Iron Deficiency Anemia in Celiac Disease
Abstract
1. Introduction
2. Pathogenesis
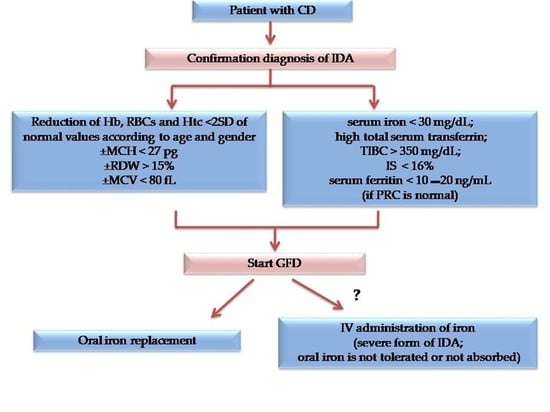
3. Diagnostic Workup for IDA Diagnosis in CD Patients
4. Prevention
5. Treatment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Franceschi, L.; Iolascon, A.; Taherd, A.; Cappellini, M.D. Clinical management of iron deficiency anemia in adults: Sistemic review on advances in diagnosis and treatment. Eur. J. Intern. Med. 2017, 42, 16–23. [Google Scholar] [CrossRef]
- Lerner, N.B.; Sills, R. Iron deficiency anemia. In Nelson Text of Pediatrics, 20th ed.; Kliegman, R.M., Stanton, B.F., Schor, N.F., St. Gemelli, G.V., Behrman, R.E., Eds.; Elseviere: Amsterdam, The Netherlands, 2016; pp. 2322–2326. [Google Scholar]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Simonetti, M.; Marconi, E.; Brignoli, O.; Cancian, M.; Masotti, A.; Pegoraro, V.; Heiman, F.; Cricelli, C.; Lapi, F. Gender differences in determinants of iron-deficiency anemia: A population-based study conducted in four European countries. Ann. Hematol. 2019, 98, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Honar, N.; Yousefi, A.; Javaherizadeh, H. Association of potential celiac disease and refractory iron deficiency anemia in children and adolescents. Arq. Gastroenterol. 2018, 55, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Bhattacharya, M. Celiac Disease and Anemia. Indian Pediatr. 2018, 24, 23–24. [Google Scholar] [CrossRef]
- Kreutz, J.M.; Adriaanse, M.P.M.; vander Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Iron deficiency anemia in celiac disease. World J. Gastroenterol. 2015, 21, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.; Kelly, C.P.; Silvester, J.A. Celiac Disease Extraintestinal Manifestations and Associated Conditions. J. Clin. Gastroenterol. 2020, 54, 8–21. [Google Scholar] [CrossRef]
- Abdallaa, A.; Saifullahb, S.M.; Osmana, M.; Baniyaa, R.; Sidahmedc, S.; LaChanced, J.; Bachuwaa, G. Prevalence of occult celiac disease in females with iron deficiency in the United States: An NHANES analysis. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 347–350. [Google Scholar] [CrossRef]
- Jericho, H.; Sansotta, N.; Guandalini, S. Extraintestinal Manifestations of Celiac Disease. JPGN 2017, 65, 75–79. [Google Scholar] [CrossRef]
- Nardecchia, S.; Auricchio, R.; Discepolo, V.; Troncone, R. Extra-Intestinal Manifestations of Coeliac Disease in Children: Clinical Features and Mechanisms. Front. Pediatr. 2019, 7, 56. [Google Scholar] [CrossRef]
- Kolho, K.L.; Färkkilä, M.A.; Savilahti, E. Undiagnosed celiac disease is commonin Finnish adults. Scand. J. Gastroenterol. 1998, 33, 1280–1283. [Google Scholar] [PubMed]
- Bergamaschi, G.; Markopoulos, K.; Albertini, R.; Di Sabatino, A.; Biagi, F.; Ciccocioppo, R.; Arbustini, E.; Corazza, G.R. Anemia of chronic disease and defective erythropoiet in production in patients with celiac disease. Haematologica 2008, 93, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.; Basha, J.; Varma, N.; Varma, S.; Prasad, K.K.; Vaiphei, K.; Dhaka, N.; Sinha, S.K.; Kochhar, R. Anemia in celiac disease is multifactorial in etiology: A prospective study from India: Anemia in celiac disease. JGH Open 2018, 2, 196–200. [Google Scholar] [CrossRef]
