12 (% of RI) | 242.5 (181.3–455.0) | 237.5 (153.7–355.0) | n.s. | 300.0 (195.0–530.0) | 275.0 (187.5–480.0) | n.s. | 230.0 (160.0–405.0) | 215.0 (142.5–282.5) | n.s. |
| Niacin (% of RI) | 103.6 (84.8–125.5) | 98.9 (86.5–128.2) | n.s. | 111.6 (85.5–125.8) | 93.7 (78.7–105.6) | n.s. | 102.9 (84.7–125.0) | 101.3 (90.0–134.6) | n.s. |
| Folic Acid (% of RI) | 64.7 (53.0–83.5) | 61.25 (48.7–72.2) | n.s. | 71.50 * (62.1–88.3) | 59.5 (48.5–72.1) | 0.032 | 61.3 (49.5–78.8) | 62.8 (48.4–72.6) | n.s. |
| Vitamin C (% of RI) | 243.30 * (180.0–304.2) | 205.8 (134.3–254.5) | 0.029 | 293.3 * (178.3–332.5) | 196.7 (110.2–230.8) | 0.012 | 226.7 (180.0–290.0) | 211.7 (142.4–285.0) | n.s. |
| Vitamin A (% of RI) | 122.05 * (89.2–152.9) | 91.6 (69.8–117.4) | 0.00 | 120.0 * (85.8–167.1) | 79.0 (63.7–98.2) | 0.001 | 123.0 * (92.6–148.3) | 101.9 (73.4–137.9) | 0.028 |
| Vitamin D (% of RI) | 21.35 (11.3–47.0) | 22.7 (9.1–36.3) | n.s. | 27.3 (12.0–56.3) | 22.0 (9.0–35.6) | n.s. | 20.0 (9.30–46.0) | 22.7 (9.0–37.3) | n.s. |
| Vitamin E (% of RI) | 69.60 (57.7–83.1) | 62.1 (49.2–85.6) | n.s. | 81.7 (66.7–88.7) | 67.5 (54.6–96.6) | n.s. | 65.8 (56.7–77.5) | 60.0 (46.2–75.0) | n.s. |
| Vitamin K (% of RI) | 138.9 * (103.4–219.8) | 109.0 (77.5–165.2) | 0.006 | 149.2 * (118.7–185.0) | 100.00 (60.0–155.8) | 0.024 | 135.6 (100.6–242.2) | 115.6 (84.2–172.7) | n.s. |
| Total Sample | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | |
| Weight (kg) | 64.0 (55.4–80.6) | 64.6 (54.2–73.8) | n.s. | 72.3 * (65.4–76.4) | 81.4 (72.9–89.3) | 0.003 | 57.4 (51.7–66.5) | 56.0 (51.2–64.6) | n.s. |
| Height (cm) | 167.9 (162.0–183.1) | 166.9 (160.4–179.9) | n.s. | 179.6 (171.7–184.2) | 177.5 (174.2–180.2) | n.s. | 163.8 (158.0–168.6) | 163.1 (158.1–166.9) | n.s. |
| Body Fat (%) | 30.6 (22.1–38.7) | 28.7 (23.2–38.7) | n.s. | 21.7 * (17.5–26.3) | 26.0 (21.6–29.1) | 0.019 | 33.4 * (29.8–36.5) | 29.6 (25.0–35.0) | 0.027 |
| BMI (kg/m2) | 21.8 (20.2–27.5) | 22.7 (20.5–28.2) | n.s. | 22.6 * (21.2–24.1) | 24.9 (23.3–27.9) | 0.000 | 21.3 (20.0–23.9) | 21.7 (18.9–23.2) | n.s. |
| Total Sample | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | |
| BMD (g/cm2) | 0.520 (0.440–0.610) | 0.560 (0.460–0.650) | n.s. | 0.540 (0.450–0.650) | 0.630 (0.530–0.700) | n.s. | 0.510 (0.440–0.600) | 0.530 (0.440–0.620) | n.s. |
| Total Sample | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | Reference Value | |
| Red blood cells/μL (×106) | 4.64 (4.4–4.8) | 4.73 (4.4–5.1) | n.s. | 4.98* (4.7–5.0) | 5.17 (4.9–5.4) | 0.022 | 4.5 (4.2–4.7) | 4.6 (4.3–4.8) | n.s. | M:4.6–6.20 W:4.20–5.40/μL (×106) |
| Hemoglobin (g/dL) | 14.30 (13.6–15.1) | 14.20 (13.5–15.2) | n.s. | 15.20 (14.9–15.8) | 15.50 (14.8–16.3) | n.s. | 13.7 (13.3–14.3) | 13.8 (13.0–14.3) | n.s. | M:13.5–18 W:12–16 g/dL |
| Hematocrit (%) | 42.5 (40.0–44.2) | 42.6 (39.5–45.0) | n.s. | 45.0 (43.9–46.2) | 45.7 (43.9–48.0) | n.s. | 40.9 (39.6–42.9) | 41.1 (38.9–42.9) | n.s. | M:42–52% W:37–47% |
