Nutrition for Older Athletes: Focus on Sex-Differences
Abstract
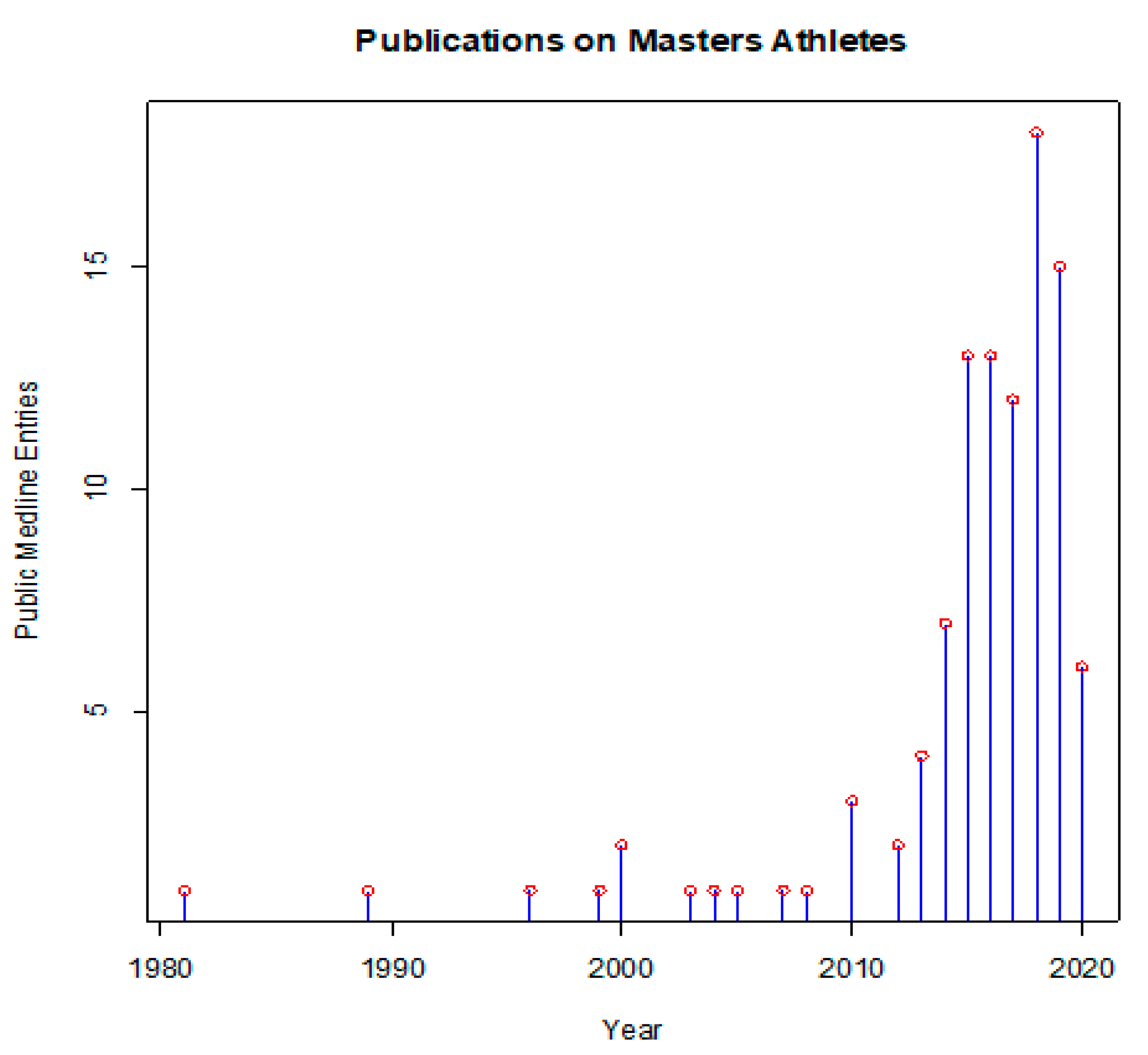
1. Introduction
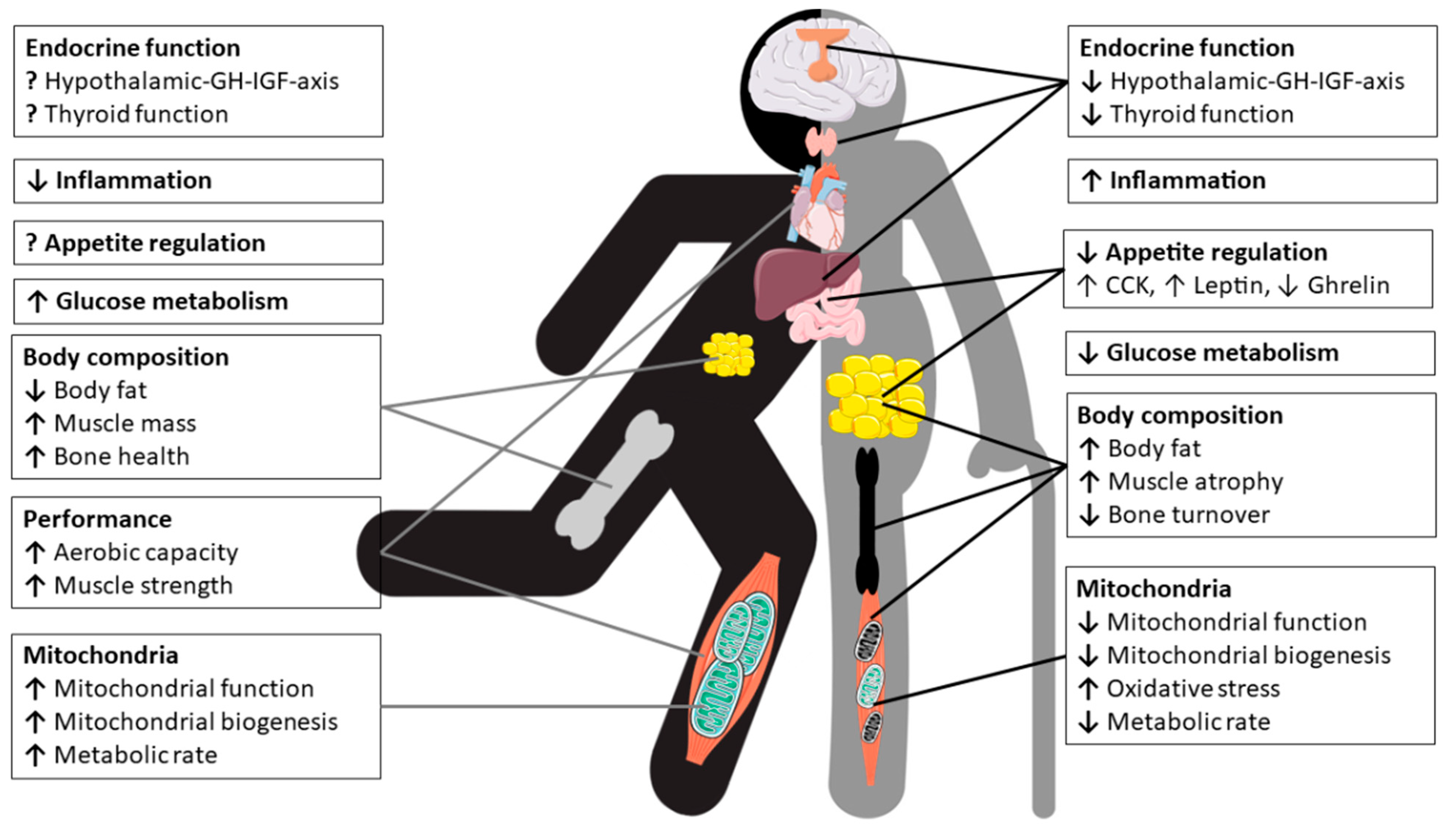
2. Physiological Changes in Older Athletes
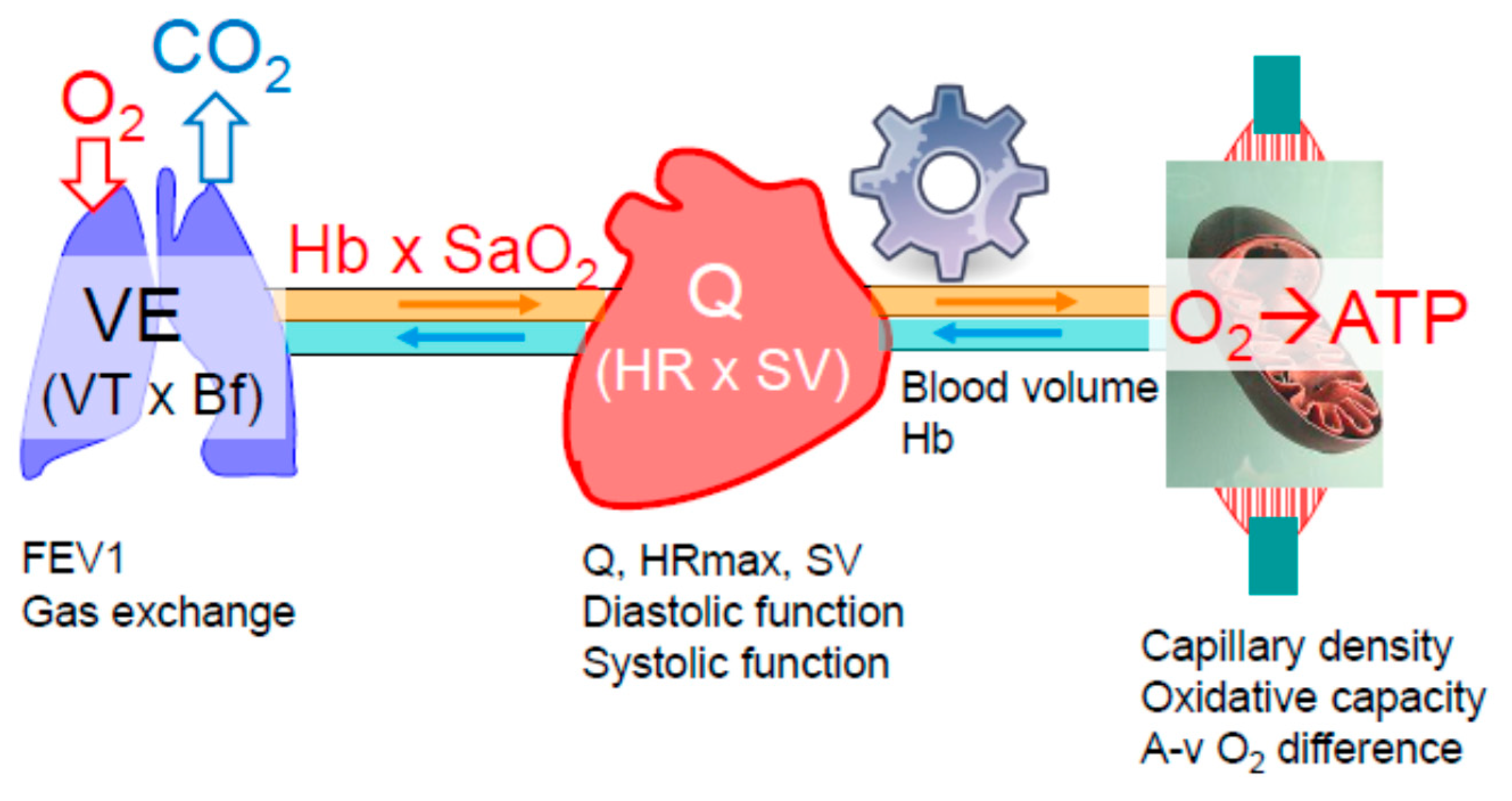
2.1. Oxygen Delivery and Utilization Systems
2.1.1. The Pulmonary System
2.1.2. The Cardiovascular System
2.1.3. Skeletal Muscle and Mitochondria
2.2. Body Composition and Metabolism
2.3. Effects of Aging on the Endocrine System and Metabolic Pathways
2.3.1. Thyroid Hormones
2.3.2. Hypothalamic Growth Hormone-Insulin-Like Growth Factor-I Axis
2.3.3. Hormones Regulating Appetite and Food Intake
2.3.4. Insulin, Glucose and Metabolic Pathways Mediating Glucose Homeostasis
2.4. Bone and Bone Metabolism
3. Performance and Trainability of Older Athletes
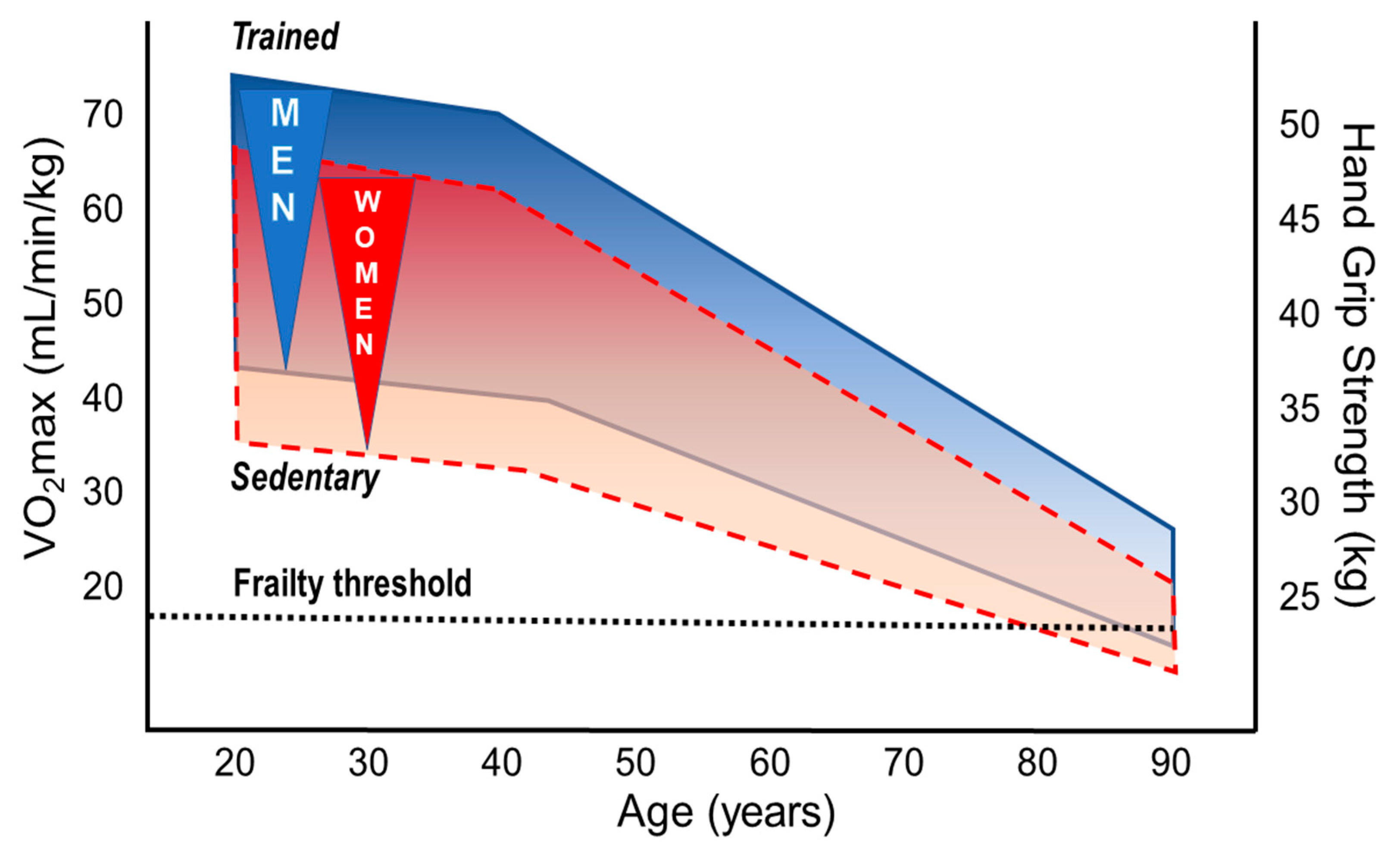
3.1. Performance Decline in the Aging Athlete
3.2. Sex Differences in Performance, Decline Rates, and Training Effects
3.2.1. Aerobic Fitness
3.2.2. Muscular Strength
3.2.3. Mitochondria
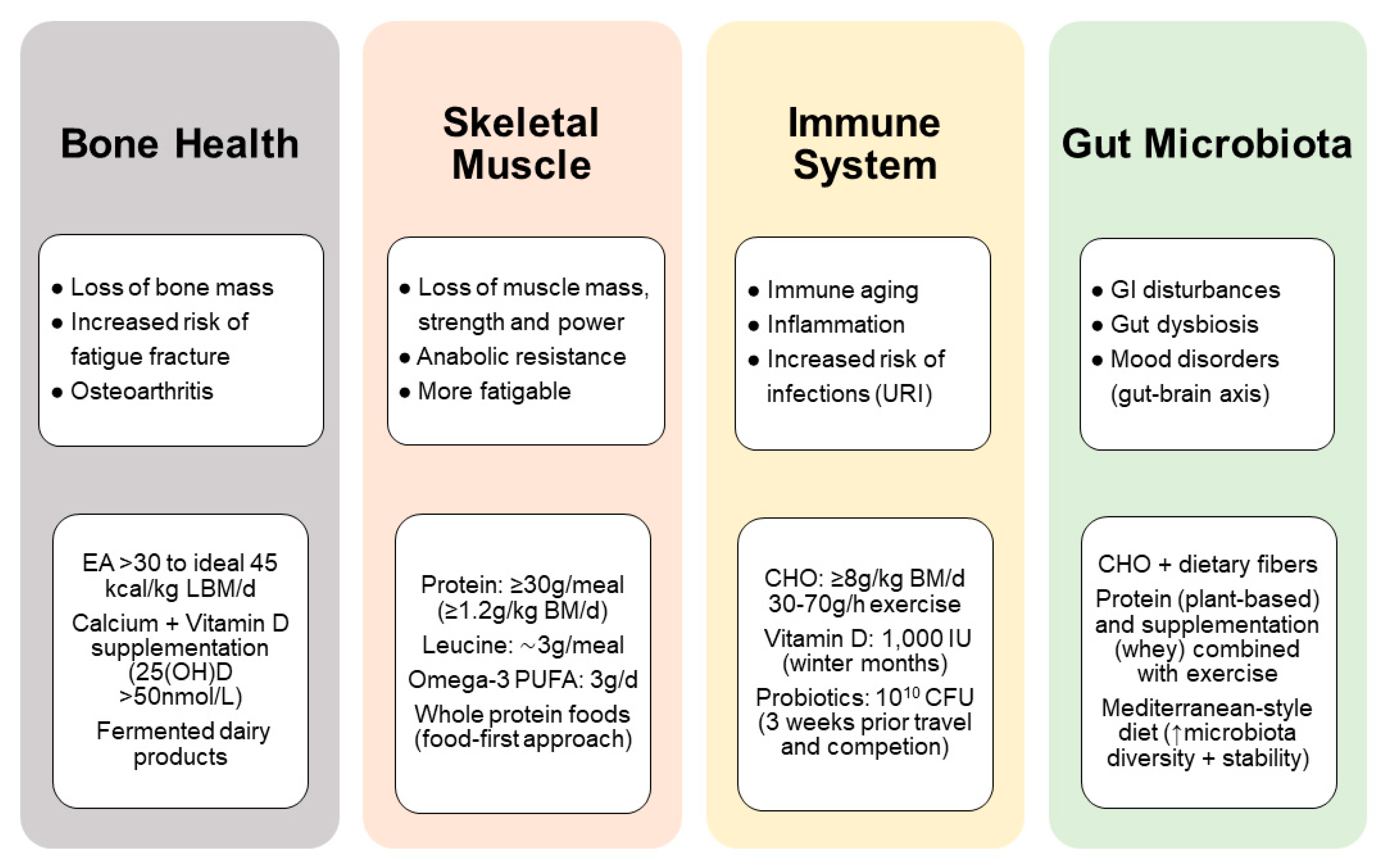
4. Nutritional Considerations for Masters Athletes
4.1. Dietary Protein and Energy Requirements
4.1.1. Optimizing Post-Exercise Recovery
4.1.2. Mastering Anabolic Resistance
4.2. Bone Health and Injury Recovery
4.2.1. Muscle Disuse Atrophy
4.2.2. Role of Nutrition for Injury Recovery
4.3. Immune Function and Risk of Infection
4.3.1. Anti-Inflammatory Vitamin D
4.3.2. Gut Microbiota and Probiotics
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Bowden Davies, K.A.; Sprung, V.S.; Norman, J.A.; Thompson, A.; Mitchell, K.L.; Halford, J.C.G.; Harrold, J.A.; Wilding, J.P.H.; Kemp, G.J.; Cuthbertson, D.J. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: Effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia 2018, 61, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Buehring, B.; Krueger, D.; Anderson, R.M.; Schoeller, D.A.; Binkley, N. Electrical Properties Assessed by Bioelectrical Impedance Spectroscopy as Biomarkers of Age-related Loss of Skeletal Muscle Quantity and Quality. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef] [PubMed]
- Health effects of dietary risks in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [CrossRef]
- World Health Organization. Physical Activity for Health. Report to the Director-General. WHO Executive Board, 142nd Session; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Valenzuela, P.L.; Maffiuletti, N.A.; Joyner, M.J.; Lucia, A.; Lepers, R. Lifelong Endurance Exercise as a Countermeasure Against Age-Related [Formula: See text] Decline: Physiological Overview and Insights from Masters Athletes. Sports Med. 2020, 50, 703–716. [Google Scholar] [CrossRef]
- Burtscher, M. Exercise limitations by the oxygen delivery and utilization systems in aging and disease: Coordinated adaptation and deadaptation of the lung-heart muscle axis-a mini-review. Gerontology 2013, 59, 289–296. [Google Scholar] [CrossRef]
- Habedank, D.; Reindl, I.; Vietzke, G.; Bauer, U.; Sperfeld, A.; Gläser, S.; Wernecke, K.D.; Kleber, F.X. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 421–426. [Google Scholar] [CrossRef] [PubMed]
- McClaran, S.R.; Babcock, M.A.; Pegelow, D.F.; Reddan, W.G.; Dempsey, J.A. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J. Appl. Physiol. 1995, 78, 1957–1968. [Google Scholar] [CrossRef]
- Lavin, K.M.; Straub, A.M.; Uhranowsky, K.A.; Smoliga, J.M.; Zavorsky, G.S. Alveolar-membrane diffusing capacity limits performance in Boston marathon qualifiers. PLoS ONE 2012, 7, e44513. [Google Scholar] [CrossRef] [PubMed]
- Amann, M. Pulmonary system limitations to endurance exercise performance in humans. Exp. Physiol. 2012, 97, 311–318. [Google Scholar] [CrossRef]
- Taylor, B.J.; Johnson, B.D. The pulmonary circulation and exercise responses in the elderly. Semin. Respir. Crit. Care Med. 2010, 31, 528–538. [Google Scholar] [CrossRef]
- Burtscher, M.; Schocke, M.; Koch, R. Ventilation-limited exercise capacity in a 59-year-old athlete. Respir. Physiol. Neurobiol. 2011, 175, 181–184. [Google Scholar] [CrossRef]
- Degens, H.; Maden-Wilkinson, T.M.; Ireland, A.; Korhonen, M.T.; Suominen, H.; Heinonen, A.; Radak, Z.; McPhee, J.S.; Rittweger, J. Relationship between ventilatory function and age in master athletes and a sedentary reference population. Age 2013, 35, 1007–1015. [Google Scholar] [CrossRef][Green Version]
- Ogawa, T.; Spina, R.J.; Martin, W.H.; Kohrt, W.M.; Schechtman, K.B.; Holloszy, J.O.; Ehsani, A.A. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992, 86, 494–503. [Google Scholar] [CrossRef]
- Di Prampero, P.E. Factors limiting maximal performance in humans. Eur. J. Appl. Physiol. 2003, 90, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Dijk, E.; Prasad, A.; Fu, Q.; Torres, P.; Zhang, R.; Thomas, J.D.; Palmer, D.; Levine, B.D. Effect of aging and physical activity on left ventricular compliance. Circulation 2004, 110, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.P.; Nyberg, M.; Gliemann, L.; Thaning, P.; Saltin, B.; Hellsten, Y. Exercise training modulates functional sympatholysis and α-adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J. Physiol. 2014, 592, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Hamer, H.M.; Snijders, T.; van Kranenburg, J.; Frijns, D.; Vink, H.; van Loon, L.J. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 2014, 116, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Bori, Z.; Zhao, Z.; Koltai, E.; Fatouros, I.G.; Jamurtas, A.Z.; Douroudos, I.I.; Terzis, G.; Chatzinikolaou, A.; Sovatzidis, A.; Draganidis, D.; et al. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp. Gerontol. 2012, 47, 417–424. [Google Scholar] [CrossRef]
- Iversen, N.; Krustrup, P.; Rasmussen, H.N.; Rasmussen, U.F.; Saltin, B.; Pilegaard, H. Mitochondrial biogenesis and angiogenesis in skeletal muscle of the elderly. Exp. Gerontol. 2011, 46, 670–678. [Google Scholar] [CrossRef]
- Saltin, B. Hemodynamic adaptations to exercise. Am. J. Cardiol. 1985, 55, 42D–47D. [Google Scholar] [CrossRef]
- Stathokostas, L.; Jacob-Johnson, S.; Petrella, R.J.; Paterson, D.H. Longitudinal changes in aerobic power in older men and women. J. Appl. Physiol. 2004, 97, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Forster, H.; Burtscher, J. Superior endurance performance in aging mountain runners. Gerontology 2008, 54, 268–271. [Google Scholar] [CrossRef]
