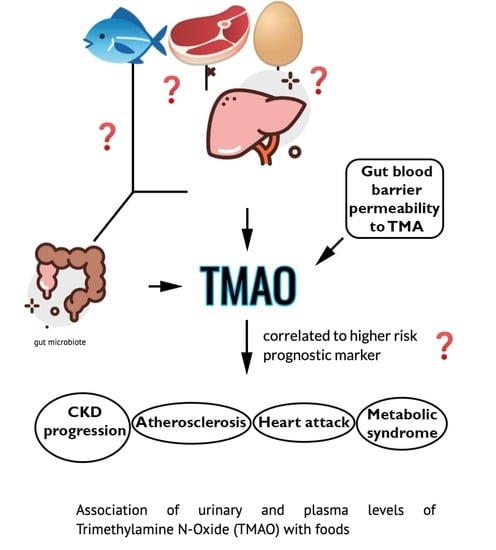
Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods
Abstract
1. Introduction
Search Strategy and Selection Criteria
2. Results
2.1. Fish
2.2. Eggs
2.3. Meat
2.4. Dairy
2.5. Plant-Based Foods
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMAO | trimethylamine N-oxide |
| TMA | trimethylamine |
| RCT | randomised controlled trial |
| CVD | cardiovascular disease |
| T2DM | type-2 diabetes mellitus |
References
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Baker, R.; Chaykin, S. The Biosynthesis of Trimethylamine-N-Oxide. J. Biol. Chem. 1962, 237, 1309–1313. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: Findings from an updated systematic review and meta-analysis. Nutrients 2020, 78, 110856. [Google Scholar] [CrossRef]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota–Dependent Trimethylamine N -Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arter. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent TrimethylamineN-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Gruppen, E.G.; Garcia, E.; Connelly, M.A.; Jeyarajah, E.J.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. TMAO is Associated with Mortality: Impact of Modestly Impaired Renal Function. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Vajdi, M. Novel findings of the association between gut microbiota–derived metabolite trimethylamine N-oxide and inflammation: Results from a systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2801–2823. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Awwad, H.M.; Rabagny, Y.; Graeber, S.; Herrmann, W.; Geisel, J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016, 103, 703–711. [Google Scholar] [CrossRef]
- Svensson, B.; Åkesson, B.; Nilsson, A.; Paulsson, K. Urinary excretion of methylamines in men with varying intake of fish from the baltic sea. J. Toxicol. Environ. Health Part A 1994, 41, 411–420. [Google Scholar] [CrossRef]
- Zhang, A.; Mitchell, S.; Smith, R. Dietary Precursors of Trimethylamine in Man: A Pilot Study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Corbin, K.D.; Da Costa, K.-A.; Zhang, S.; Zhao, X.; Galanko, J.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef]
- West, A.A.; Shih, Y.; Wang, W.; Oda, K.; Jaceldo-Siegl, K.; Sabaté, J.; Haddad, E.; Rajaram, S.; Caudill, M.A.; Burns-Whitmore, B. Egg n-3 Fatty Acid Composition Modulates Biomarkers of Choline Metabolism in Free-Living Lacto-Ovo-Vegetarian Women of Reproductive Age. J. Acad. Nutr. Diet. 2014, 114, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yde, C.C.; Clausen, M.R.; Kristensen, M.; Lorenzen, J.K.; Astrup, A.; Bertram, H.C. Metabolomics Investigation To Shed Light on Cheese as a Possible Piece in the French Paradox Puzzle. J. Agric. Food Chem. 2015, 63, 2830–2839. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef]
- DiMarco, D.M.; Missimer, A.; Murillo, A.G.; Lemos, B.S.; Malysheva, O.V.; Caudill, M.A.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids 2017, 52, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Lutter, C.K.; Waters, W.F.; Riofrío, C.A.G.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K.; et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: A randomized controlled trial in Ecuador. Am. J. Clin. Nutr. 2017, 106, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.; Merz, B.; Rist, M.J.; Ferrario, P.G.; Bub, A.; Kulling, S.E.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 37, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Missimer, A.; Fernandez, M.L.; DiMarco, D.M.; Norris, G.H.; Blesso, C.N.; Murillo, A.G.; Vergara-Jimenez, M.; Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; et al. Compared to an Oatmeal Breakfast, Two Eggs/Day Increased Plasma Carotenoids and Choline without Increasing Trimethyl AmineN-Oxide Concentrations. J. Am. Coll. Nutr. 2017, 37, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Schmedes, M.; Balderas, C.; Aadland, E.K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Holthe, A.; Mellgren, G.; Young, J.F.; et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fasting and Postprandial Serum Metabolites and Lipid Species: Results from a Randomized Crossover Intervention Study in Healthy Adults. Nutrients 2018, 10, 598. [Google Scholar] [CrossRef]