- Binicier, O.B.; Tosun, F. Evaluation of adult celiac disease from a tertiary reference center: A retrospective analysis. Rev. Assoc. Med. Bras. 2020, 66, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, G.; Cataldo, F.; Rotolo, N.; Spina, M.; Corazza, G.R. The clinical pattern of subclinical/silent celiac disease: Analyses is on 1026 consecutive cases. Am. J. Gastroenterol. 1999, 94, 691–696. [Google Scholar] [CrossRef]
- AbuDaya, H.; Lebwohl, B.; Lewis, S.K.; Green, P.H. Celiac disease patients presenting with anemia have more severe disea than those presenting with diarrhea. Clin. Gastroenterol. Hepatol. 2013, 11, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac Disease Symptom Resolution: Effectiveness of the Gluten-free Diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef]
- De Falco, L.; Tortora, R.; Imperatore, N.; Bruno, M.; Capasso, M.; Girelli, D.; Castagna, A.; Caporaso, N.; Iolascon, A.; Rispo, A. The role of TMPRSS6 and HFE variants in iron deficiency anemia in celiac disease. Am. J. Hematol. 2018, 93, 383–393. [Google Scholar] [CrossRef]
- Akbari, M.; Moosazadeh, M.; Tabrizi, R.; Khatibi, S.R.; Khodadost, M.; Heydari, S.T.; Tahami, A.N.; Lankarani, K.B. Estimation of iron deficiency anemia in Iranian children and adolescents: A systematic review and meta-analysis. Hematology 2017, 22, 231–239. [Google Scholar] [CrossRef]
- Kochhar, R.; Jain, K.; Thapa, B.R.; Rawal, P.; Khaliq, A.; Kochhar, R.; Bhadada, S.; Vaiphei, K.; Varma, S.; Dutta, U.; et al. Clinical presentation of celiac disease among pediatric compared to adolescent and adult patients. Indian J. Gastroenterol. 2012, 31, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Tolone, C.; Bellini, G.; Punzo, F.; Papparella, A.; Miele, E.; Vitale, A.; Nobili, B.; Strisciuglio, C.; Rossi, F. The DMT1 IVS4+44C>A polymorphis mand the risk of iron deficiency anemia in children with celiac disease. PLoS ONE 2017, 12, e0185822. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Iannitto, E.; Cavataio, F.; Montalto, G.; Tumminello, M.; Campagna, P.; Lipari, M.G.; Notarbartolo, A.; Iacono, G. Sideropenic anemia and celiac disease: One study, two points of view. Dig. Dis. Sci. 1998, 43, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, Z.; Kirsaçlioglu, C.T.; Kansu, A.; Ensari, A.; Girgin, N. Celiac disease: Presentation of 109 children. Yonsei Med. J. 2009, 50, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Sanseviero, M.T.; Mazza, G.A.; Pullano, M.N.; Oliveiro, A.C.; Altomare, F.; Pedrelli, L.; Dattilo, B.; Miniero, R.; Meloni, G.; Giancotti, L.; et al. Iron deficiency anemia in newly diagnosed celiac disease in children. Minerva Pediatr. 2016, 68, 1–4. [Google Scholar] [PubMed]
- Mahadev, S.; Laszkowska, M.; Sundström, J.; Björkholm, M.; Lebwohl, B.; Green, P.H.R.; Ludvigsson, J.F. Prevalence of Celiac Disease in Patients with Iron Deficiency Anemia–a Systematic Review with Meta-analysis. Gastroenterology 2018, 155, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, V.; Tozun, M.S.; Küçük, N. The prevalence of celiac disease in children with iron-deficiency anemia. Turk. J. Gastroenterol. 2013, 24, 334–338. [Google Scholar] [CrossRef]
- Karaman, K.; Akbayram, S.; Kar, S.; Demirören, K.J. Prevalence of Celiac Disease in Children with Iron Deficiency Anemia in Van Lake Region of Turkey. Pediatr. Hematol. Oncol. 2016, 38, 143–146. [Google Scholar] [CrossRef]