- Lucía, A.; Hoyos, J.; Pérez, M.; Santalla, A.; Chicharro, J.L. Inverse relationship between VO2max and economy/efficiency in world-class cyclists. Med. Sci. Sports Exerc. 2002, 34, 2079–2084. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fien, S.; Climstein, M.; Quilter, C.; Buckley, G.; Henwood, T.; Grigg, J.; Keogh, J.W.L. Anthropometric, physical function and general health markers of Masters athletes: A cross-sectional study. PeerJ 2017, 5, e3768. [Google Scholar] [CrossRef]
- Wroblewski, A.P.; Amati, F.; Smiley, M.A.; Goodpaster, B.; Wright, V. Chronic exercise preserves lean muscle mass in masters athletes. Phys. Sportsmed 2011, 39, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Pantelić, S.; Trajković, N.; Sporiš, G.; Kostić, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.R.; Pearson, J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J. Physiol. 1969, 204, 63p–66p. [Google Scholar]
- Grassi, B.; Cerretelli, P.; Narici, M.V.; Marconi, C. Peak anaerobic power in master athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Young, A.; Macaluso, A.; Devito, G.; Nimmo, M.A.; Cobbold, M.; Harridge, S.D. Muscle function in elite master weightlifters. Med. Sci. Sports Exerc. 2002, 34, 1199–1206. [Google Scholar] [CrossRef]
- Ireland, A.; Mittag, U.; Degens, H.; Felsenberg, D.; Ferretti, J.L.; Heinonen, A.; Koltai, E.; Korhonen, M.T.; McPhee, J.S.; Mekjavic, I.; et al. Greater maintenance of bone mineral content in male than female athletes and in sprinting and jumping than endurance athletes: A longitudinal study of bone strength in elite masters athletes. Arch. Osteoporos. 2020, 15, 87. [Google Scholar] [CrossRef]
- Alvero Cruz, J.R.; Brikis, M.; Chilibeck, P.; Frings-Meuthen, P.; Vico Guzmán, J.; Mittag, U.; Michely, S.; Mulder, E.; Tanaka, H.; Tank, J.; et al. Age-Related Decline in Vertical Jumping Performance in Masters Track and Field Athletes: Concomitant Influence of Body Composition. Front. Physiol. 2021, in press. [Google Scholar] [CrossRef]
- Piasecki, J.; Ireland, A.; Piasecki, M.; Deere, K.; Hannam, K.; Tobias, J.; McPhee, J.S. Comparison of Muscle Function, Bone Mineral Density and Body Composition of Early Starting and Later Starting Older Masters Athletes. Front. Physiol. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Singh, M.A.F. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef]
- Dubé, J.J.; Broskey, N.T.; Despines, A.A.; Stefanovic-Racic, M.; Toledo, F.G.S.; Goodpaster, B.H.; Amati, F. Muscle Characteristics and Substrate Energetics in Lifelong Endurance Athletes. Med. Sci. Sports Exerc. 2016, 48, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Piers, L.S.; Soares, M.J.; McCormack, L.M.; O’Dea, K. Is there evidence for an age-related reduction in metabolic rate? J. Appl. Physiol. 1998, 85, 2196–2204. [Google Scholar] [CrossRef]
- Krems, C.; Lührmann, P.M.; Straßburg, A.; Hartmann, B.; Neuhäuser-Berthold, M. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur. J. Clin. Nutr. 2005, 59, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Frings-Meuthen, P.; Henkel, S.; Boschmann, M.; Chilibeck, P.D.; Alvero Cruz, J.R.; Hoffmann, F.; Möstl, S.; Mittag, U.; Mulder, E.; Rittweger, N.; et al. Resting Energy Expenditure of Master Athletes: Accuracy of Predictive Equations and Primary Determinants. Front. Physiol. 2021, 12, 641455. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, R.E.; Dinneno, F.A.; Seals, D.R.; Jones, P.P. Age-related decline in RMR in physically active men: Relation to exercise volume and energy intake. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E633–E639. [Google Scholar] [CrossRef]
- Sullo, A.; Cardinale, P.; Brizzi, G.; Fabbri, B.; Maffulli, N. Resting metabolic rate and post-prandial thermogenesis by level of aerobic power in older athletes. Clin. Exp. Pharmacol. Physiol. 2004, 31, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, R.E.; Jones, P.P.; Davy, K.P.; DeSouza, C.A.; Tanaka, H.; Davy, B.M.; Seals, D.R. Regular Exercise and the Age-Related Decline in Resting Metabolic Rate in Women*. J. Clin. Endocrinol. Metab. 1997, 82, 3208–3212. [Google Scholar] [CrossRef]
- Kim, B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid Off. J. Am. Thyroid Assoc. 2008, 18, 141–144. [Google Scholar] [CrossRef]
- Cooper, D.S.; Biondi, B. Subclinical thyroid disease. Lancet 2012, 379, 1142–1154. [Google Scholar] [CrossRef]
- Bremner, A.P.; Feddema, P.; Leedman, P.J.; Brown, S.J.; Beilby, J.P.; Lim, E.M.; Wilson, S.G.; O’Leary, P.C.; Walsh, J.P. Age-related changes in thyroid function: A longitudinal study of a community-based cohort. J. Clin. Endocrinol. Metab. 2012, 97, 1554–1562. [Google Scholar] [CrossRef]
- Waring, A.C.; Arnold, A.M.; Newman, A.B.; Bùzková, P.; Hirsch, C.; Cappola, A.R. Longitudinal changes in thyroid function in the oldest old and survival: The cardiovascular health study all-stars study. J. Clin. Endocrinol. Metab. 2012, 97, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, S.; Barbesino, G.; Caturegli, P.; Bartalena, L.; Sansoni, P.; Fagnoni, F.; Monti, D.; Fagiolo, U.; Franceschi, C.; Pinchera, A. Complex alteration of thyroid function in healthy centenarians. J. Clin. Endocrinol. Metab. 1993, 77, 1130–1134. [Google Scholar] [CrossRef]
- Suzuki, S.; Nishio, S.-I.; Takeda, T.; Komatsu, M. Gender-specific regulation of response to thyroid hormone in aging. Thyroid Res. 2012, 5, 1. [Google Scholar] [CrossRef]
- Meunier, N.; Beattie, J.H.; Ciarapica, D.; O’Connor, J.M.; Andriollo-Sanchez, M.; Taras, A.; Coudray, C.; Polito, A. Basal metabolic rate and thyroid hormones of late-middle-aged and older human subjects: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59 (Suppl. 2), S53–S57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le Roith, D.; Butler, A.A. Insulin-like growth factors in pediatric health and disease. J. Clin. Endocrinol. Metab. 1999, 84, 4355–4361. [Google Scholar] [CrossRef]
