Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia?
Abstract
1. Introduction
- Overweight, obes*, BMI;
- Mother*, maternal, preg*, neonat*;
- Anaem*, anem*iron status, ferritin, hepcidin;
- Inflamm*.
2. Iron Deficiency and Iron-Deficiency Anaemia in Pregnancy
3. Does Obesity in Pregnancy Trigger an Inflammatory Response?
3.1. Prevalence of Obesity in Pregnancy
3.2. Inflammatory Status in Healthy Pregnancies
3.3. Obesity, the Inflammatory Response and Iron Status
3.4. Is Inflammation Playing a Role in the Development of ID/IDA in Pregnant Women Who Are Obese?
3.5. Can Physical Activity Counteract the Negative Effects of Inflammation in Obese Individuals?
3.6. Risks and Complications Associated with Anaemia in Pregnancy May Be Compounded by an Increased BMI in the Overweight/Obese Range
- •
- Iron deficiency and iron-deficiency anaemia in pregnancy remain a widespread issue [2].
- •
- •
- •
- Women who are overweight/obese in the first trimester tend to gain more weight during gestation than lean women [83].
- •
- •
- Obese mothers had lower serum ferritin and higher hepcidin levels when compared to women with normal BMI. Maternal ferritin did not correlate with CRP across all BMI groups suggesting that, in this cohort, ferritin levels were likely reflecting iron status rather than inflammation.
- •
- In young mothers (13–18 years of age) with class I obesity, inflammation does not appear to exert control over iron metabolism in pregnancy [87].
- •
- Pregnant women are not meeting the guidelines for physical activity [90].
- •
- •
- There is potential to use low-carbohydrate diets to reduce the levels of CRP in obese pregnancy [94].
- •
4. Effect of Maternal Overweight/Obesity on Infants’ Iron Status
- •
- Lower ferritin expression in placentas from obese mothers may lead to increased levels of free iron, increased formation of reactive oxygen species and oxidative stress. Additionally, insufficient placental ferritin expression may lead to reduced iron transfer from mother to fetus [106].
- •
- •
- Babies born to young obese mothers (BMI ≥ 30 kg/m2) had significantly higher body iron levels and haemoglobin when compared to lean (BMI range between 18.5 and 24.9 kg/m2) mothers [87]. Conversely others reported that maternal obesity and excessive weight gain during pregnancy negatively affect neonatal iron status [36,58,83,114,115] indicating an iron deficient profile.
- •
- Placenta’s adaptation (increased level of pTfR 1) may play a key role in ensuring sufficient levels of fetal iron across different groups of pre-pregnancy maternal BMI [89].
5. Iron Status in Pregnancy and the Effect of Dietary Interventions and Iron Supplements
- •
- Impaired absorption of dietary iron may be caused by elevated hepcidin [82] and contribute to ID/IDA in overweight/obese pregnant women.
- •
- In women who received iron supplementation throughout pregnancy:
- ○
- Hepcidin, CRP and leptin were significantly higher in obese pregnant women compared to lean women, and serum iron was significantly lower;
- ○
- Inflammatory markers (CRP, IL-6 and leptin) were not associated with hepcidin;
- ○
- Pre-pregnancy BMI was not associated with iron status [88].
- •
- Infants born to ID mothers are more likely to remain ID beyond 9 months of age despite an iron rich diet [21].
6. Diagnostics—Correcting Iron Status for Inflammation
- •
- Demonstrating the presence of inflammation with the use of inflammation biomarkers (CRP and AGP) and:
- ○
- For research purposes, exclusion of the data, or,
- ○
- For clinical practice, careful interpretation of the data obtained from participants with elevated inflammation biomarkers.
- •
- •
- •
- There is a clear need to employ an appropriate diagnostic method to correctly identify the presence of ID/IDA, particularly in presence of inflammation and to match iron repletion interventions accordingly. This may be possible through future routine analysis of inflammatory markers and ferritin.
- •
- Future research is required to develop algorithms that can be used as diagnostic tools, particularly where inflammation is present.
7. Clinical Guidelines and Strategies to Diagnose and Correct ID/IDA in Pregnancy
Intravenous (IV) Iron as an Alternative to Oral Iron Supplementation in Pregnancy
- •
- •
- Routine screening processes to identify iron deficiency in the ante- and postpartum periods are needed.
- •
- Procedures for trimester-specific iron supplementation strategies for ID/anaemic pregnant women may help alleviate the current burden of ID/IDA in pregnancy [132].
8. Conclusions
- (i)
- Do women who are living with overweight or obesity respond differently to iron supplementation?
- (ii)
- Are women who are living with overweight or obesity more likely to enter pregnancy with ID/IDA and thus should they be considered to be at higher risk of developing IDA?