- Kalayci, A.G.; Kanber, Y.; Birinci, A.; Yildiz, L.; Albayrak, D. The prevalence of celiac diseaseas detected by screening in children with iron deficiency anaemia. Acta Paediatr. 2005, 94, 678–681. [Google Scholar] [CrossRef]
- Abd El Dayem, S.M.; Ahmed Aly, A.; Abd El Gafar, E.; Kamel, H. Screening for celiac disease among Egyptian children. Arch. Med. Sci. 2010, 6, 226–235. [Google Scholar] [CrossRef]
- Bansal, D.; Trehan, A.; Gupta, M.K.; Varma, N.; Marwaha, R.K. Serodiagnosis of celiac disease in children referred for evaluation of anemia: A pediatric hematology unit's experience. Indian J. Pathol. Microbiol. 2011, 54, 756–760. [Google Scholar] [PubMed]
- Dubé, C.; Rostom, A.; Sy, R.; Cranney, A.; Saloojee, N.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: A systematic review. Gastroenterology 2005, 128, S57–S67. [Google Scholar] [CrossRef]
- Hershko, C.; Patz, J. Iron in gout the mechanism of anemia in celiac disease. Haematologica 2008, 93, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Valentini, R.A.; Andreani, M.L.; D’Anchino, M.; Leva, M.T.; Ginaldi, L.; DeFeudis, L.; Quaglino, D.; Gasbarrini, G. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand. J. Gastroenterol. 1995, 30, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Severi, C.; Chistolini, A.; Antonelli, G.; Lahner, E.; Marcheggiano, A.; Iannoni, C.; Monarca, B.; DelleFave, G. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef]
- Howard, M.R.; Turnbull, A.J.; Morley, P.; Hollier, P.; Webb, R.; Clarke, A. A prospective study of the prevalence of undiagnosed celiac disease in laboratory defined iron and folate deficiency. J. Clin. Pathol. 2002, 55, 754–757. [Google Scholar] [CrossRef]
- Mandal, A.K.; Mehdi, I.; Munshi, S.K.; Lo, T.C. Value of routine duodenal biopsy in diagnosing celiac disease in patients with iron deficiency anaemia. Postgrad. Med. J. 2004, 80, 475–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carter, D.; Bardan, E.; Derazne, E.; Tzur, D.; Avidan, B. The incidence of gastrointestinal pathology and subsequent anemia in young men presenting with iron deficiency without anemia. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Lasa, J.S.; Olivera, P.; Soifer, L.; Moore, M. Iron-Deficiency anemia as asubclinical celiac disease presentation in an Argentinian population. Rev. Gastroenterol. México 2017, 82, 270–273. [Google Scholar] [CrossRef]
- Paez, M.A.; Gramel spacher, A.M.; Sinacore, J.; Winterfield, L.; Venu, M. Delay in Diagnosis of Celiac Disease in Patients Without Gastrointestinal Complaints. Am. J. Med. 2017, 130, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition. Guidelines for Diagnosing Coeliac Disease. JPGN 2020, 70, 141–157. [Google Scholar]
- Ko, C.W.; Siddique, S.M.; Patel, A.; Harris, H.; Sultan, S.; Altayar, O.; Falck-Ytter, Y. AGA Clinical Practice Guidelines on th e Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1085–1094. [Google Scholar] [CrossRef]
- Goddard, A.F.; James, M.W.; Mc Intyre, A.S.; Scott, B.B. British Society of Gastroenterology. Guidelines for the management of iron –deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Norsa, L.; Zullo, A.; Carroccio, A.; Girelli, C.; Oliva, S.; Romano, C.; Leandro, G.; Bellini, M.; Marmo, R.; et al. Diagnosis of chronic anaemia in gastrointestinal disorders: A guideline by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) and the Italian Society of Paediatric Gastroenterology Hepatology and Nutrition (SIGENP). Dig. Liver Dis. 2019, 51, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Smukalla, S.; Lebwohl, B.; Mears, J.G.; Leslie, L.A.; Green, P.H. How Often Do Hematologists Consider Celiac Disease in Iron-Deficiency Anemia? Results of a National Survey. Clin. Adv. Hematol. Oncol. 2014, 12, 100–105. [Google Scholar] [PubMed]