- Ghigo, E.; Arvat, E.; Gianotti, L.; Lanfranco, F.; Broglio, F.; Aimaretti, G.; Maccario, M.; Camanni, F. Hypothalamic growth hormone-insulin-like growth factor-I axis across the human life span. J. Pediatric Endocrinol. Metab. 2000, 13 (Suppl. 6), 1493–1502. [Google Scholar] [CrossRef]
- Gilbert, K.L.; Stokes, K.A.; Hall, G.M.; Thompson, D. Growth hormone responses to 3 different exercise bouts in 18- to 25- and 40- to 50-year-old men. Appl. Physiol. Nutr. Metab. 2008, 33, 706–712. [Google Scholar] [CrossRef]
- Craig, B.W.; Brown, R.; Everhart, J. Effects of progressive resistance training on growth hormone and testosterone levels in young and elderly subjects. Mech. Ageing Dev. 1989, 49, 159–169. [Google Scholar] [CrossRef]
- Hameed, M.; Orrell, R.W.; Cobbold, M.; Goldspink, G.; Harridge, S.D. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J. Physiol. 2003, 547, 247–254. [Google Scholar] [CrossRef]
- Hameed, M.; Lange, K.H.; Andersen, J.L.; Schjerling, P.; Kjaer, M.; Harridge, S.D.; Goldspink, G. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J. Physiol. 2004, 555, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- MacIntosh, C.G.; Andrews, J.M.; Jones, K.L.; Wishart, J.M.; Morris, H.A.; Jansen, J.B.; Morley, J.E.; Horowitz, M.; Chapman, I.M. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am. J. Clin. Nutr. 1999, 69, 999–1006. [Google Scholar] [CrossRef]
- Giezenaar, C.; Hutchison, A.T.; Luscombe-Marsh, N.D.; Chapman, I.; Horowitz, M.; Soenen, S. Effect of Age on Blood Glucose and Plasma Insulin, Glucagon, Ghrelin, CCK, GIP, and GLP-1 Responses to Whey Protein Ingestion. Nutrients 2017, 10, 2. [Google Scholar] [CrossRef]
- Chapman, I.M. Endocrinology of anorexia of ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 437–452. [Google Scholar] [CrossRef]
- Johnson, K.O.; Shannon, O.M.; Matu, J.; Holliday, A.; Ispoglou, T.; Deighton, K. Differences in circulating appetite-related hormone concentrations between younger and older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2020, 32, 1233–1244. [Google Scholar] [CrossRef]
- Zhang, Y.; Scarpace, P.J. The role of leptin in leptin resistance and obesity. Physiol. Behav. 2006, 88, 249–256. [Google Scholar] [CrossRef]
- Nicholson, E.; Allison, D.J.; Bullock, A.; Heisz, J.J. Examining the obesity paradox: A moderating effect of fitness on adipose endocrine function in older adults. Mech. Ageing Dev. 2020, 193, 111406. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, N.T.; Møller, B.K.; Raben, A.; Kristensen, S.T.; Holm, L.; Flint, A.; Astrup, A. Determinants of appetite ratings: The role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr. Res. 2011, 55. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M.; Chen, N.; Hollenbeck, C.; Chen, Y.D. Effect of age on glucose tolerance and glucose uptake in healthy individuals. J. Am. Geriatr. Soc. 1989, 37, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Van den Beld, A.W.; Kaufman, J.M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; van der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.U. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Valenta, V.; Wagner, R.; Tschritter, O.; Machann, J.; Häring, H.-U.; Preissl, H.; Fritsche, A.; Heni, M. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat. Commun. 2020, 11, 1841. [Google Scholar] [CrossRef]
- Meneilly, G.S.; Veldhuis, J.D.; Elahi, D. Disruption of the pulsatile and entropic modes of insulin release during an unvarying glucose stimulus in elderly individuals. J. Clin. Endocrinol. Metab. 1999, 84, 1938–1943. [Google Scholar] [CrossRef]
- Basu, R.; Dalla Man, C.; Campioni, M.; Basu, A.; Klee, G.; Toffolo, G.; Cobelli, C.; Rizza, R.A. Effects of age and sex on postprandial glucose metabolism: Differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 2006, 55, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Elahi, D.; Muller, D.C.; Tzankoff, S.P.; Andres, R.; Tobin, J.D. Effect of age and obesity on fasting levels of glucose, insulin, glucagon, and growth hormone in man. J. Gerontol. 1982, 37, 385–391. [Google Scholar] [CrossRef]
- Simonson, D.C.; DeFronzo, R.A. Glucagon physiology and aging: Evidence for enhanced hepatic sensitivity. Diabetologia 1983, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Hagberg, J.M.; Allen, W.K.; Hurley, B.F.; Dalsky, G.P.; Ehsani, A.A.; Holloszy, J.O. Glucose tolerance in young and older athletes and sedentary men. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 1521–1525. [Google Scholar] [CrossRef]
- Amati, F.; Dubé, J.J.; Coen, P.M.; Stefanovic-Racic, M.; Toledo, F.G.S.; Goodpaster, B.H. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009, 32, 1547–1549. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Morino, K.; Alves, T.C.; Kibbey, R.G.; Dufour, S.; Sono, S.; Yoo, P.S.; Cline, G.W.; Shulman, G.I. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11330–11334. [Google Scholar] [CrossRef]
- Consitt, L.A.; Van Meter, J.; Newton, C.A.; Collier, D.N.; Dar, M.S.; Wojtaszewski, J.F.; Treebak, J.T.; Tanner, C.J.; Houmard, J.A. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes 2013, 62, 3437–3447. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526 Pt 1, 203–210. [Google Scholar] [CrossRef]
- Gianni, P.; Jan, K.J.; Douglas, M.J.; Stuart, P.M.; Tarnopolsky, M.A. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp. Gerontol. 2004, 39, 1391–1400. [Google Scholar] [CrossRef]
- Sharma, A.; Smith, H.J.; Yao, P.; Mair, W.B. Causal roles of mitochondrial dynamics in longevity and healthy aging. Embo. Rep. 2019, 20, e48395. [Google Scholar] [CrossRef]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local communication on and within bone controls bone remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Mundy, G.R. The effects of TGF-beta on bone. Ciba Found. Symp. 1991, 157, 137–143, discussion 143–151. [Google Scholar]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.M. Chapter 19-Histomorphometric Analysis of Bone Remodeling. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; p. 303. [Google Scholar]
- Burr, D.B.; Martin, R.B.; Schaffler, M.B.; Radin, E.L. Bone remodeling in response to in vivo fatigue microdamage. J. Biomech 1985, 18, 189–200. [Google Scholar] [CrossRef]