- (iii)
- Does pregnancy dampen the response to inflammation or is there homeostatic adaptation by the placenta and fetus to become more efficient in acquiring iron?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Australian Bureau of Statistics |
| AGP | α-1 acid glycoprotein |
| AI | anaemia of inflammation |
| BMI | body mass index |
| CRP | C-reactive protein |
| EPO | erythropoietin |
| hsCRP | high-sensitivity CRP |
| ID | iron deficiency |
| IDA | iron-deficiency anaemia |
| il-6 | interleukin-6 |
| pTfR | placental transferrin receptor |
| RBC | red blood cells |
| sTfR | soluble transferrin receptor |
| TNF-α | tumor necrosis factor |
| TSAT | transferrin saturation |
| WHO | World Health Organization |
| ZnPP/H | erythrocyte zinc protoporphyrin/heme |
References
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, 16–25. [Google Scholar] [CrossRef]
- WHO. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2015; pp. 1–48. [Google Scholar]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Yip, R.; Binkin, N.J.; Fleshood, L.; Trowbridge, F.L. Declining prevalence of anemia among low-income children in the United States. J. Am. Med. Assoc. 1987, 258, 1619–1623. [Google Scholar] [CrossRef]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef]
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Iron Review. J. Nutr. 2018, 148, 1001S–1067S. [Google Scholar] [CrossRef]
- Hallberg, L.; Rossander-Hulten, L. Iron requirements in menstruating women. Am. J. Clin. Nutr. 1991, 54, 1047–1058. [Google Scholar] [CrossRef]
- Aguree, S.; Gernand, A.D. Plasma volume expansion across healthy pregnancy: A systematic review and meta-analysis of longitudinal studies. BMC Pregnancy Childbirth 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Ferguson, M.T.; Dennis, A.T. Defining peri-operative anaemia in pregnant women—Challenging the status quo. Anaesthesia 2019, 74, 237–245. [Google Scholar] [CrossRef]
- Milman, N. Iron in pregnancy—How do we secure an appropriate iron status in the mother and child? Ann. Nutr. Metab. 2011, 59, 50–54. [Google Scholar] [CrossRef]
- Milman, N.; Byg, K.-E.; Agger, A.O. Hemoglobin and erythrocyte indices during normal pregnancy and postpartum in 206 women with and without iron supplementation. Acta Obstet. Gynecol. Scand. 2000, 79, 89–98. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 1–527, CD004736. [Google Scholar] [CrossRef]
- Brabin, B.J.; Hakimi, M.; Pelletier, D. An Analysis of Anemia and Pregnancy-Related Maternal Mortality. J. Nutr. 2001, 131, 604S–615S. [Google Scholar] [CrossRef]
- Brabin, B.J.; Premji, Z.; Verhoeff, F. An Analysis of Anemia and Child Mortality. J. Nutr. 2001, 131, 636S–648S. [Google Scholar] [CrossRef]
- Daru, J.; Zamora, J.; Fernández-Félix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tunçalp, Ö.; Torloni, M.R.; Mittal, S.; Jayaratne, K.; et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. Lancet Glob. Health 2018, 6, e548–e554. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal Med. 2007, 12, 54–63. [Google Scholar] [CrossRef]
- Grantham-McGregor, S.; Ani, C. A Review of Studies on the Effect of Iron Deficiency on Cognitive Development in Children. J. Nutr. 2001, 131, 649S–668S. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-Lasting Neural and Behavioral Effects of Iron Deficiency in Infancy. Nutr. Rev. 2006, 64, S34–S91. [Google Scholar] [CrossRef]
- Falkingham, M.; Abdelhamid, A.; Curtis, P.; Fairweather-Tait, S.; Dye, L.; Hooper, L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr. J. 2010, 9, 4. [Google Scholar] [CrossRef]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef]
- Bar-Zeev, S.J.; Kruske, S.G.; Barclay, L.M.; Bar-Zeev, N.; Kildea, S.V. Adherence to management guidelines for growth faltering and anaemia in remote dwelling Australian Aboriginal infants and barriers to health service delivery. BMC Health Serv. Res. 2013, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.J.; Gibson, R.A.; Gibson, R.S.; Makrides, M. Nutrient intakes and status of preschool children in Adelaide, South Australia. Med. J. Aust. 2012, 196, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.A.; McNaughton, S.A.; Campbell, K.J.; Szymlek-Gay, E.A. Iron intakes of Australian infants and toddlers: Findings from the Melbourne Infant Feeding, Activity and Nutrition Trial (InFANT) Program. Br. J. Nutr. 2016, 115, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Bentham, J.; Cesare, D.; Bllano, M.; Boddy, V.; LM and NCD-RisC Group. Worldwide trends in children’s and adolescents’ body mass index, underweight and obesity, in comparison with adults, from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies with 128.9 million participants. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Wellman, N.S.; Friedberg, B. Causes and consequences of adult obesity: Health, social and economic impacts in the United States. Asia Pac. J. Clin. Nutr. 2002, 11, S705–S709. [Google Scholar] [CrossRef]
- WHO. Population-Based Approaches to Childhood Obesity Prevention; WHO: Geneva, Switzerland, 2012; pp. 1–45. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2015 in Brief. Perinatal Statistics Series Number 33; Australian Institute of Health and Welfare: Darlinghurst, Australia, 2017. [Google Scholar]
- Sheil, W.; Scott, J.; Catcheside, B.; Sage, L. Pregnancy Outcome in South Australia 2010; Pregnancy Outcome Unit, SA Health: Adelaide, Australia, 2012. [Google Scholar]
- Australian Institute of Health and Welfare. A Picture of Overweight and Obesity in Australia. Cat. No. PHE 216; Australian Institute of Health and Welfare: Canberra, Australia, 2017. [Google Scholar]
- Dodd, J.M.; Grivell, R.M.; Nguyen, A.-M.; Chan, A.; Robinson, J.S. Maternal and perinatal health outcomes by body mass index category. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 136–140. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press (US): Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Peaceman, A.M. Gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 642–651. [Google Scholar] [CrossRef]
- Khambule, L.; Anna George, J. Reviews on Biomarker Studies of Metabolic and Metabolism-Related Disorders. 12 The role of Inflammation in the developement ofGDM and the use of Markers of Inflammation in GDM screening. Adv. Exp. Med. Biol. 2019, 217–242. [Google Scholar] [CrossRef]
- Korlesky, C.; Kling, P.J.; Pham, D.Q.D.; Ovasapyan, A.A.; Leyns, C.E.G.; Weber, M.B.; Coe, C.L. Cord Blood Erythropoietin and Hepcidin Reflect Lower Newborn Iron Stores due to Maternal Obesity during Pregnancy. Am. J. Perinatol. 2018, 36, 511–516. [Google Scholar] [CrossRef]
- Chen, A.; Feresu, S.A.; Fernandez, C.; Rogan, W.J. Maternal Obesity and the Risk of Infant Death in the United States. Epidemiology 2009, 20, 74–81. [Google Scholar] [CrossRef]
- Kristensen, J.; Vestergaard, M.; Wisborg, K.; Kesmodel, U.; Secher, J. Pre-pregnancy weight and the risk of stillbirth and neonatal death. Int. J. Obstet. Gyneacol. 2005, 112, 403–408. [Google Scholar] [CrossRef]
- Ovesen, P.; Rasmussen, S.; Kesmodel, U. Effect of Prepregnancy Maternal Overweight and Obesity on Pregnancy Outcome. Obstet. Gynecol. 2011, 118, 305–312. [Google Scholar] [CrossRef]
- Nohr, E.A.; Timpson, N.J.; Andersen, C.S.; Smith, G.D.; Olsen, J.; Sørensen, T.I.A. Severe Obesity in Young Women and Reproductive Health: The Danish National Birth Cohort. PLoS ONE 2009, 4, e8444. [Google Scholar] [CrossRef]
- Leddy, M.A.; Power, M.L.; Schulkin, J. Impact of maternal obesity on fetal health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar]
- Radulescu, L.; Munteanu, O.; Popa, F.; Cirstoiu, M. The implications and consequences of maternal obesity on fetal intrauterine growth restriction. J. Med. Life 2013, 6, 292–298. [Google Scholar]
- Overweight and Obesity. Australian Bureau of Statistics; 4338.0—Profiles of Health, Australia. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/bySubject/4338.0~2011-13 (accessed on 24 September 2014).