- Nestares, T.; Martín-Masot, R.; Labella, A.; Aparicio, V.A.; Flor-Alemany, M.; López-Frías, M.; Maldonado, J. Isa Gluten-Free Diet Enough to Maintain Correct Micronutrients Status in Young Patients with Celiac Disease? Nutrients 2020, 12, 844. [Google Scholar] [CrossRef]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Rauci, U.; Parisei, P. Nutritional deficiencies in children with celiac disease resulting from a gluten-free diet: A systematic review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef]
- Saukkonen, J.; Kaukinen, K.; Koivisto, A.M.; Mäki, M.; Laurila, K.; Sievänen, H.; Collin, P.; Kurppa, K. Clinical Characteristics and the Dietary Response in Celiac Disease Patients Presenting with or Without Anemia. Clin. Gastroenterol. 2017, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, S.; Kivelä, L.; Huhtala, H.; Kaukinen, K.; Kurppa, K. Extraintestinal manifestations were common in children with celiac disease and were more prevalent in patients with more severe clinical and histological presentation. Acta Paediatr. 2019, 108, 681–687. [Google Scholar] [CrossRef]
- Harper, J.W.; Holleran, S.F.; Ramakrishnan, R.; Bhagat, G.; Green, P.H. Anemia in celiac disease is multifactorial in etiology. Am. J. Hematol. 2007, 82, 996–1000. [Google Scholar] [CrossRef]
- Repo, M.; Rajalahti, T.; Hiltunen, P.; Sotka, A.; Kivelä, L.; Huhtala, H.; Kaukinen, K.; Lindfors, K.; Kurppa, K. Diagnostic findings and long-term prognosis in children with anemia undergoing GI endoscopies. Gastrointest. Endosc. 2020, 91, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Maimaris, S.; Nicolardi, M.L.; Alimenti, E.; Vernero, M.; Costetti, M.; Costa, S.; Biagi, F. Determinants and Trends of Adherence to a Gluten-Free Diet in Adult Celiac Patients on a Long-Term Follow-Up (2000–2020). Clin. Gastroenterol. Hepatol. 2020, S1542-3565, 31672–33174. [Google Scholar]
- Stefanelli, G.; Viscido, A.; Longo, S.; Magistroni, M.; Latella, G. Persistent Iron Deficiency Anemia in Patients with Celiac Disease Despite a Gluten-Free Diet. Nutrients 2020, 12, 2176. [Google Scholar] [CrossRef]
- Repo, M.; Hannula, M.; Taavela, J.; Hyttinen, J.; Isola, J.; Hiltunen, P.; Popp, A.; Kaukinen, K.; Kurppa, K.; Lindfors, K. Iron Transporter Protein Expressions in Children with Celiac Disease. Nutrients 2021, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Barisani, D.; Parafioriti, A.; Bardella, M.T.; Zoller, H.; Conte, D.; Armiraglio, E.; Trovato, C.; Koch, R.O.; Weiss, G. Adaptive changes of duodenal iron transport proteins in celiac disease. Physiol. Genom. 2004, 17, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Singh, P.; Agnihotri, A.; Das, P.; Mishra, A.; Verma, A.K.; Ahuja, A.; Sreenivas, V.; Khadgawat, R.; Gupta, S.D.; et al. Celiac disease: A disease with varied manifestations in adults and adolescents. J. Dig. Dis. 2013, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Martín-Masot, R.; Nestares, M.T.; Diaz-Castro, J.; López-Aliaga, I.; MuñozAlférez, M.J.; Moreno-Fernandez, J.; Maldonado, J. Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients 2019, 11, 2557. [Google Scholar] [CrossRef]