- Pignanelli, M.; Bogiatzi, C.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.L.; Ruetz, K.N.; Velenosi, T.J.; Spence, J.D. Moderate Renal Impairment and Toxic Metabolites Produced by the Intestinal Microbiome: Dietary Implications. J. Ren. Nutr. 2019, 29, 55–64. [Google Scholar] [CrossRef]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Hear. J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Yu, D.; Shu, X.; Rivera, E.S.; Zhang, X.; Cai, Q.; Calcutt, M.W.; Xiang, Y.; Li, H.; Gao, Y.; Wang, T.J.; et al. Urinary Levels of Trimethylamine-N-Oxide and Incident Coronary Heart Disease: A Prospective Investigation Among Urban Chinese Adults. J. Am. Hear. Assoc. 2019, 8, e010606. [Google Scholar] [CrossRef]
- Andraos, S.; Lange, K.; Clifford, S.; Jones, B.; Thorstensen, E.B.; Kerr, J.; Wake, M.; Saffery, R.; Burgner, D.P.; O’Sullivan, J.M. Plasma Trimethylamine N-Oxide and Its Precursors: Population Epidemiology, Parent–Child Concordance, and Associations with Reported Dietary Intake in 11- to 12-Year-Old Children and Their Parents. Curr. Dev. Nutr. 2020, 4, nzaa103. [Google Scholar] [CrossRef]
- Burton, K.J.; Krüger, R.; Scherz, V.; Münger, L.H.; Picone, G.; Vionnet, N.; Bertelli, C.; Greub, G.; Capozzi, F.; Vergères, G. Trimethylamine-N-Oxide Postprandial Response in Plasma and Urine Is Lower After Fermented Compared to Non-Fermented Dairy Consumption in Healthy Adults. Nutrients 2020, 12, 234. [Google Scholar] [CrossRef]
- De Souza, R.J.; Shanmuganathan, M.; Lamri, A.; Atkinson, S.; Becker, A.; Desai, D.; Gupta, M.; Mandhane, P.J.; Moraes, T.J.; Morrison, K.M.; et al. Maternal Diet and the Serum Metabolome in Pregnancy: Robust Dietary Biomarkers Generalizable to a Multiethnic Birth Cohort. Curr. Dev. Nutr. 2020, 4. [Google Scholar] [CrossRef]
- Gessner, A.; Di Giuseppe, R.; Koch, M.; Fromm, M.F.; Lieb, W.; Maas, R. Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: Distribution and correlates in the population-based PopGen cohort. Clin. Chem. Lab. Med. 2020, 58, 733–740. [Google Scholar] [CrossRef]
- Gibson, R.; Lau, C.-H.E.; Loo, R.L.; Ebbels, T.M.D.; Chekmeneva, E.; Dyer, A.R.; Miura, K.; Ueshima, H.; Zhao, L.; Daviglus, M.L.; et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am. J. Clin. Nutr. 2019, 111, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Hagen, I.V.; Helland, A.; Bratlie, M.; Midttun, Ø.; McCann, A.; Sveier, H.; Rosenlund, G.; Mellgren, G.; Ueland, P.M.; Gudbrandsen, O.A. TMAO, creatine and 1-methylhistidine in serum and urine are potential biomarkers of cod and salmon intake: A randomised clinical trial in adults with overweight or obesity. Eur. J. Nutr. 2019, 59, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, R.; Ivey, K.L.; Lee, D.H.; Wang, M.; Li, J.; Franke, A.; Sun, Q.; Rimm, E.B. Association of diet with circulating trimethylamine-N-oxide concentration. Am. J. Clin. Nutr. 2020, 112, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, M.E.; Hov, J.R.; Ueland, T.; Dahl, T.B.; Kummen, M.; Otterdal, K.; Holm, K.; Berge, R.K.; Mollnes, T.E.; Trøseid, M.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Associates with Inflammation in Common Variable Immunodeficiency. Front. Immunol. 2020, 11, 2217. [Google Scholar] [CrossRef]
- Yin, X.; Gibbons, H.; Rundle, M.; Frost, G.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Brennan, L. The Relationship between Fish Intake and Urinary Trimethylamine-N-Oxide. Mol. Nutr. Food Res. 2020, 64, e1900799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. TMAO and Heart Disease: The New Red Meat Risk? JAMA 2019, 321, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Galliazzo, S.; Molaro, M.; Visconti, E.; Pennella, B.; Gaudio, G.V.; Lupi, A.; Grandi, A.M.; Squizzato, A. TMAO as a biomarker of cardiovascular events: A systematic review and meta-analysis. Intern. Emerg. Med. 2021, 16, 201–207. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Kashukh, Y. Impact of L-carnitine and phosphatidylcholine containing products on the proatherogenic metabolite TMAO production and gut microbiome changes in patients with coronary artery disease. Vopr Pitan 2019, 88, 25–33. [Google Scholar]