- Frost, H.M. Skeletal structural adaptations to mechanical usage (SATMU): 1. Redefining Wolff’s law: The bone modeling problem. Anat. Rec. 1990, 226, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Buehlmeier, J.; Frings-Meuthen, P.; Mohorko, N.; Lau, P.; Mazzucco, S.; Ferretti, J.L.; Biolo, G.; Pisot, R.; Simunic, B.; Rittweger, J. Markers of bone metabolism during 14 days of bed rest in young and older men. J. Musculoskelet. Neuronal. Interact. 2017, 17, 399–408. [Google Scholar] [PubMed]
- Diab, T.; Condon, K.W.; Burr, D.B.; Vashishth, D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone 2006, 38, 427–431. [Google Scholar] [CrossRef]
- Schiessl, H.; Frost, H.M.; Jee, W.S. Estrogen and bone-muscle strength and mass relationships. Bone 1998, 22, 1–6. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: Function as a determinant for adaptive remodeling in bone. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1987, 5, 300–310. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP signaling controls muscle mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef]
- Regan, J.N.; Trivedi, T.; Guise, T.A.; Waning, D.L. The Role of TGFβ in Bone-Muscle Crosstalk. Curr. Osteoporos. Rep. 2017, 15, 18–23. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Venken, K.; Ophoff, J.; Bouillon, R.; Boonen, S. Clinical Review: Sex steroids and the periosteum—Reconsidering the roles of androgens and estrogens in periosteal expansion. J. Clin. Endocrinol. Metab. 2006, 91, 378–382. [Google Scholar] [CrossRef]
- Prentice, A. Calcium intakes and bone densities of lactating women and breast-fed infants in The Gambia. Adv. Exp. Med. Biol. 1994, 352, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef] [PubMed]
- Khan, K. Physical Activity and Bone Health; Human Kinetics: Champaign, IL, USA, 2001. [Google Scholar]
- Milgrom, C.; Finestone, A.; Simkin, A.; Ekenman, I.; Mendelson, S.; Millgram, M.; Nyska, M.; Larsson, E.; Burr, D. In-vivo strain measurements to evaluate the strengthening potential of exercises on the tibial bone. J. Bone Jt. Surg. Br. Vol. 2000, 82, 591–594. [Google Scholar] [CrossRef]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Kannus, P. Femoral neck structure in adult female athletes subjected to different loading modalities. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 520–528. [Google Scholar] [CrossRef]
- Nichols, J.F.; Palmer, J.E.; Levy, S.S. Low bone mineral density in highly trained male master cyclists. Osteoporos. Int. 2003, 14, 644–649. [Google Scholar] [CrossRef]
- Wilks, D.C.; Gilliver, S.F.; Rittweger, J. Forearm and tibial bone measures of distance- and sprint-trained master cyclists. Med. Sci. Sports Exerc. 2009, 41, 566–573. [Google Scholar] [CrossRef]
- Ireland, A.; Maden-Wilkinson, T.; McPhee, J.; Cooke, K.; Narici, M.; Degens, H.; Rittweger, J. Upper limb muscle-bone asymmetries and bone adaptation in elite youth tennis players. Med. Sci. Sports Exerc. 2013, 45, 1749–1758. [Google Scholar] [CrossRef]
- Warden, S.J. Extreme skeletal adaptation to mechanical loading. J. Orthop Sports Phys. 2010, 40, 188. [Google Scholar] [CrossRef]
- Ireland, A.; Korhonen, M.; Heinonen, A.; Suominen, H.; Baur, C.; Stevens, S.; Degens, H.; Rittweger, J. Side-to-side differences in bone strength in master jumpers and sprinters. J. Musculoskelet. Neuronal. Interact. 2011, 11, 298–305. [Google Scholar]
- Riggs, B.L.; Melton, L.J.; Robb, R.A.; Camp, J.J.; Atkinson, E.J.; McDaniel, L.; Amin, S.; Rouleau, P.A.; Khosla, S. A population-based assessment of rates of bone loss at multiple skeletal sites: Evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008, 23, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Mantila Roosa, S.M.; Kersh, M.E.; Hurd, A.L.; Fleisig, G.S.; Pandy, M.G.; Fuchs, R.K. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc. Natl. Acad. Sci. USA 2014, 111, 5337–5342. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.; Maden-Wilkinson, T.; Ganse, B.; Degens, H.; Rittweger, J. Effects of age and starting age upon side asymmetry in the arms of veteran tennis players: A cross-sectional study. Osteoporos. Int. 2014, 25, 1389–1400. [Google Scholar] [CrossRef]
- Wilks, D.C.; Winwood, K.; Gilliver, S.F.; Kwiet, A.; Chatfield, M.; Michaelis, I.; Sun, L.W.; Ferretti, J.L.; Sargeant, A.J.; Felsenberg, D.; et al. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: A pQCT study. Bone 2009, 45, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wilks, D.C.; Winwood, K.; Gilliver, S.F.; Kwiet, A.; Sun, L.W.; Gutwasser, C.; Ferretti, J.L.; Sargeant, A.J.; Felsenberg, D.; Rittweger, J. Age-dependency in bone mass and geometry: A pQCT study on male and female master sprinters, middle and long distance runners, race-walkers and sedentary people. J. Musculoskelet. Neuronal Interact. 2009, 9, 236–246. [Google Scholar] [PubMed]
- Strasser, B.; Burtscher, M. Survival of the fittest: VO. Front. BioSci. 2018, 23, 1505–1516. [Google Scholar] [CrossRef]
- Burtscher, J.; Burtscher, M. Run for your life: Tweaking the weekly physical activity volume for longevity. Br. J. Sports Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Seals, D.R. Invited Review: Dynamic exercise performance in Masters athletes: Insight into the effects of primary human aging on physiological functional capacity. J. Appl. Physiol. 2003, 95, 2152–2162. [Google Scholar] [CrossRef]
- Tanaka, H.; Seals, D.R. Endurance exercise performance in Masters athletes: Age-associated changes and underlying physiological mechanisms. J. Physiol. 2008, 586, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.B.; Tang, Y.Q. Aging performance for masters records in athletics, swimming, rowing, cycling, triathlon, and weightlifting. Exp. Aging Res. 2010, 36, 453–477. [Google Scholar] [CrossRef] [PubMed]
- Ganse, B.; Drey, M.; Hildebrand, F.; Knobe, M.; Degens, H. Performance Declines Are Accelerated in the Oldest-Old Track and Field Athletes 80 to 94 Years of Age. Rejuvenation Res. 2020. [Google Scholar] [CrossRef]
- Wright, V.J.; Perricelli, B.C. Age-related rates of decline in performance among elite senior athletes. Am. J. Sports Med. 2008, 36, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J.; Di Prampero, P.E.; Maffulli, N.; Narici, M.V. Sprint and endurance power and ageing: An analysis of master athletic world records. Proc. Biol. Sci. 2009, 276, 683–689. [Google Scholar] [CrossRef]