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. Inflammation and implantation. Am. J. Reprod. Immunol. 2010, 63, 17–21. [Google Scholar] [CrossRef]
- Mor, G. Inflammation and pregnancy: The role of toll-like receptors in trophoblast-immune interaction. Ann. N. Y. Acad. Sci. 2008, 1127, 121–128. [Google Scholar] [CrossRef]
- Belo, L.; Santos-Silva, A.; Rocha, S.; Caslake, M.; Cooney, J.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 46–51. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kwon, J.Y.; Kim, M.A.; Park, Y.W.; Kim, Y.H. Maternal serum highly sensitive C-reactive protein in normal pregnancy and pre-eclampsia. Int. J. Gyanecol. Obstet. 2007, 98, 105–109. [Google Scholar] [CrossRef]
- Simavli, S.; Derbent, A.U.; Uysal, S.; Turhan, N.O. Hepcidin, iron status, and inflammation variables among healthy pregnant women in the Turkish population. J. Matern. Fetal Neonatal Med. 2013, 27, 75–79. [Google Scholar] [CrossRef]
- Christian, P.; Jiang, T.; Khatry, S.K.; LeClerq, S.C.; Shrestha, S.R.; West, K.P. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am. J. Clin. Nutr. 2006, 83, 788–794. [Google Scholar] [CrossRef]
- Lee, S.; Guillet, R.; Cooper, E.M.; Westerman, M.; Orlando, M.; Pressman, E.; O’Brien, K.O. Maternal Inflammation at Delivery Affects Assessment of Maternal Iron Status. J. Nutr. 2014, 144, 1524–1532. [Google Scholar] [CrossRef]
- Christian, L.M.; Porter, K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 2014, 70, 1–18. [Google Scholar] [CrossRef]
- Picklesimer, A.H.; Jared, H.L.; Moss, K.; Offenbacher, S.; Beck, J.D.; Boggess, K.A. Racial differences in C-reactive protein levels during normal pregnancy. Am. J. Obstet. Gynecol. 2008, 199, 523.e1–523.e6. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Siega-Riz, A.M.; Miller, W.C.; Cogswell, M.E.; McDonald, T. Who should be screened for postpartum anemia? An evaluation of current recommendations. Am. J. Epidemiol. 2002, 156, 903–912. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Scanlon, K.S.; Freedman, D.S.; Siega-Riz, A.M.; Cogswell, M.E. High prevalence of postpartum anemia among low-income women in the United States. Am. J. Obstet. Gynecol. 2001, 185, 438–443. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Siega-Riz, A.M.; Cogswell, M.E. High Prepregnancy BMI Increases the Risk of Postpartum Anemia. Obes. Res. 2004, 12, 941–948. [Google Scholar] [CrossRef]
- del Giudice, E.M.; Santoro, N.; Amato, A.; Brienza, C.; Calabro, P.; Wiegerinck, E.T.; Cirillo, G.; Tartaglione, N.; Grandone, A.; Swinkels, D.W.; et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J. Clin. Endocrinol. Metab. 2009, 94, 5102–5107. [Google Scholar] [CrossRef]
- Dao, M.C.; Sen, S.; Iyer, C.; Klebenov, D.; Meydani, S.N. Obesity during pregnancy and fetal iron status: Is Hepcidin the link? J. Perinatol. 2013, 33, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Matak, P.; McKie, A.T.; Sharp, P. Leptin Increases the Expression of the Iron Regulatory Hormone Hepcidin in HuH7 Human Hepatoma Cells. J. Nutr. 2007, 137, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Anemia of Inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef]
- Srole, D.N.; Ganz, T. Erythroferrone structure, function, and physiology: Iron homeostasis and beyond. J. Cell. Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.A.; Emeis, J.J.; Coppack, S.W. C-Reactive Protein in Healthy Subjects: Associations with Obesity, Insulin Resistance, and Endothelial Dysfunction: A Potential Role for Cytokines Originating From Adipose Tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Feelders, R.A.; Vreugdenhil, G.; Eggermont, A.M.; Kuiper-Kramer, P.A.; van Eijk, H.G.; Swaak, A.J. Regulation of iron metabolism in the acute-phase response: Interferon gamma and tumour necrosis factor alpha induce hypoferraemia, ferritin production and a decrease in circulating transferrin receptors in cancer patients. Eur. J. Clin. Investig. 1998, 28, 520–527. [Google Scholar] [CrossRef]