| MCV (µ3) | 91.1 (88.5–94.5) | 90.4 (86.7–93.4) | n.s. | 91.7 * (89.8–95.6) | 89.8 (86.1–92.4) | 0.031 | 90.4 (88.0–94.0) | 90.6 (86.8–94.2) | n.s. | 80–96 (µ3) |
| MCH (pg) | 30.7 (30.1–31.9) | 30.4 (29.1–31.6) | n.s. | 31.2 * (30.3–32.7) | 30.3 (29.3–31.5) | 0.018 | 30.7 (30.0–31.7) | 30.5 (28.9–31.7) | n.s. | 27–33 pg |
| MCHC (%) | 33.9 (33.2–34.3) | 33.6 (33.0–34.2) | n.s. | 34.1 (33.3–34.5) | 33.8 (33.4–34.5) | n.s. | 33.7 (33.0–34.3) | 33.3 (32.9–34.0) | n.s. | 33–37% |
| RDW (%) | 11.7 (11.5–12.2) | 11.9 (11.6–12.5) | n.s. | 11.7 (11.4–12.1) | 11.9 (11.5–12.2) | n.s. | 11.9 (11.5–12.2) | 12.1 (11.6–12.7) | n.s. | 11–18% |
| Platelets /μL (×103) | 219.0 (181.7–247.0) | 214.5 (190.7–256.2) | n.s. | 195.0 (182.0–235.5) | 206.0 (185.5–226.5) | n.s. | 230.0 (181.0–318.6) | 229.0 (199.5–299.0) | n.s. | 130–450/μL (×103) |
| MPV (µ3) | 8.5 * (8.0–9.2) | 8.8 (8.1–9.3) | 0.126 | 8.6 (8.1–9.1) | 9.0 (8.2–9.2) | n.s. | 8.4 (7.9–9.2) | 8.8 (8.0–9.3) | n.s. | 7–13 (µ3) |
| Leukocytes /μL (×103) | 5.5 * (4.6–6.6) | 6.2 (5.0–7.0) | 0.014 | 5.5 * (4.6–6.2) | 6.8 (5.4–7.8) | 0.003 | 5.6 (4.6–6.7) | 5.8 (5.0–6.8) | n.s. | 4.00–11.00/μL (×103) |
| Lymphocytes /μL | 1837.00 (1567.0–2326.0) | 2017.5 (1728.7–2393.0) | n.s. | 1740.0 * (1571.0–2246.0) | 2100.0 (1732.5–2522.0) | 0.050 | 1941.0 (1561.0–2385.0) | 1994.0 (1705.0–2361.5) | n.s. | 1000–4500/μL |
| Monocytes /μL | 426.0 (362.5–487.2) | 456.5 (376.2–534.5) | n.s. | 428.0 * (353.0–478.0) | 473.0 (411.0–578.0) | 0.050 | 424.0 (362.0–528.0) | 446.0 (357.0–525.0) | n.s. | 1800–7500/μL |
| Neutrophils /μL | 2926.5 * (2258.5–3567.5) | 3361.5 (2645.7–4153.0) | 0.024 | 3038.0 * (2280.0–3458.5) | 3452.0 (2914.5–4579.5) | 0.028 | 2854.0 (2230.0–3684.0) | 3150.0 (2537.5–3962.5) | n.s. | <800/μL |
| Eosinophils /μL | 148.5 (82.7–225.7) | 163.5 (100.2–241.0) | n.s. | 167.0.0 (84.5–225.0) | 195.0 (107.5–245.0) | n.s. | 148.0 (77.0–239.0) | 150.0 (99.5–241.5) | n.s. | <800/μL |
| Basophils /μL | 43.0 (23.5–61.5) | 51.0 (38.0–63.2) | n.s. | 43.0 (22.5–58.5) | 51.0 (37.0–74.5) | n.s. | 40.0 (25.0–63.0) | 52.0 (39.0–62.5) | n.s. | <200/μL |
| Total Sample | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Celiac (n = 64) | Non-Celiac (n = 74) | p | Celiac (n = 21) | Non-Celiac (n = 25) | p | Celiac (n = 43) | Non-Celiac (n = 49) | p | Reference Value | |
| Basal Glucose (mg/dL) | 83.0 (77.0–88.0) | 81.5 (76.7–87.2) | n.s. | 86.0 (78.0–90.0) | 84.0 (79.0–91.0) | n.s. | 82.0 (77.0–88.0) | 80.0 (75.0–86.0) | n.s. | 60–110 mg/dL |
| Albumin (g/dL) | 4.5 (4.3–4.7) | 4.5 (4.3–4.6) | n.s. | 4.7* (4.5–4.9) | 4.5 (4.4–4.6) | 0.039 | 4.5 (4.3–4.6) | 4.5 (4.3–4.6) | n.s. | 3.5–5.2 g/dL |
| Iron (μg/dL) | 100.5 (81.2–121.7) | 107.5 (84.7–132.0) | n.s. | 106.0 (90.5–133.0) | 108.0 (91.0–128.5) | n.s. | 97.0 (76.0–113.0) | 104.0 (81.0–133.5) | n.s. | 37–160 (μg/dL) |
| Folate (ng/mL) | 8.0 (4.3–10.3) | 6.3 (4.60–8.62) | n.s. | 6.7 (4.0–9.9) | 5.5 (4.4–7.3) | n.s. | 8.1 (5.7–10.7) | 7.0 (4.8–9.2) | n.s. | 3–17 ng/mL |