- Fitzgerald, M.D.; Tanaka, H.; Tran, Z.V.; Seals, D.R. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J. Appl. Physiol. 1997, 83, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Zwetsloot, K.A. Basic concepts about genes, inactivity and aging. Scand. J. Med. Sci. Sports 2010, 20, 1–4. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Fusco, D.; Sisto, A.; Ortolani, E.; Savera, G.; Salini, S.; Marzetti, E. Age-Related Variations of Muscle Mass, Strength, and Physical Performance in Community-Dwellers: Results From the Milan EXPO Survey. J. Am. Med. Dir. Assoc. 2017, 18, 88.e17–88.e24. [Google Scholar] [CrossRef]
- Carr, D.B.; Flood, K.; Steger-May, K.; Schechtman, K.B.; Binder, E.F. Characteristics of frail older adult drivers. J. Am. Geriatr. Soc. 2006, 54, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Nachbauer, W.; Wilber, R. The upper limit of aerobic power in humans. Eur. J. Appl. Physiol. 2011, 111, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- Sandbakk, Ø.; Ettema, G.; Holmberg, H.C. Gender differences in endurance performance by elite cross-country skiers are influenced by the contribution from poling. Scand. J. Med. Sci. Sports 2014, 24, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Hayes, E.; Galpin, A.; Kaminsky, L.; Jemiolo, B.; Fink, W.; Trappe, T.; Jansson, A.; Gustafsson, T.; Tesch, P. New records in aerobic power among octogenarian lifelong endurance athletes. J. Appl. Physiol. 2013, 114, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cattagni, T.; Gremeaux, V.; Lepers, R. The Physiological Characteristics of an 83-Year-Old Champion Female Master Runner. Int. J. Sports Physiol. Perform. 2019, 1–5. [Google Scholar] [CrossRef]
- Billat, V.; Dhonneur, G.; Mille-Hamard, L.; Le Moyec, L.; Momken, I.; Launay, T.; Koralsztein, J.P.; Besse, S. Case Studies in Physiology: Maximal oxygen consumption and performance in a centenarian cyclist. J. Appl. Physiol. 2017, 122, 430–434. [Google Scholar] [CrossRef]
- Seiler, S.; De Koning, J.J.; Foster, C. The fall and rise of the gender difference in elite anaerobic performance 1952–2006. Med. Sci. Sports Exerc. 2007, 39, 534–540. [Google Scholar] [CrossRef]
- Coast, J.R.; Blevins, J.S.; Wilson, B.A. Do gender differences in running performance disappear with distance? Can. J. Appl. Physiol. 2004, 29, 139–145. [Google Scholar] [CrossRef]
- Calbet, J.A.; Joyner, M.J. Disparity in regional and systemic circulatory capacities: Do they affect the regulation of the circulation? Acta Physiol. 2010, 199, 393–406. [Google Scholar] [CrossRef]
- Yilmaz, D.C.; Buyukakilli, B.; Gurgul, S.; Rencuzogullari, I. Adaptation of heart to training: A comparative study using echocardiography & impedance cardiography in male & female athletes. Indian J. Med. Res. 2013, 137, 1111–1120. [Google Scholar]
- Karjalainen, J.; Mäntysaari, M.; Viitasalo, M.; Kujala, U. Left ventricular mass, geometry, and filling in endurance athletes: Association with exercise blood pressure. J. Appl. Physiol. 1997, 82, 531–537. [Google Scholar] [CrossRef]
- Korhonen, M.T.; Cristea, A.; Alén, M.; Häkkinen, K.; Sipilä, S.; Mero, A.; Viitasalo, J.T.; Larsson, L.; Suominen, H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J. Appl. Physiol. 2006, 101, 906–917. [Google Scholar] [CrossRef]
- Messa, G.A.M.; Piasecki, M.; Rittweger, J.; McPhee, J.S.; Koltai, E.; Radak, Z.; Simunic, B.; Heinonen, A.; Suominen, H.; Korhonen, M.T.; et al. Absence of an aging-related increase in fiber type grouping in athletes and non-athletes. Scand. J. Med. Sci. Sports 2020, 30, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, J.; Inns, T.B.; Bass, J.J.; Scott, R.; Stashuk, D.W.; Phillips, B.E.; Atherton, P.J.; Piasecki, M. Influence of sex on the age-related adaptations of neuromuscular function and motor unit properties in elite masters athletes. J. Physiol. 2020. [Google Scholar] [CrossRef]
- Strasser, B.; Volaklis, K.; Fuchs, D.; Burtscher, M. Role of Dietary Protein and Muscular Fitness on Longevity and Aging. Aging Dis. 2018, 9, 119–132. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, I.; Kwiet, A.; Gast, U.; Boshof, A.; Antvorskov, T.; Jung, T.; Rittweger, J.; Felsenberg, D. Decline of specific peak jumping power with age in master runners. J. Musculoskelet Neuronal. Interact. 2008, 8, 64–70. [Google Scholar]
- Runge, M.; Rittweger, J.; Russo, C.R.; Schiessl, H.; Felsenberg, D. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin. Physiol. Funct. Imaging 2004, 24, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol Sci. Med. Sci. 1995, 50, 11–16. [Google Scholar] [CrossRef]
- Galloway, M.T.; Kadoko, R.; Jokl, P. Effect of aging on male and female master athletes’ performance in strength versus endurance activities. Am. J. Orthop 2002, 31, 93–98. [Google Scholar] [PubMed]
- Faulkner, J.A.; Davis, C.S.; Mendias, C.L.; Brooks, S.V. The aging of elite male athletes: Age-related changes in performance and skeletal muscle structure and function. Clin. J. Sport Med. 2008, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Cureton, K.; Collins, M. Sex difference in muscular strength in equally-trained men and women. Ergonomics 1987, 30, 675–687. [Google Scholar] [CrossRef]
- Cureton, K.J.; Collins, M.A.; Hill, D.W.; McElhannon, F.M. Muscle hypertrophy in men and women. Med. Sci. Sports Exerc. 1988, 20, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lindle, R.S.; Metter, E.J.; Lynch, N.A.; Fleg, J.L.; Fozard, J.L.; Tobin, J.; Roy, T.A.; Hurley, B.F. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J. Appl. Physiol. 1997, 83, 1581–1587. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Ogborn, D.I.; Vigotsky, A.D.; Franchi, M.V.; Krieger, J.W. Hypertrophic Effects of Concentric vs. Eccentric Muscle Actions: A Systematic Review and Meta-analysis. J. Strength Cond Res. 2017, 31, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
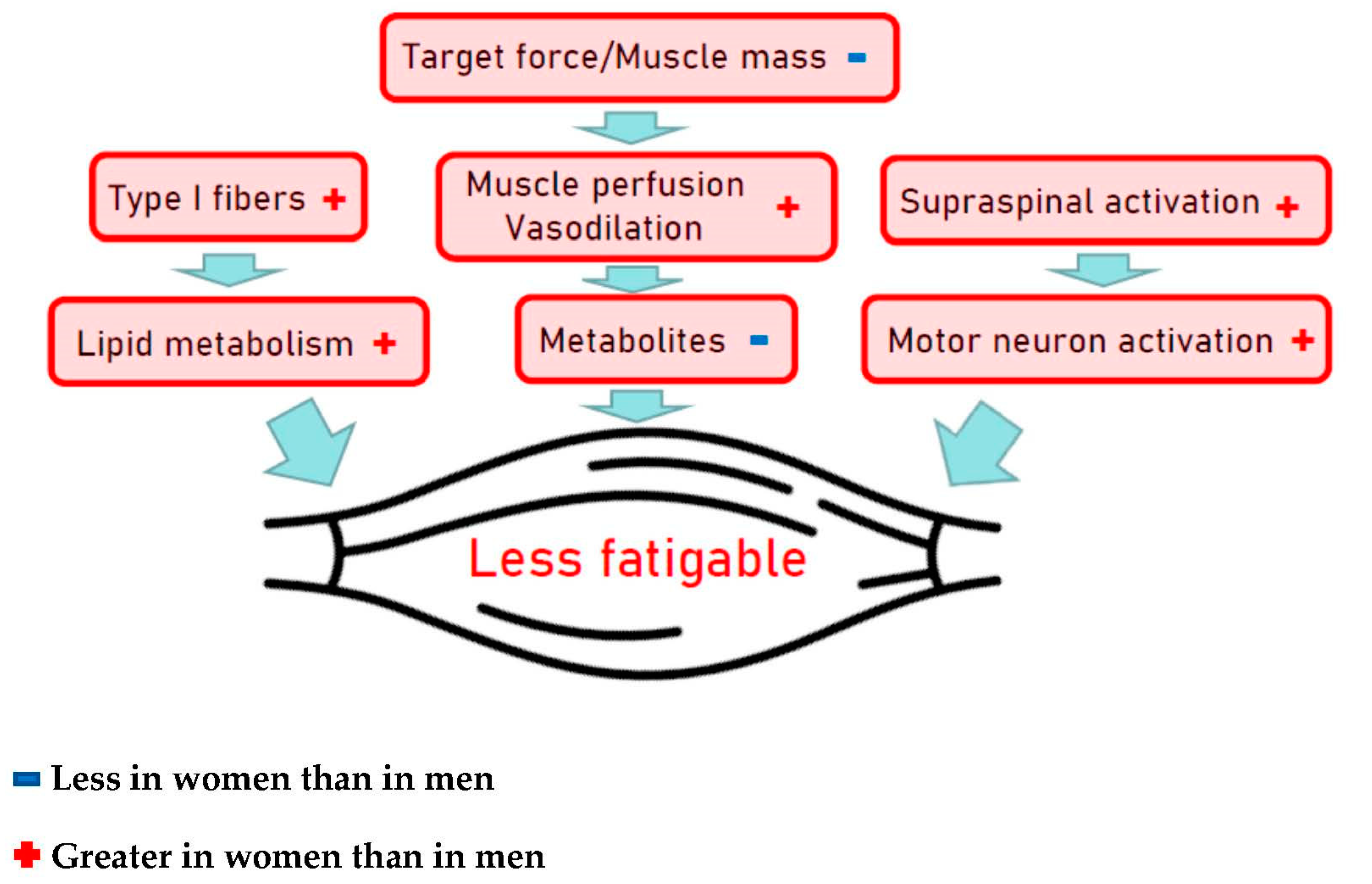