- Elli, L.; Poggiali, E.; Tomba, C.; Andreozzi, F.; Nava, I.; Bardella, M.T.; Campostrini, N.; Girelli, D.; Conte, D.; Cappellini, M.D. Does TMPRSS6RS855791 polymorphism contribute to iron deficiency in treated celiac disease? Am. J. Gastroenterol. 2015, 110, 200–202. [Google Scholar] [CrossRef]
- Hoppe, M.; Onning, G.; Berggren, A.; Hulthen, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef]
- Rosen, G.M.; Morrissette, S.; Larson, A.; Stading, P.; Griffin, K.H.; Barnes, T.L. Use of a Probiotic to Enhance Iron Absorption in a Randomized Trial of Pediatric Patients Presenting with Iron Deficiency. J. Pediatr. 2019, 207, 192–197. [Google Scholar] [CrossRef]
- Ferus, K.; Drabinska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. Randomized, Placebo-Controlled, Pilot Clinical Trial to Evaluate the Effect of Supplementation with Prebiotic Synergy 1 on Iron Homeostasis in Children and Adolescents with Celiac Disease Treated with a Gluten-Free Diet. Nutrients 2018, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P. Treatment of mil non chemotherapy induced iron deficiency anemia in cancer patients: Comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomed. Pharm. 2012, 66, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Bagna, R.; Spada, E.; Mazzone, R.; Saracco, P.; Boetti, T.; Cester, E.A.; Bertino, E.; Coscia, A. Efficacy of Supplementation with Iron Sulfate Compared to Iron Bisglycinate Chelate in Preterm Infants. Curr. Pediatr. Rev. 2018, 14, 123–129. [Google Scholar] [CrossRef] [PubMed]
- JoãoName, J.; Rodrigues Vasconcelos, A.; Valzachi Rocha Maluf, M.C. Iron Bisglycinate Chelate and Polymaltose Iron for the Treatment of Iron Deficiency Anemia: A Pilot Randomized Trial. Curr. Pediatric. Rev. 2018, 14, 261–268. [Google Scholar]
- Hacibekiroglu, T.; Akinci, S.; Basturk, A.R.; Bakanay, S.M.; Ulas, T.; Guney, T.; Dilek, I.A. Forgotten screening test for iron deficiency anemia: Oral iron absorbtion test. Clin. Ter. 2013, 164, 495–497. [Google Scholar]
- Mazza, G.A.; Pedrelli, L.; Battaglia, E.; Giancotti, L.; Miniero, R. Oral iron absorption test with ferrous bisglycinate chelate in children with celiac disease: Preliminary results. Minerva Pediatr. 2019, 10, 139–143. [Google Scholar] [CrossRef]
- Rondinelli, M.B.; Di Bartolomei, A.; De Rosa, A.; Pirelli, L. Oral Iron Absorption Test (OIAT): A forgotten screening test for iron absorption from the gastrointestinal tract. A casa series of Iron Deficiency Anemia (IDA) patients treated with FERALGINE®. J. Blood Disord. Med. 2017, 2, 1. [Google Scholar]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral iron supplementation with Feralgine® in inflammatory bowel disease: A retrospective observational study. Minerva Gastroenterol. Dietol. 2019, 65, 200–203. [Google Scholar] [CrossRef]
- Giancotti, L.; Talarico, V.; Mazza, G.A.; Marrazzo, S.; Gangemi, G.; Miniero, R.; Bertini, M. FERALGINE™ a new approach for Iron Deficiency Anemia in Celiac Patients. Nutrients 2019, 11, 887. [Google Scholar] [CrossRef]