- Baynes, R.; Bezwoda, W.; Bothwell, T.; Khan, Q.; Mansoor, N. The non-immune inflammatory response: Serial changes in plasma iron, iron-binding capacity, lactoferrin, ferritin and C-reactive protein. Scand. J. Clin. Lab. Investig. 1986, 46, 695–704. [Google Scholar] [CrossRef]
- World Health Organization & Centre for Disease Control and Prevention, C.A.N.-C. The interpretation of indicators of iron status during an acute phase response. Assess. Iron Status Popul. 2007, Annex 4, 95–108. [Google Scholar]
- Cooke, A.A.; Connaughton, R.M.; Lyons, C.L.; McMorrow, A.M.; Roche, H.M. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur. J. Pharmacol. 2016, 785, 207–214. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Gustafson, B. Adipose Tissue, Inflammation and Atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 1–12. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Pendeloski, K.P.T.; Ono, E.; Torloni, M.R.; Mattar, R.; Daher, S. Maternal obesity and inflammatory mediators: A controversial association. Am. J. Reprod. Immunol. 2017, 77, 1–8. [Google Scholar] [CrossRef]
- Micozzi, M.S.; Albanes, D.; Stevens, R.G. Relation of body and hematologic size and composition to clinical indices in US men and women. Am. J. Clin. Nutr. 1989, 50, 1276–1281. [Google Scholar] [CrossRef]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight Children and Adolescents: A Risk Group for Iron Deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, H.; Bidad, K.; Zadhoush, S.; Gholami, N.; Anari, S. Increasing prevalence of iron deficiency in overweight and obese children and adolescents (Tehran Adolescent Obesity Study). Eur. J. Pediatr. 2006, 165, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. 2008, 32, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Newfield, R.S.; Koren, I.; Agmon, A.; Lilos, P.; Phillip, M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. 2003, 27, 416–418. [Google Scholar] [CrossRef]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an Emerging Risk Factor for Iron Deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef]
- Jones, A.D.; Zhao, G.; Jiang, Y.-P.; Zhou, M.; Xu, G.; Kaciroti, N.; Zhang, Z.; Lozoff, B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur. J. Clin. Nutr. 2016, 70, 918–924. [Google Scholar] [CrossRef]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef]
- Namaste, S.M.; Rohner, F.; Huang, J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 359S–371S. [Google Scholar] [CrossRef]
- Flynn, A.C.; Begum, S.; White, S.L.; Dalrymple, K.; Gill, C.; Alwan, N.A.; Kiely, M.; Latunde-Dada, G.; Bell, R.; Briley, A.L.; et al. Relationships between Maternal Obesity and Maternal and Neonatal Iron Status. Nutrients 2018, 10, 1000. [Google Scholar] [CrossRef]
- Cao, C.; Pressman, E.K.; Cooper, E.M.; Guillet, R.; Westerman, M.; O’Brien, K.O. Prepregnancy Body Mass Index and Gestational Weight Gain Have No Negative Impact on Maternal or Neonatal Iron Status. Reprod. Sci. 2016, 23, 613–622. [Google Scholar] [CrossRef]
- Flores-Quijano, M.E.; Vega-Sánchez, R.; Tolentino-Dolores, M.C.; López-Alarcón, M.G.; Flores-Urrutia, M.C.; López-Olvera, A.D.; Talavera, J.O. Obesity Is Associated with Changes in Iron Nutrition Status and Its Homeostatic Regulation in Pregnancy. Nutrients 2019, 11, 693. [Google Scholar] [CrossRef]
- Garcia-Valdes, L.; Campoy, C.; Hayes, H.; Florido, J.; Rusanova, I.; Miranda, M.T.; McArdle, H.J. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int. J. Obes. 2015, 39, 571–578. [Google Scholar] [CrossRef]