- Hunter, S.K. The Relevance of Sex Differences in Performance Fatigability. Med. Sci. Sports Exerc. 2016, 48, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.-D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Schrepfer, E.; Scorrano, L. Mitofusins, from Mitochondria to Metabolism. Mol. Cell 2016, 61, 683–694. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016, 30, 3413–3423. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Rasmussen, P.; Siebenmann, C.; Díaz, V.; Gassmann, M.; Pesta, D.; Gnaiger, E.; Nordsborg, N.B.; Robach, P.; Lundby, C. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J. Appl. Physiol. 2011, 111, 1422–1430. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Lundby, C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J. Appl. Physiol. 2013, 114, 344–350. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Reznick, R.M.; Zong, H.; Li, J.; Morino, K.; Moore, I.K.; Hannah, J.Y.; Liu, Z.-X.; Dong, J.; Mustard, K.J.; Hawley, S.A. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007, 5, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Hood, D.A. The regulation of autophagy during exercise in skeletal muscle. J. Appl. Physiol. 2016, 120, 664–673. [Google Scholar] [CrossRef]
- Guevara, R.; Santandreu, F.M.; Valle, A.; Gianotti, M.; Oliver, J.; Roca, P. Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress. Free Radic. Biol. Med. 2009, 46, 169–175. [Google Scholar] [CrossRef]
- Valle, A.; Guevara, R.; Garcia-Palmer, F.J.; Roca, P.; Oliver, J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am. J. Physiol. Cell Physiol. 2007, 293, C1302–C1308. [Google Scholar] [CrossRef]
- Justo, R.; Boada, J.; Frontera, M.; Oliver, J.; Bermúdez, J.; Gianotti, M. Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am. J. Physiol. Cell Physiol. 2005, 289, C372–C378. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007, 74, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Alcolea, M.; Valle, A.; Oliver, J.; Roca, P.; García-Palmer, F. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell. Physiol. Biochem. 2007, 19, 205–212. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef]
- Zawada, I.; Masternak, M.M.; List, E.O.; Stout, M.B.; Berryman, D.E.; Lewinski, A.; Kopchick, J.J.; Bartke, A.; Karbownik-Lewinska, M.; Gesing, A. Gene expression of key regulators of mitochondrial biogenesis is sex dependent in mice with growth hormone receptor deletion in liver. Aging 2015, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, N.; Beekman, M.; Deelen, J.; van den Akker, E.B.; de Craen, A.J.; Slagboom, P.E.; t Hart, L.M. Low mitochondrial DNA content associates with familial longevity: The Leiden Longevity Study. Age 2014, 36, 9629. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Irving, B.A.; Short, K.R.; O’Brien, P.; Nair, K.S. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 2010, 59, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Howald, H.; Hoppeler, H.; Claassen, H.; Mathieu, O.; Straub, R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Arch. 1985, 403, 369–376. [Google Scholar] [CrossRef]
- Howald, H.; Boesch, C.; Kreis, R.; Matter, S.; Billeter, R.; Essen-Gustavsson, B.; Hoppeler, H. Content of intramyocellular lipids derived by electron microscopy, biochemical assays, and 1H-MR spectroscopy. J. Appl. Physiol. 2002, 92, 2264–2272. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Rennie, C.D.; Robertshaw, H.A.; Fedak-Tarnopolsky, S.N.; Devries, M.C.; Hamadeh, M.J. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1271–R1278. [Google Scholar] [CrossRef]
- Tanaka, H.; Tarumi, T.; Rittweger, J. Aging and Physiological Lessons from Master Athletes. Compr. Physiol. 2019, 10, 261–296. [Google Scholar] [CrossRef]
- Sloane, P.D.; Marzetti, E.; Landi, F.; Zimmerman, S. Understanding and Addressing Muscle Strength, Mass, and Function in Older Persons. J. Am. Med. Dir. Assoc. 2019, 20, 1–4. [Google Scholar] [CrossRef]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med. Sci. Sports Exerc. 2008, 40, 648–654. [Google Scholar] [CrossRef]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.M.; Reaburn, P.R.; Cox, G.R.; Jenkins, D.G. Comparison of Postexercise Nutrition Knowledge and Postexercise Carbohydrate and Protein Intake Between Australian Masters and Younger Triathletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29 (Suppl. 1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Mitmesser, S.H. Potential Impact of Nutrition on Immune System Recovery from Heavy Exertion: A Metabolomics Perspective. Nutrients 2017, 9, 513. [Google Scholar] [CrossRef]
- Tipton, K.D. Efficacy and consequences of very-high-protein diets for athletes and exercisers. Proc. Nutr Soc. 2011, 70, 205–214. [Google Scholar] [CrossRef]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- Burd, N.A.; Beals, J.W.; Martinez, I.G.; Salvador, A.F.; Skinner, S.K. Food-First Approach to Enhance the Regulation of Post-exercise Skeletal Muscle Protein Synthesis and Remodeling. Sports Med. 2019, 49, 59–68. [Google Scholar] [CrossRef]
- Holwerda, A.M.; Paulussen, K.J.M.; Overkamp, M.; Goessens, J.P.B.; Kramer, I.F.; Wodzig, W.K.W.H.; Verdijk, L.B.; van Loon, L.J.C. Dose-Dependent Increases in Whole-Body Net Protein Balance and Dietary Protein-Derived Amino Acid Incorporation into Myofibrillar Protein During Recovery from Resistance Exercise in Older Men. J. Nutr. 2019, 149, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Szwiega, S.; Pencharz, P.B.; Rafii, M.; Lebarron, M.; Chang, J.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. Dietary leucine requirement of older men and women is higher than current recommendations. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S.M. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Deane, C.S.; Bass, J.J.; Crossland, H.; Phillips, B.E.; Atherton, P.J. Animal, Plant, Collagen and Blended Dietary Proteins: Effects on Musculoskeletal Outcomes. Nutrients 2020, 12, 2670. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.; Burd, N.A.; Van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Villareal, D.T.; Frimel, T.N.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65-80 year old men and women. PLoS ONE 2008, 3, e1875. [Google Scholar] [CrossRef]