| Homocysteine (µmol/L) | 10.0 (8.4–11.1) | 9.6 (8.1–12.1) | n.s. | 11.5 (9.7–13.0) | 11.7 (9.7–13.4) | n.s. | 9.7 (8.4–10.6) | 8.8 (7.7–10.4) | n.s. | <15.4 µmol/L |
| Calcium (mg/dL) | 9.4 (9.1–9.6) | 9.3 (9.0–9.6) | n.s. | 9.4 (9.1–9.5) | 9.4 (9.1–9.6) | n.s. | 9.4 (9.0–9.6) | 9.3 (8.9–9.6) | n.s. | 8.2–10.6 mg/dL |
| Phosphorus (mg/dL) | 3.7 (3.4–3.9) | 3.6 (3.3–4.0) | n.s. | 3.5 (3.0–3.7) | 3.4 (3.2–3.6) | n.s. | 3.8 (3.7–4.0) | 3.8 (3.4–4.1) | n.s. | 2.5–5 mg/dL |
| Cholesterol (mg/dL) | 183.5 (148.2–205.5) | 177.5 (155.0–206.0) | n.s. | 191.0 (145.0–220.0) | 186.0 (164.0–210.5) | n.s. | 180.0 (152.0–201.0) | 174.0 (149.0–204.5) | n.s. | <200 mg/dL |
| Triglycerides (mg/dL) | 59.0 (47.25–74.0) | 70.5 (50.5–97.5) | 0.044 | 61.0 (46.5–94.5) | 72.0 (60.0–113.5) | n.s. | 59.0 (48.0–69.0) | 64.0 (48.0–95.0) | n.s. | <200 mg/dL |
| HDL (mg/dL) | 63.0 (51.7–73.0) | 65.5 (54.2–74.2) | n.s. | 56.0 (47.5–68.0) | 52.0 (44.0–67.5) | n.s. | 66.0 (56.0–74.0) | 68.0 (61.0–77.0) | n.s. | >40 mg/dL |
| LDL (mg/dL) | 100.0 (83.2–123.0) | 96.0 (76.7–124.0) | n.s. | 104.0 (76.0–135.0) | 111.0 (96.0–133.5) | n.s. | 99.0 (84.0–112.0) | 87.0 (69.5–115.0) | n.s. | <130 mg/dL: Primary prevention <100 mg/dL Secondary prevention |
| Cholesterol/HDL | 2.8 (2.4–3.2) | 2.7 (2.2–3.4) | n.s. | 2.8 (2.5–3.4) | 3.4 (2.8–4.2) | n.s. | 2.8 (2.4–3.1) | 2.5 (2.1–2.9) | n.s. | <4.5 |
| LDL/HDL | 1.6 (1.3–1.9) | 1.4 (0.97–2.15) | n.s. | 1.6 (1.4–2.1) | 2.1 (1.6–2.8) | n.s. | 1.5 * (1.3–1.9) | 1.3 (0.9–1.7) | 0.032 | M: <3.55 W: <3.22 |
| Vitamin D (ng/mL) | 34.7 (24.5–59.9) | 33.7 (22.2–66.8) | n.s. | 43.7 (26.8–55.3) | 31.6 (19.8–63.2) | n.s. | 33.8 (22.1–60.6) | 38.0 (22.3–68.8) | n.s. | <10 ng/mL: Moderate deficit; 10–30 ng/mL: Severe deficit; 30–96 ng/mL Recommended values; >96 ng/mL excess |
| Parathormone (pg/mL) | 32.85 (24.0–51.3) | 34.20 (18.9–48.8) | n.s. | 31.30 (24.9–54.9) | 36.7 (18.8–46.6) | n.s. | 32.9 (23.4–29.9) | 34.2 (18.8–51.5) | n.s. | 14.5–87.1 pg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballestero-Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac. Nutrients 2021, 13, 1626. https://doi.org/10.3390/nu13051626
Ballestero-Fernández C, Varela-Moreiras G, Úbeda N, Alonso-Aperte E. Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac. Nutrients. 2021; 13(5):1626. https://doi.org/10.3390/nu13051626
Chicago/Turabian StyleBallestero-Fernández, Catalina, Gregorio Varela-Moreiras, Natalia Úbeda, and Elena Alonso-Aperte. 2021. "Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac" Nutrients 13, no. 5: 1626. https://doi.org/10.3390/nu13051626
APA StyleBallestero-Fernández, C., Varela-Moreiras, G., Úbeda, N., & Alonso-Aperte, E. (2021). Nutritional Status in Spanish Adults with Celiac Disease Following a Long-Term Gluten-Free Diet Is Similar to Non-Celiac. Nutrients, 13(5), 1626. https://doi.org/10.3390/nu13051626