- Evenson, K.R.; Wen, F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev. Med. 2011, 53, 39–43. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Fitzgerald, E.M.; Woekel, E.; Cardinal, B.J. Association of Physical Activity and Sedentary Behavior with Biological Markers among U.S. Pregnant Women. J. Women’s Health 2013, 22, 953–958. [Google Scholar] [CrossRef]
- Hawkins, M.; Pekow, P.; Chasan-Taber, L. Physical Activity, Sedentary Behavior, and C-reactive Protein in Pregnancy. Med. Sci. Sports Exerc. 2014, 46, 284–292. [Google Scholar] [CrossRef]
- Tinius, R.A.; Cahill, A.G.; Strand, E.A.; Todd Cade, W. Maternal inflammation during late pregnancy is lower in physically active compared to inactive obese women. Appl. Physiol. Nutr. Metab. 2016, 41, 191–198. [Google Scholar] [CrossRef]
- Renault, K.M.; Carlsen, E.M.; Hædersdal, S.; Nilas, L.; Secher, N.J.; Eugen-Olsen, J.; Cortes, D.; Olsen, S.F.; Halldorsson, T.I.; Nørgaard, K. Impact of lifestyle intervention for obese women during pregnancy on maternal metabolic and inflammatory markers. Int. J. Obes. 2017, 41, 598–605. [Google Scholar] [CrossRef]
- Wang, Y.; Cupul-Uicab, L.A.; Rogan, W.J.; Eggesbo, M.; Travlos, G.; Wilson, R.; Longnecker, M.P. Recreational Exercise Before and During Pregnancy in Relation to Plasma C-Reactive Protein Concentrations in Pregnant Women. J. Phys. Act. Health 2015, 12, 770–775. [Google Scholar] [CrossRef]
- Frass, K.A. Postpartum hemorrhage is related to the hemoglobin levels at labor: Observational study. Alex. J. Med. 2015, 51, 333–337. [Google Scholar] [CrossRef]
- Rukuni, R.; Bhattacharya, S.; Murphy, M.F.; Roberts, D.; Stanworth, S.J.; Knight, M. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: A retrospective cohort study. Acta Obstet. Gynecol. Scand. 2016, 95, 555–564. [Google Scholar] [CrossRef]
- Aly, H.; Hammad, T.A.; Nada, A.; Mohamed, M.A.; Bathgate, S.; El-Mohandes, A. Maternal obesity, associated complications and risk of prematurity. J. Perinatol. 2009, 30, 447–451. [Google Scholar] [CrossRef]
- Wang, L.-F.; Wang, H.-J.; Ao, D.; Liu, Z.; Wang, Y.; Yang, H.-X. Influence of pre-pregnancy obesity on the development of macrosomia and large for gestational age in women with or without gestational diabetes mellitus in Chinese population. J. Perinatol. 2015, 35, 985–990. [Google Scholar] [CrossRef]
- Lynch, C.; Sexton, D.; Hession, M.; Morrison, J.J. Obesity and Mode of Delivery in Primigravid and Multigravid Women. Am. J. Perinatol. 2008, 25, 163–167. [Google Scholar] [CrossRef]
- Fallatah, A.M.; Babatin, H.M.; Nassibi, K.M.; Banweer, M.K.; Fayoumi, M.N.; Oraif, A.M. Maternal and Neonatal Outcomes among Obese Pregnant Women in King Abdulaziz University Hospital: A Retrospective Single-Center Medical Record Review. Med. Arch. 2019, 73, 425–432. [Google Scholar] [CrossRef]
- Blomberg, M. Maternal Obesity and Risk of Postpartum Hemorrhage. Obstet. Gynecol. 2011, 118, 561–568. [Google Scholar] [CrossRef]
- Allard, S.; Green, L.; Hunt, B.J. How we manage the haematological aspects of major obstetric haemorrhage. Br. J. Haematol. 2013, 164, 177–188. [Google Scholar] [CrossRef]
- Shields, L.E.; Goffman, D.; Caughey, A.B. Postpartum hemorrhage. Am. Coll. Obstet. Gynecol. Pract. Bull. 2017, 130, 168–186. [Google Scholar]
- Butwick, A.J.; Abreo, A.; Bateman, B.T.; Lee, H.C.; El-Sayed, Y.Y.; Stephansson, O.; Flood, P. Effect of Maternal Body Mass Index on Postpartum Hemorrhage. Anesthesiology 2018, 128, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Oliva, K.; Barker, G.; Riley, C.; Bailey, M.J.; Permezel, M.; Rice, G.E.; Lappas, M. The effect of pre-existing maternal obesity on the placental proteome: Two-dimensional difference gel electrophoresis coupled with mass spectrometry. J. Mol. Endocrinol. 2012, 48, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sewell, M.F.; Huston-Presley, L.; Super, D.M.; Catalano, P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am. J. Obstet. Gynecol. 2006, 195, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Goldenberg, R.L.; Hou, J.; Johnston, K.E.; Cliver, S.P.; Ramey, S.L.; Nelson, K.G. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J. Pediatr. 2002, 140, 165–170. [Google Scholar] [CrossRef]