- Doering, T.M.; Jenkins, D.G.; Reaburn, P.R.; Borges, N.R.; Hohmann, E.; Phillips, S.M. Lower Integrated Muscle Protein Synthesis in Masters Compared with Younger Athletes. Med. Sci. Sports Exerc. 2016, 48, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- McKendry, J.; Shad, B.J.; Smeuninx, B.; Oikawa, S.Y.; Wallis, G.; Greig, C.; Phillips, S.M.; Breen, L. Comparable Rates of Integrated Myofibrillar Protein Synthesis Between Endurance-Trained Master Athletes and Untrained Older Individuals. Front. Physiol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- McKendry, J.; Joanisse, S.; Baig, S.; Liu, B.; Parise, G.; Greig, C.A.; Breen, L. Superior Aerobic Capacity and Indices of Skeletal Muscle Morphology in Chronically Trained Master Endurance Athletes Compared With Untrained Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, F.G.; Situlin, R.; Fiotti, N.; Tence, M.; De Colle, P.; Mearelli, F.; Minetto, M.A.; Ghigo, E.; Pagani, M.; Lucini, D.; et al. Higher protein intake is associated with improved muscle strength in elite senior athletes. Nutrition 2017, 42, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Dolan, E.; Elliott-Sale, K.J.; Sale, C. Reduced energy availability: Implications for bone health in physically active populations. Eur. J. Nutr. 2018, 57, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Ihle, R.; Loucks, A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Min. Res. 2004, 19, 1231–1240. [Google Scholar] [CrossRef]
- Hammond, K.M.; Sale, C.; Fraser, W.; Tang, J.; Shepherd, S.O.; Strauss, J.A.; Close, G.L.; Cocks, M.; Louis, J.; Pugh, J.; et al. Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: Implications for training adaptation. J. Physiol. 2019, 597, 4779–4796. [Google Scholar] [CrossRef]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; Kenny, A.M.; Insogna, K.L. Dietary protein and skeletal health: A review of recent human research. Curr. Opin. Lipidol. 2011, 22, 16–20. [Google Scholar] [CrossRef]
- Holm, L.; Olesen, J.L.; Matsumoto, K.; Doi, T.; Mizuno, M.; Alsted, T.J.; Mackey, A.L.; Schwarz, P.; Kjaer, M. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J. Appl. Physiol. 2008, 105, 274–281. [Google Scholar] [CrossRef]
- Bowen, J.; Noakes, M.; Clifton, P.M. A high dairy protein, high-calcium diet minimizes bone turnover in overweight adults during weight loss. J. Nutr. 2004, 134, 568–573. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Mølgaard, C.; Iuliano, S.; Rizzoli, R.; Manios, Y.; Van Loon, L.J.C.; Lecerf, J.M.; Moschonis, G.; Reginster, J.Y.; Givens, I.; et al. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporos Int. 2020, 31, 601–615. [Google Scholar] [CrossRef]
- Frassetto, L.; Morris, R.C.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging—The pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef]
- New, S.A.; Robins, S.P.; Campbell, M.K.; Martin, J.C.; Garton, M.J.; Bolton-Smith, C.; Grubb, D.A.; Lee, S.J.; Reid, D.M. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am. J. Clin. Nutr. 2000, 71, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Russell, J.; Cobley, J.N.; Owens, D.J.; Wilson, G.; Gregson, W.; Fraser, W.D.; Morton, J.P. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef]
- Ruohola, J.P.; Laaksi, I.; Ylikomi, T.; Haataja, R.; Mattila, V.M.; Sahi, T.; Tuohimaa, P.; Pihlajamäki, H. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J. Bone Min. Res. 2006, 21, 1483–1488. [Google Scholar] [CrossRef]
- Lappe, J.; Cullen, D.; Haynatzki, G.; Recker, R.; Ahlf, R.; Thompson, K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Min. Res. 2008, 23, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef]
- Edouard, P.; Branco, P.; Alonso, J.M. Muscle injury is the principal injury type and hamstring muscle injury is the first injury diagnosis during top-level international athletics championships between 2007 and 2015. Br. J. Sports Med. 2016, 50, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Jennings, F.; Beaulieu, C.; Matheson, G.O. Stress fractures in athletes. Top. Magn. Reson. Imaging 2006, 17, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Soligard, T.; Schwellnus, M.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 1) International Olympic Committee consensus statement on load in sport and risk of injury. Br. J. Sports Med. 2016, 50, 1030–1041. [Google Scholar] [CrossRef]
- Ganse, B.; Degens, H.; Drey, M.; Korhonen, M.T.; McPhee, J.; Müller, K.; Johannes, B.W.; Rittweger, J. Impact of age, performance and athletic event on injury rates in master athletics-first results from an ongoing prospective study. J. Musculoskelet Neuronal. Interact. 2014, 14, 148–154. [Google Scholar]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.; Dolmans, J.; van Loon, L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef]
- LeBlanc, A.D.; Schneider, V.S.; Evans, H.J.; Pientok, C.; Rowe, R.; Spector, E. Regional changes in muscle mass following 17 weeks of bed rest. J. Appl. Physiol. 1992, 73, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Stephens, F.B.; Senden, J.M.; Verscheijden, M.L.; van Loon, L.J. Short-term muscle disuse atrophy is not associated with increased intramuscular lipid deposition or a decline in the maximal activity of key mitochondrial enzymes in young and older males. Exp. Gerontol. 2015, 61, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Bowden Davies, K.A.; Pickles, S.; Sprung, V.S.; Kemp, G.J.; Alam, U.; Moore, D.R.; Tahrani, A.A.; Cuthbertson, D.J. Reduced physical activity in young and older adults: Metabolic and musculoskeletal implications. Adv. Endocrinol. Metab. 2019, 10. [Google Scholar] [CrossRef]
- Demling, R.H. Nutrition, anabolism, and the wound healing process: An overview. Eplasty 2009, 9, e9. [Google Scholar] [PubMed]
- Close, G.L.; Sale, C.; Baar, K.; Bermon, S. Nutrition for the Prevention and Treatment of Injuries in Track and Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 189–197. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Rehabilitation Nutrition for Injury Recovery of Athletes: The Role of Macronutrient Intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Hirsch, K.R.; Saylor, H.E.; Gould, L.M.; Blue, M.N.M. Nutritional Considerations and Strategies to Facilitate Injury Recovery and Rehabilitation. J. Athl. Train. 2020, 55, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sports Med. 2015, 45 (Suppl. 1), S93–S104. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Van Loon, L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef]