- Talarico, V.; Giancotti, L.; Miniero, R.; Bertini, M. Iron Deficiency Anemia Refractory to Conventional Therapy but Responsive to Feralgine® in a Young Woman with Celiac Disease. Int. Med. Case Rep. J. 2021, 14, 89–93. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Ding, H.; Zhao, Y.; Feng, J. Iron Transport from Ferrous Bisglycinate and Ferrous Sulfate in DMT1-Knockout Human Intestinal Caco-2 Cells. Nutrients 2019, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.C.; Guan, W.T.; Chen, F.; Hou, D.X.; Wang, C.X.; Lv, Y.T.; Qiao, H.Z.; Chen, J.; Han, J.H. Ferrous bisglycinate increased iron transportation through DMT1 and PepT1 in pig intestinal epithelial cells compared with ferrous sulphate. J. Anim. Feed Sci. 2014, 23, 153–159. [Google Scholar] [CrossRef]
- Auerbach, M.; Ballard, H. Clinical use of intravenous iron: Administration, efficacy, and safety. Hematol. Am. Soc. Hematol. Educ. Program. 2010, 2010, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, U.D.; Bayraktar, S. Treatment of iron deficiency anemia associated with gastrointestinal tract disease. Word J. Gastroenterol. 2010, 16, 2720–2725. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Lopez, A.; Cummings, J.R.; Dignass, A.; Detlie, T.E.; Danese, S. Review article: Treating–to –target for inflammatory bowel disease-associated anaemia. Aliment. Pharmacol. Ther. 2018, 48, 610. [Google Scholar] [CrossRef] [PubMed]
- Carman, N.; Muir, R.; Lewindon, P. Ferriccarboxymaltose in the treatment of iron deficiency in pediatric inflammatory bowel disease. Transl. Pediatr. 2019, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
| Authors | Country | No. Of Patients | % IDA | Year of the Study |
|---|---|---|---|---|
| ADULTS | ||||
| Koho, et al. [13] | Finland | 8 | 25 | 1998 |
| Bergamaschi et al. [14] | Italy | 132 | 34 | 2008 |
| Berry et al. [15] | India | 103 | 81 | 2018 |
| Binicier et al. [16] | Turkey | 195 | 53 | 2020 |
| Bottaro et al. [17] | Italy | 315 | 46 | 1999 |
| Abu Daya et al. [18] | USA | 727 | 21 | 2013 |
| Sansotta et al. [19] | USA | 327 | 48 | 2018 |
| De Falco et al. [20] | Italy | 505 | 45 | 2018 |
| Akbari et al. [21] | Iran | 27 | 52 | 2006 |
| Kockar et al. [22] | India | 434 | 84 | 2012 |
| CHILDREN | ||||
| Bottaro et al. [17] | Italy | 485 | 35 | 1999 |
| Sansotta et al. [19] | USA | 227 | 12 | 2018 |
| Tolone et al. [23] | Italy | 385 | 35 | 2017 |
| Carroccio et al. [24] | Italy | 130 | 70 | 1998 |
| Kullogu et al. [25] | Turkey | 109 | 82 | 2009 |
| Sanseviero et al. [26] | Italy | 518 | 22 | 2016 |
| (a) Red cell parameters values for diagnosis of IDA |
|
| (b) Biochemical parameters values for diagnosis of IDA |
|
| (c) Other parameters evaluable for diagnosis of IDA |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talarico, V.; Giancotti, L.; Mazza, G.A.; Miniero, R.; Bertini, M. Iron Deficiency Anemia in Celiac Disease. Nutrients 2021, 13, 1695. https://doi.org/10.3390/nu13051695
Talarico V, Giancotti L, Mazza GA, Miniero R, Bertini M. Iron Deficiency Anemia in Celiac Disease. Nutrients. 2021; 13(5):1695. https://doi.org/10.3390/nu13051695
Chicago/Turabian StyleTalarico, Valentina, Laura Giancotti, Giuseppe Antonio Mazza, Roberto Miniero, and Marco Bertini. 2021. "Iron Deficiency Anemia in Celiac Disease" Nutrients 13, no. 5: 1695. https://doi.org/10.3390/nu13051695
APA StyleTalarico, V., Giancotti, L., Mazza, G. A., Miniero, R., & Bertini, M. (2021). Iron Deficiency Anemia in Celiac Disease. Nutrients, 13(5), 1695. https://doi.org/10.3390/nu13051695