Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study
Abstract
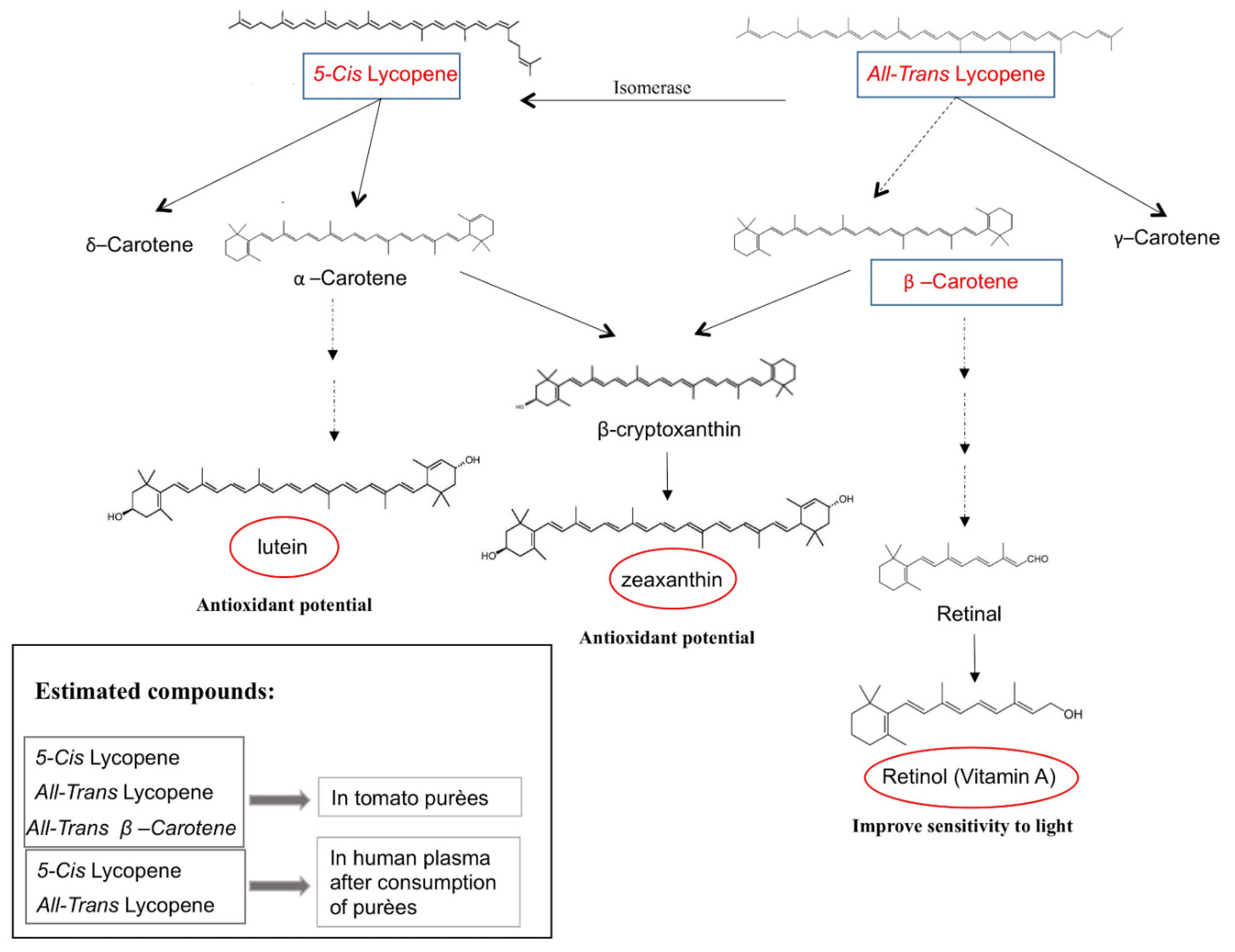
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Nutritional Characteristics of Tomato Purées
2.3. Test Meal Preparation and Composition
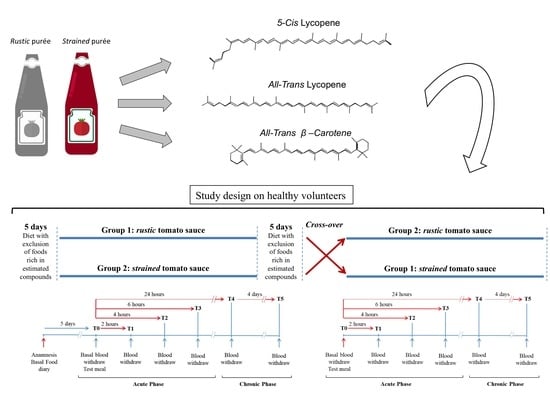
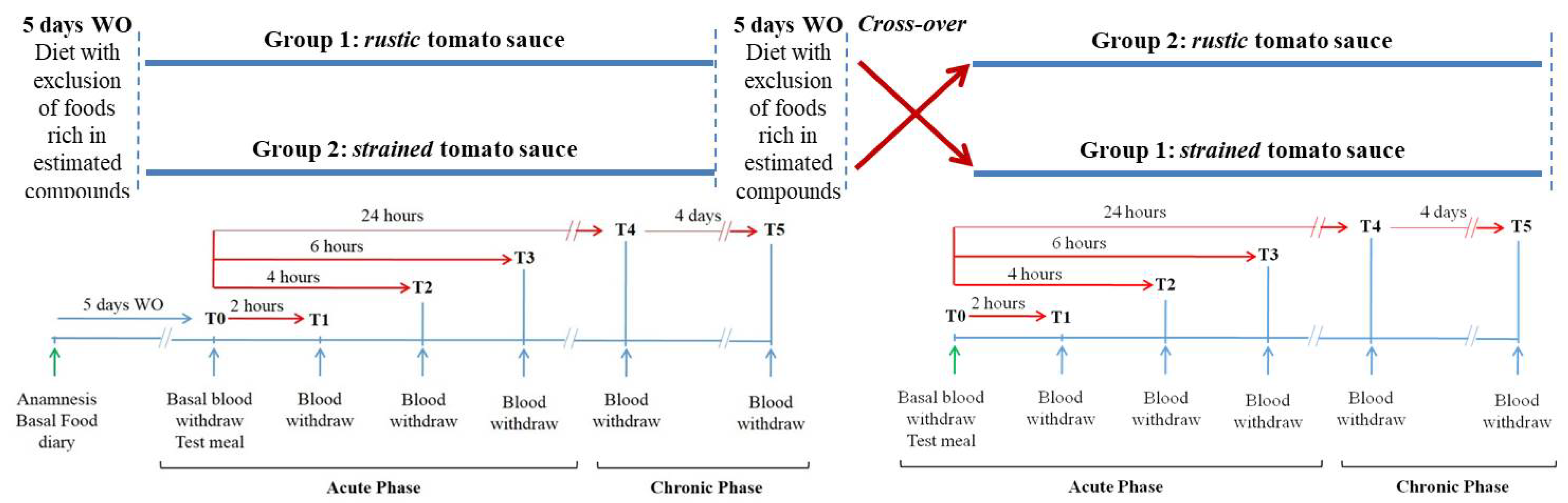