- Wall, B.T.; Morton, J.P.; van Loon, L.J. Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. Eur. J. Sport Sci. 2015, 15, 53–62. [Google Scholar] [CrossRef]
- Trommelen, J.; Van Loon, L.J. Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training. Nutrients 2016, 8, 763. [Google Scholar] [CrossRef]
- Weyh, C.; Krüger, K.; Strasser, B. Physical Activity and Diet Shape the Immune System during Aging. Nutrients 2020, 12, 622. [Google Scholar] [CrossRef]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Rama, L.; Chupel, M.U.; Rosado, F.; Dos Santos, J.V.; Simpson, R.; Martinho, A.; Paiva, A.; Teixeira, A.M. Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc. Immunol. Rev. 2018, 24, 72–84. [Google Scholar]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Sha, W. Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sports Med. 2011, 45, 987–992. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Passos, B.N.; Lima, M.C.; Sierra, A.P.R.; Oliveira, R.A.; Maciel, J.F.S.; Manoel, R.; Rogante, J.I.; Pesquero, J.B.; Cury-Boaventura, M.F. Association of Daily Dietary Intake and Inflammation Induced by Marathon Race. Mediat. Inflamm. 2019, 2019, 1537274. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gross, S.J.; Jenkins, D.P.; Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Dumke, C.L.; Utter, A.C.; McAnulty, S.R.; et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007, 39, 1561–1569. [Google Scholar] [CrossRef]
- Witard, O.C.; Turner, J.E.; Jackman, S.R.; Kies, A.K.; Jeukendrup, A.E.; Bosch, J.A.; Tipton, K.D. High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists. Brain Behav. Immun. 2014, 39, 211–219. [Google Scholar] [CrossRef]
- Walsh, N.P. Recommendations to maintain immune health in athletes. Eur. J. Sport Sci. 2018, 18, 820–831. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of vitamin D inadequacy in athletes: A systematic-review and meta-analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef] [PubMed]
- He, C.S.; Handzlik, M.; Fraser, W.D.; Muhamad, A.; Preston, H.; Richardson, A.; Gleeson, M. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 2013, 19, 86–101. [Google Scholar] [PubMed]
- He, C.S.; Bishop, N.C.; Handzlik, M.K.; Muhamad, A.S.; Gleeson, M. Sex differences in upper respiratory symptoms prevalence and oral-respiratory mucosal immunity in endurance athletes. Exerc. Immunol. Rev. 2014, 20, 8–22. [Google Scholar] [PubMed]
- He, C.S.; Aw Yong, X.H.; Walsh, N.P.; Gleeson, M. Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 2016, 22, 42–64. [Google Scholar] [PubMed]
- Roubenoff, R. Catabolism of aging: Is it an inflammatory process? Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 295–299. [Google Scholar] [CrossRef]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar]
- Logan, V.F.; Gray, A.R.; Peddie, M.C.; Harper, M.J.; Houghton, L.A. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br. J. Nutr. 2013, 109, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Bezirtzoglou, E. Human microbiota in aging and infection: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 537–545. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Cohrs, J.; Salmonson, C.; Fryer, J.D.; Nehra, V.; Hale, V.L.; Kashyap, P.; White, B.A.; Woods, J.A. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes 2018, 9, 115–130. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, S.; Du, G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiologyopen 2020, 9, e1053. [Google Scholar] [CrossRef]
- Fart, F.; Rajan, S.K.; Wall, R.; Rangel, I.; Ganda-Mall, J.P.; Tingö, L.; Brummer, R.J.; Repsilber, D.; Schoultz, I.; Lindqvist, C.M. Differences in Gut Microbiome Composition between Senior Orienteering Athletes and Community-Dwelling Older Adults. Nutrients 2020, 12, 2610. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Tana, C.; Nouvenne, A.; Ridolo, E.; Meschi, T. Exercise and immune system as modulators of intestinal microbiome: Implications for the gut-muscle axis hypothesis. Exerc. Immunol. Rev. 2019, 25, 84–95. [Google Scholar] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic Supplements Beneficially Affect Tryptophan-Kynurenine Metabolism and Reduce the Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef]
- Pyne, D.B.; West, N.P.; Cox, A.J.; Cripps, A.W. Probiotics supplementation for athletes-clinical and physiological effects. Eur. J. Sport Sci. 2015, 15, 63–72. [Google Scholar] [CrossRef]
- Colbey, C.; Cox, A.J.; Pyne, D.B.; Zhang, P.; Cripps, A.W.; West, N.P. Upper Respiratory Symptoms, Gut Health and Mucosal Immunity in Athletes. Sports Med. 2018, 48, 65–77. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Hopkins, W.G.; Eskesen, D.C.; Jairath, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011, 10, 30. [Google Scholar] [CrossRef]
- Moro-García, M.A.; Alonso-Arias, R.; Baltadjieva, M.; Fernández Benítez, C.; Fernández Barrial, M.A.; Díaz Ruisánchez, E.; Alonso Santos, R.; Alvarez Sánchez, M.; Saavedra Miján, J.; López-Larrea, C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 2013, 35, 1311–1326. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Guillemard, E.; Tanguy, J.; Flavigny, A.; de la Motte, S.; Schrezenmeir, J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J. Am. Coll. Nutr. 2010, 29, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasser, B.; Pesta, D.; Rittweger, J.; Burtscher, J.; Burtscher, M. Nutrition for Older Athletes: Focus on Sex-Differences. Nutrients 2021, 13, 1409. https://doi.org/10.3390/nu13051409
Strasser B, Pesta D, Rittweger J, Burtscher J, Burtscher M. Nutrition for Older Athletes: Focus on Sex-Differences. Nutrients. 2021; 13(5):1409. https://doi.org/10.3390/nu13051409
Chicago/Turabian StyleStrasser, Barbara, Dominik Pesta, Jörn Rittweger, Johannes Burtscher, and Martin Burtscher. 2021. "Nutrition for Older Athletes: Focus on Sex-Differences" Nutrients 13, no. 5: 1409. https://doi.org/10.3390/nu13051409
APA StyleStrasser, B., Pesta, D., Rittweger, J., Burtscher, J., & Burtscher, M. (2021). Nutrition for Older Athletes: Focus on Sex-Differences. Nutrients, 13(5), 1409. https://doi.org/10.3390/nu13051409