- Shao, J.; Lou, J.; Rao, R.; Georgieff, M.K.; Kaciroti, N.; Felt, B.T.; Zhao, Z.-Y.; Lozoff, B. Maternal Serum Ferritin Concentration Is Positively Associated with Newborn Iron Stores in Women with Low Ferritin Status in Late Pregnancy. J. Nutr. 2012, 142, 2004–2009. [Google Scholar] [CrossRef]
- Amin, S.B.; Orlando, M.; Wang, H. Latent Iron Deficiency in Utero Is Associated with Abnormal Auditory Neural Myelination in ≥35 Weeks Gestational Age Infants. J. Perinatol. 2013, 163, 1267–1271. [Google Scholar] [CrossRef]
- Armony-Sivan, R.; Eidelman, A.I.; Lanir, A.; Sredni, D.; Yehuda, S. Iron Status and Neurobehavioral Development of Premature Infants. J. Perinatol. 2004, 24, 757–762. [Google Scholar] [CrossRef]
- Dosch, N.C.; Guslits, E.F.; Weber, M.B.; Murray, S.E.; Ha, B.; Coe, C.L.; Auger, A.P.; Kling, P.J. Maternal Obesity Affects Inflammatory and Iron Indices in Umbilical Cord Blood. Physiol. Behav. 2016, 172, 20–28. [Google Scholar] [CrossRef]
- McLimore, H.M.; Phillips, A.K.; Blohowiak, S.E.; Pham, D.Q.-D.; Coe, C.L.; Fischer, B.A.; Kling, P.J. Impact of Multiple Prenatal Risk Factors on Newborn Iron Status at Delivery. J. Pediatr. Hematol. 2013, 35, 473–477. [Google Scholar] [CrossRef]
- MacQueen, B.C.; Christensen, R.D.; Baer, V.L.; Ward, D.M.; Snow, G.L. Screening umbilical cord blood for congenital Iron deficiency. Blood Cells Mol. Dis. 2019, 77, 95–100. [Google Scholar] [CrossRef]
- Phillips, A.K.; Roy, S.C.; Lundberg, R.; Guilbert, T.W.; Auger, A.P.; Blohowiak, S.E.; Coe, C.L.; Kling, P.J. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J. Perinatol. 2014, 34, 513–518. [Google Scholar] [CrossRef]
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and Iron Homeostasis during Pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef]
- Barrett, J.F.; Whittaker, P.G.; Williams, J.G.; Lind, T. Absorption of non-haem iron from food during normal pregnancy. BMJ 1994, 309, 79–82. [Google Scholar] [CrossRef]
- Flores-Quijano, M.E.; Montalvo-Velarde, I.; Vital-Reyes, V.S.; Rodríguez-Cruz, M.; Rendón-Macías, M.E.; López-Alarcón, M. Longitudinal Analysis of the Interaction Between Obesity and Pregnancy on Iron Homeostasis: Role of Hepcidin. Arch. Med. Res. 2016, 47, 550–556. [Google Scholar] [CrossRef]
- Koenig, M.D.; Klikuszowian, E.; O’Brien, K.O.; Pauls, H.; Steffen, A.; Demartelly, V.; Ruchob, R.; Welke, L.; Hemphill, N.; LaBomascus, B.; et al. Prepregnancy Obesity Is Not Associated with Iron Utilization during the Third Trimester. J. Nutr. 2020, 150, 1397–1404. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Wewerka, S.W.; Nelson, C.A.; Deregnier, R.-A. Iron status at 9 months of infants with low iron stores at birth. J. Pediatr. 2002, 141, 405–409. [Google Scholar] [CrossRef]
- Lukowski, A.F.; Koss, M.; Burden, M.J.; Jonides, J.; Nelson, C.A.; Kaciroti, N.; Jimenez, E.; Lozoff, B. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr. Neurosci. 2010, 13, 54–70. [Google Scholar] [CrossRef]
- Finch, C.A.; Stray, S.; Huebers, H.A.; Bellotti, V.; Lipschitz, D.A.; Cook, J.D.; Pippard, M.J. Plasma Ferritin Determination as a Diagnostic Tool. West. J. Med. 1986, 145, 657–663. [Google Scholar]
- Nemeth, E.; Ganz, T. The Role of Hepcidin in Iron Metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef]