- Mente, A.; Chalcraft, K.; Ak, H.; Davis, A.D.; Lonn, E.; Miller, R.; Potter, M.A.; Yusuf, S.; Anand, S.S.; McQueen, M.J. The Relationship Between Trimethylamine-N-Oxide and Prevalent Cardiovascular Disease in a Multiethnic Population Living in Canada. Can. J. Cardiol. 2015, 31, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nat. Cell Biol. 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Q.; Li, L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015, 16, S4. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Moré, M.; Bellamine, A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Croyal, M.; Saulnier, P.-J.; Aguesse, A.; Gand, E.; Ragot, S.; Roussel, R.; Halimi, J.-M.; Ducrocq, G.; Cariou, B.; Montaigne, D.; et al. Plasma Trimethylamine N-Oxide and Risk of Cardiovascular Events in Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Naghipour, S.; Cox, A.J.; Peart, J.N.; Du Toit, E.F.; Headrick, J.P. TrimethylamineN-oxide: Heart of the microbiota–CVD nexus? Nutr. Res. Rev. 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Shah, S.; Kalantar-Zadeh, K. Adequacy of Plant-Based Proteins in Chronic Kidney Disease. J. Ren. Nutr. 2019, 29, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Parra-Soto, S.; Gray, S.; Anderson, J.; Welsh, P.; Gill, J.; Sattar, N.; Ho, F.K.; Celis-Morales, C.; Pell, J.P. Vegetarians, fish, poultry, and meat-eaters: Who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur. Hear. J. 2021, 42, 1136–1143. [Google Scholar] [CrossRef]
- Ufnal, M.; Nowiński, A. Is increased plasma TMAO a compensatory response to hydrostatic and osmotic stress in cardiovascular diseases? Med. Hypotheses 2019, 130, 109271. [Google Scholar] [CrossRef] [PubMed]
- Gawrys-Kopczynska, M.; Konop, M.; Maksymiuk, K.; Kraszewska, K.; Derzsi, L.; Sozanski, K.; Holyst, R.; Pilz, M.; Samborowska, E.; Dobrowolski, L.; et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. eLife 2020, 9, e57028. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Datab. Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Mccarty, M.; Okeefe, J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019, 6, e000890. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Bellia, C.; Moletto, C.; Aulisa, G.; Padua, E.; Della-Morte, D.; Caprio, M.; Bellia, A. Effects of Quality and Quantity of Protein Intake for Type 2 Diabetes Mellitus Prevention and Metabolic Control. Curr. Nutr. Rep. 2020, 9, 329–337. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year | Study Design | Sample | Methodology for TMAO Determination $ | Red Meat (e.g., Beef, Lamb) | Meat Products (e.g., Sausages, Bacon) | White Meat (e.g., Chicken) | Fish | Eggs | Milk and Other Dairy Food | Plant-Based Foods | Reference | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Svensson BG | 1994 | Comparative study | Urine | LC/MS | ↑↑ | [11] | Swedish Work Environment Fund and others | ||||||
| Zhang AQ | 1999 | Clinical trial | Urine | TMA/DMA | = | = | = | ↑↑ | = | = | = | [12] | The Leverhulme Trust |
| Tang WHW | 2013 | Prospective | Plasma and Urine | UHPLC-MS/MS | ↑↑ | [13] | National Institutes of Health and its Office of Dietary Supplements | ||||||
| Miller CA | 2014 | RCT | Plasma | LC/MS | ↑↑ | [14] | Egg Nutrition Centre | ||||||
| West AA | 2014 | Clinical trial | Plasma | UHPLC-ESI-MS/SM | = | [15] | American Egg Board and the Agriculture Research Institute at California State Polytechnic University, Pomona | ||||||
| Zheng H | 2015 | Cross-sectional | Urine | NMR | = | [16] | The Danish Council for Strategic Research, Arla Foods, and the Danish Dairy Research Foundation in the project | ||||||
| Rohrmann S | 2016 | Cross-sectional | Plasma | LC/MS | = | = | = | = | ↑ | [17] | Advancement of Human Nutrition | ||
| Di Marco DM | 2017 | Crossover randomised | Plasma | LC/MS | = | [18] | Egg Nutrition Centre | ||||||
| Iannotti LL | 2017 | RCT | Plasma | LC/MS | ↑↑ | [19] | The Mathile Institute for the Advancement of Human Nutrition | ||||||
| Kruger R | 2017 | Comparative study | Plasma | LC/MS | ↑ | ↑ | ↑↑ | [20] | Federal Ministry of Food and Agriculture | ||||
| Cho CE | 2017 | RCT | Urine and Plasma | LC-MS/MS | ↑ | ↑ | ↑↑ | = | [21] | Egg Nutrition Centre and Beef Checkoff | |||
| Lemos BS | 2018 | Crossover randomised | Plasma | LC-MS/MS | = | [22] | Egg Nutrition Centre | ||||||
| Missimer A | 2018 | RCT | Plasma | LC/MS | = | [23] | Egg Nutrition Centre | ||||||
| Schmedes M | 2018 | RCT | Plasma | LC/MS | ↑↑ § | [24] | Aarhus University project “Seafood protein in the prevention of the metabolic syndrome” | ||||||
| Pignanelli M | 2019 | Prospective cohort | Plasma | UHPLC-MS/MS | ↑↑ | [25] | Canadian Institutes of Health Research | ||||||
| Wang Z | 2019 | RCT | Plasma Urine | LC /MS | ↑↑ | [26] | National Institutes of Health and the Office of Dietary Supplements | ||||||
| Yu D | 2019 | Case-control multicentre | Urine | LC/MS | ↑↑ # | ↑↑ # | ↑↑ | = | = | = | [27] | National Institutes of Health (and others) | |
| Andraos S | 2020 | Cross-sectional | Plasma | UHPLC-MS/MS | ↑↑ | = (children) ↑↑ (adults) | = (children) ↑↑ (adults) | ↑↑ | = | [28] | The New Zealand-Australia Life Course Collaboration on Genes, Environment, Nutrition and Obesity | ||
| Burton KJ | 2020 | Crossover randomised | Plasma and Urine | Plasma: UHPLC-MS/MS Urine: NMR (urine) | ↑↑ (fermented) ç ↑ (non-fermented) | [29] | Joint Programming Initiative: A Healthy Diet for a Healthy Life | ||||||
| De Souza RJ | 2020 | Cross-sectional | Plasma | MSI-CE-MS | ↑↑ | ↑↑ | ↑↑ | ↑↑ | [30] | Canadian Institutes of Health Research (CIHR) | |||
| Gessner A | 2020 | Community-based | Plasma | LC/MS | = | = | ↑↑ ^ | = | = | [31] | None declared | ||
| Gibson R | 2020 | Cross-sectional | Urine | NMR | ↑↑ | [32] | None declared | ||||||
| Hagen IV | 2020 | RCT | Plasma and Urine | UHPLC-MS/MS | ↑↑ | [33] | Bergen Medical Research Foundation | ||||||
| Hamaya R | 2020 | Retrospective | Plasma | UPLC-ESI-MS/MS | = | ↑↑ | ↑ | [34] | US Highbush Blueberry Council | ||||
| Macpherson ME | 2020 | Prospective cohort | Plasma | UHPLC-MS/MS | = | = | = | = | = | = | [35] | South-Eastern Norway-Regional Health Authority | |
| Yin X | 2020 | RCT | Urine | NMR | ↑ | ↑↑ | [36] | NutriTech and the European Research Council | |||||
| Zhu C | 2020 | Crossover randomised | Plasma | LC/MS | = | [37] | Egg Nutrition Council |
| First Author | Year | Study Design | Cod | Farmed Salmon | Halibut | Herring | Mackerel | Sardine | swordfish | Shellfish | Clam | Tuna | Trout | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang AQ # | 1999 | Clinical trial | ↑↑ 5135.3 | ↑↑ 8230.2 | ↑↑ 4345 | ↑ 1424.1 | ↑ 2769.4 | ↑ 1562 | = 377.1 | = 301.8 | = 495.2 | [12] | ||
| Yu D | 2019 | Case-control multicentre | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | ↑↑ * | = | [27] |
| Hagen IV | 2020 | RCT | ↑↑ | ↑ | [33] | |||||||||
| Hamaya R | 2020 | Retrospective | ↑↑ | ↑? | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G.; Tarsisano, M.G.; Di Renzo, L.; Federici, M.; Caprio, M.; De Lorenzo, A. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients 2021, 13, 1426. https://doi.org/10.3390/nu13051426
Lombardo M, Aulisa G, Marcon D, Rizzo G, Tarsisano MG, Di Renzo L, Federici M, Caprio M, De Lorenzo A. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients. 2021; 13(5):1426. https://doi.org/10.3390/nu13051426
Chicago/Turabian StyleLombardo, Mauro, Giovanni Aulisa, Daniele Marcon, Gianluca Rizzo, Maria Grazia Tarsisano, Laura Di Renzo, Massimo Federici, Massimiliano Caprio, and Antonino De Lorenzo. 2021. "Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods" Nutrients 13, no. 5: 1426. https://doi.org/10.3390/nu13051426
APA StyleLombardo, M., Aulisa, G., Marcon, D., Rizzo, G., Tarsisano, M. G., Di Renzo, L., Federici, M., Caprio, M., & De Lorenzo, A. (2021). Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients, 13(5), 1426. https://doi.org/10.3390/nu13051426