- Semba, R.D.; Kumwenda, N.; Hoover, D.R.; Taha, T.E.; Mtimavalye, L.; Broadhead, R.; Eisinger, W.; Miotti, P.G.; Chiphangwi, J.D. Assessment of iron status using plasma transferrin receptor in pregnant women with and without human immunodeficiency virus infection in Malawi. Eur. J. Clin. Nutr. 2000, 54, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Menendez, C.; Quinto, L.L.; Kahigwa, E.; Alvarez, L.; Fernandez, R.; Gimenez, N.; Schellenberg, D.; Aponte, J.J.; Tanner, M.; Alonso, P.L. Effect of Malaria on Soluble transferin receptor levels in Tanzanian infants. Am. J. Trop. Med. Hyg. 2001, 65, 138–142. [Google Scholar] [CrossRef]
- Grant, F.K.E.; Suchdev, P.S.; Flores-Ayala, R.; Cole, C.R.; Ramakrishnan, U.; Ruth, L.J.; Martorell, R. Correcting for Inflammation Changes Estimates of Iron Deficiency among Rural Kenyan Preschool Children. J. Nutr. 2011, 142, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Skikne, B.S. Serum transferrin receptor. Am. J. Hematol. 2008, 83, 872–875. [Google Scholar] [CrossRef]
- Lewis, D.K.; Whitty, C.J.; Epino, H.; Letsky, E.A.; Mukiibi, J.M.; van den Broek, N.R. Interpreting tests for iron deficiency among adults in a high HIV prevalence African setting: Routine tests may lead to misdiagnosis. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 613–617. [Google Scholar] [CrossRef]
- Namaste, S.M.; Aaron, G.J.; Varadhan, R.; Peerson, J.M.; Suchdev, P.S. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 333S–347S. [Google Scholar] [CrossRef]
- Witte, D.L. Can serum ferritin be effectively interpreted in the presence of the acute-phase response? Clin. Chem. 1991, 37, 484–485. [Google Scholar] [CrossRef]
- Calje, E.; Skinner, J. The challenge of defining and treating anemia and iron deficiency in pregnancy: A study of New Zealand midwives’ management of iron status in pregnancy and the postpartum period. Birth 2017, 44, 181–190. [Google Scholar] [CrossRef]
- Parker, M.L.; Storm, S.; Sholzberg, M.; Yip, P.M.; Beriault, D.R. Revising Ferritin Lower Limits: It’s Time to Raise the Bar on Iron Deficiency. J. Appl. Lab. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Stengel, M.R.; Kraschnewski, J.L.; Hwang, S.W.; Kjerulff, K.H.; Chuang, C.H. “What My Doctor Didn’t Tell Me”: Examining Health Care Provider Advice to Overweight and Obese Pregnant Women on Gestational Weight Gain and Physical Activity. Women’s Health Issues 2012, 22, e535–e540. [Google Scholar] [CrossRef]
- Beard, J.L. Effectiveness and strategies of iron supplementation during pregnancy. Am. J. Clin. Nutr. 2000, 71, 1288S–1294S. [Google Scholar] [CrossRef]
- Bhavi, S.B.; Jaju, P.B. Intravenous iron sucrose v/s oral ferrous fumarate for treatment of anemia in pregnancy. A randomized controlled trial. BMC Pregnancy Childbirth 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Verma, S.; Cherayil, B.J. Iron and inflammation-the gut reaction. Metallomics 2017, 9, 101–111. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Achebe, M.; DeLoughery, T.G. Clinical data for intravenous iron—Debunking the hype around hypersensitivity. Transfusion 2020, 60, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Cocchiaro, C.; Saadat-Gilani, K.; Hodyl, N.; Dekker, G. Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: A randomized trial. J. Matern. Neonatal Med. 2012, 26, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Collingwood, J.; Hodyl, N.A.; Dekker, G. Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Pregnancy Childbirth 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Froessler, B.; Gajic, T.; Dekker, G.; Hodyl, N.A. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch. Gynecol. Obstet. 2018, 298, 75–82. [Google Scholar] [CrossRef]
- Wolf, M.; Chertow, G.M.; MacDougall, I.C.; Kaper, R.; Krop, J.; Strauss, W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Klein, K.; Asaad, S.; Econs, M.; Rubin, J.E. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J.; BSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef]
- Department of Health, Government of South Australia. South Australian Perinatal Practice Guidelines Anaemia in pregnancy. SA Health, 19 April 2016. [Google Scholar]
- Cepeda-Lopez, A.C.; Melse-Boonstra, A.; Zimmermann, M.B.; Herter-Aeberli, I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am. J. Clin. Nutr. 2015, 102, 1389–1397. [Google Scholar] [CrossRef]
- Baumgartner, J.; Smuts, C.M.; Aeberli, I.; Malan, L.; Tjalsma, H.; Zimmermann, M.B. Overweight impairs efficacy of iron supplementation in iron-deficient South African children: A randomized controlled intervention. Int. J. Obes. 2013, 37, 24–30. [Google Scholar] [CrossRef]
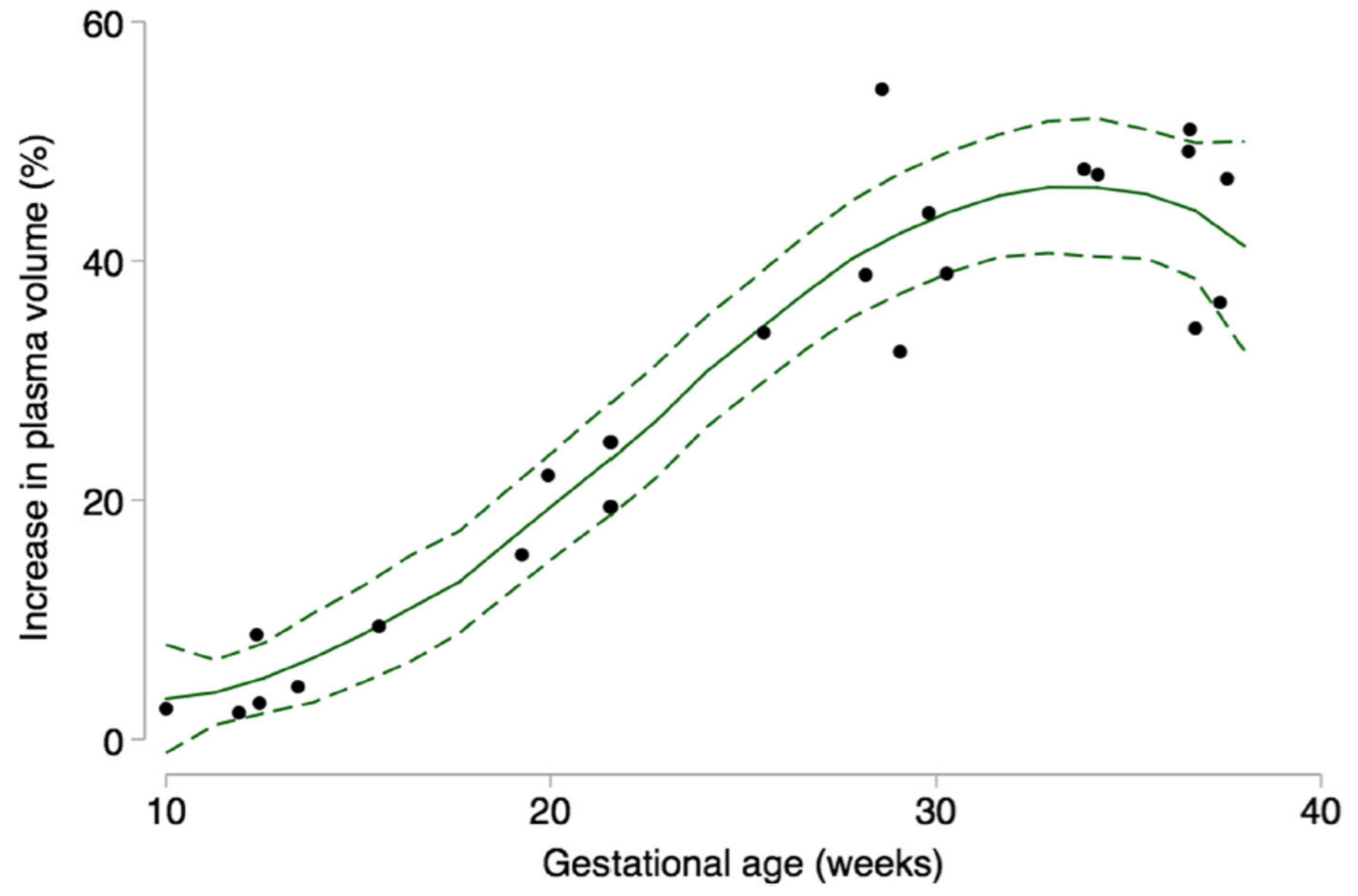
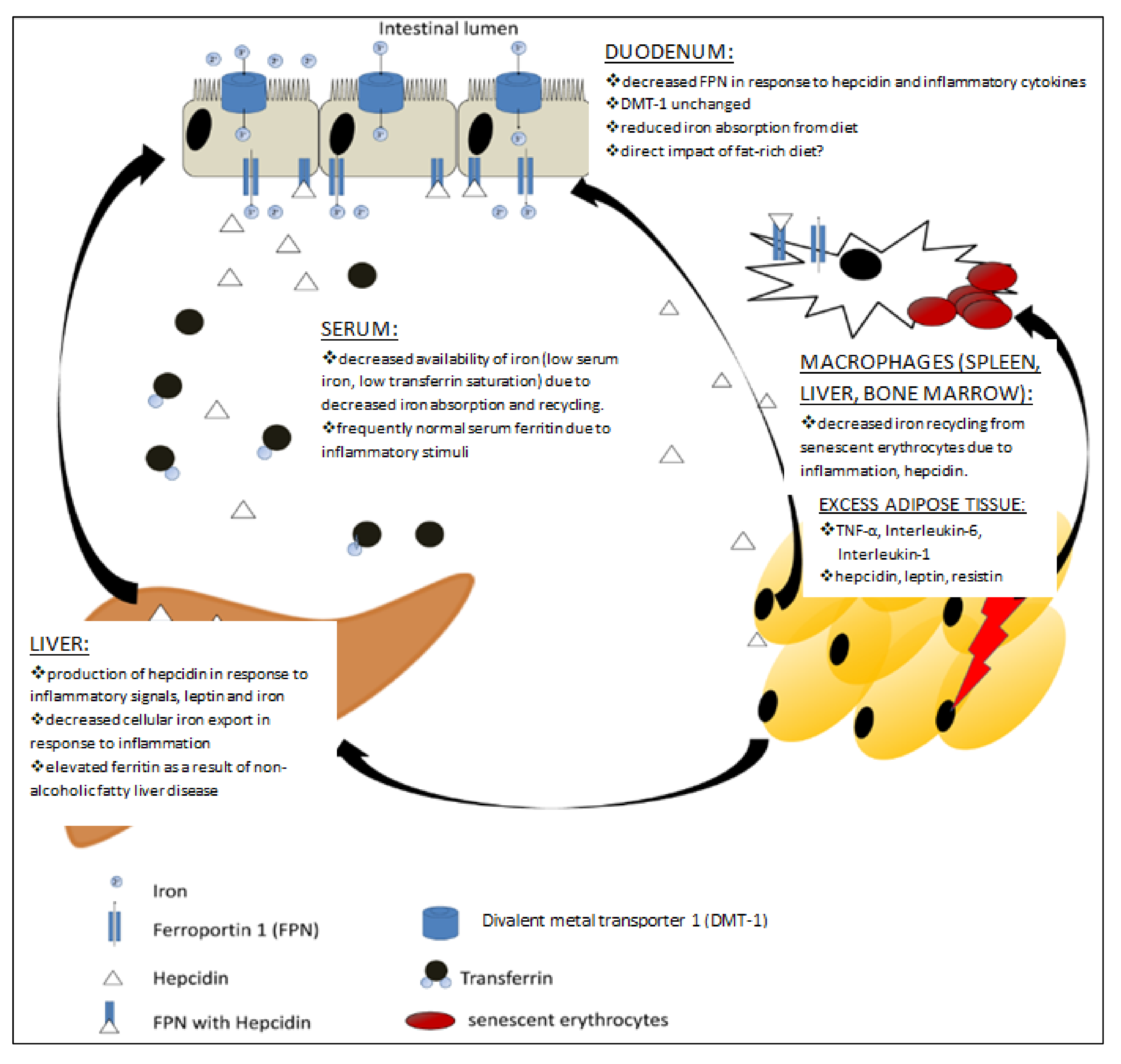
| Pre-Pregnancy | First Trimester | Second Trimester | Third Trimester | Postpartum, 24 h | |
|---|---|---|---|---|---|
| Daily iron requirements approx. (mg/d) | 1.5 | ↓ to 0.8 due to halted menstruation | ↑ to 4 towards the end of second trim | ↑ to 6–10 | --- |
| Iron absorption from a highly bioavailable diet ◊ (mg/d) | Approx. 1.5 | ↓ to 0.4 | ↑ to 1.9 | ↑ to 5 | --- |
| Circulating haemoglobin * | 120–160 g/L | ↓ by 10 g/L | ↓ | ↑ with Fe supplements ↓ without Fe supplements | ↔; individual changes depend on blood loss and fluid shifts. |
| Anaemia threshold for women Hb (g/L), WHO | 120 | ↓ to 110 | 110 (WHO) or 105 (CDC ***) | 110 | --- |
| Red blood cell mass | ↑ | ||||
| Plasma volume expansion, placental growth | --- | ↑ ** towards the end of the first trimester | ↑ | ↑ | --- |
| Serum iron a | ↓ ** | ↓ | ↓ | ↔ | |
| Serum ferritin a | ↓ ** | ↓ | ↓ | ↑ ° | |
| sTfR a | ↔ | ↔; ↑ | ↑, ? | ↔ |
| Pre-Pregnancy BMI (kg/m2) | Recommended Weight Gain (kg) | Rates of Weight Gain Second and Third Trimester, Average, (kg/wk) |
|---|---|---|
| <18.5 | 12.5–18.0 | 0.51 |
| 15.5–24.9 | 11.5–16.0 | 0.42 |
| 25.0–29.9 | 7.0–11.5 | 0.28 |
| ≥30 | 5.0–9.0 | 0.22 |
| Direction of Change in Pregnant Obese Women Compared to Pregnant Normal-Weight Women | ||||
|---|---|---|---|---|
| Marker | First Trimester | Second Trimester | Third Trimester | Delivery |
| Hepcidin [58,87,89] | --- | ↑↔↑ ↑ * | ↑ | ↔↑ |
| Serum iron [58,87] | --- | ↔↔ | --- | ↔ |
| TSAT [58] | --- | ↔ | --- | --- |
| IL-6 [58,86,87] | --- | ↔↑↑ | --- | --- |
| CRP [58,86,87] | --- | ↑↑↑ | --- | --- |
| sTfR [86,87,89] | --- | ↑↔↑ | ↑ | ↔↑ |
| ferritin [86,87,89] | --- | ↔↔↓ ↑ * | ↔ | ↔↓ |
| EPO [87] | --- | ↔ | --- | ↔ |
| leptin [87] | --- | ↑ | --- | ↑ |
| Marker | Direction of Change of Cord Blood Parameters in Infants Born to Obese vs. Lean Mother |
|---|---|
| Hepcidin [36,89] | ↔↓ |
| Ferritin [36,83,86,112,115] | ↓↓↓↓↓ |
| TSAT, serum iron, transferrin [89] | ↔ |
| CRP, IL-6 and TNF-α [112] | ↑ |
| Body iron [87] | ↑ |
| EPO, and ZnPP/H [36] | ↑ |
| sTfR [83,89] | ↑↔ |
| Hb [36,87,115] | ↑↑↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawer, A.A.; Hodyl, N.A.; Fairweather-Tait, S.; Froessler, B. Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia? Nutrients 2021, 13, 1572. https://doi.org/10.3390/nu13051572
Wawer AA, Hodyl NA, Fairweather-Tait S, Froessler B. Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia? Nutrients. 2021; 13(5):1572. https://doi.org/10.3390/nu13051572
Chicago/Turabian StyleWawer, Anna A., Nicolette A. Hodyl, Susan Fairweather-Tait, and Bernd Froessler. 2021. "Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia?" Nutrients 13, no. 5: 1572. https://doi.org/10.3390/nu13051572
APA StyleWawer, A. A., Hodyl, N. A., Fairweather-Tait, S., & Froessler, B. (2021). Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia? Nutrients, 13(5), 1572. https://doi.org/10.3390/nu13051572