2.4. Experimental Design
2.5. Blood Sampling and Analysis
2.6. Carotenoid and Lycopene Extraction and Estimation
2.7. HPLC Analysis
2.8. Biological Antioxidant Potential Test
2.9. Statistical Analysis
3. Results
3.1. Tomato Samples Treatment and Carotenoids Estimation
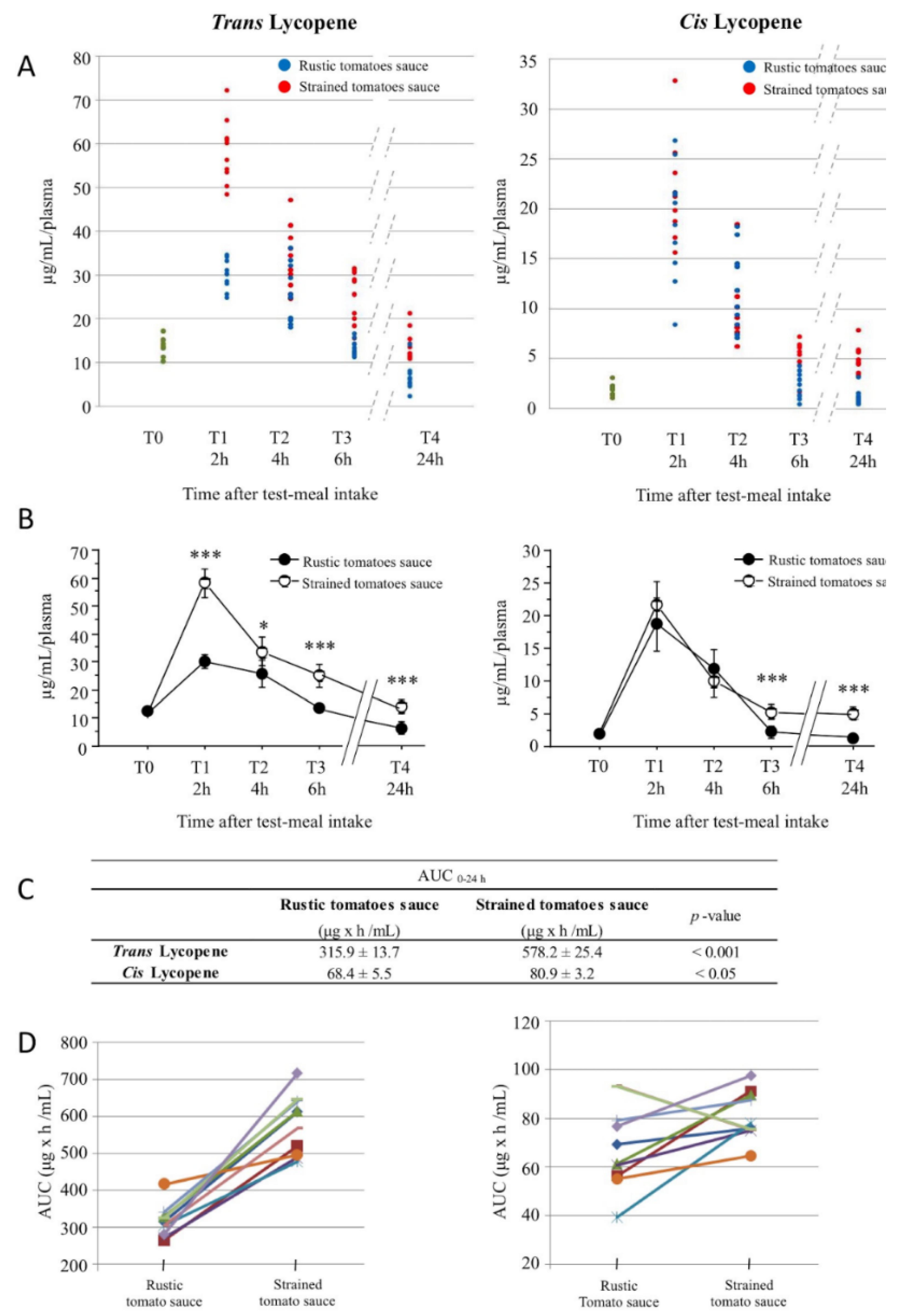
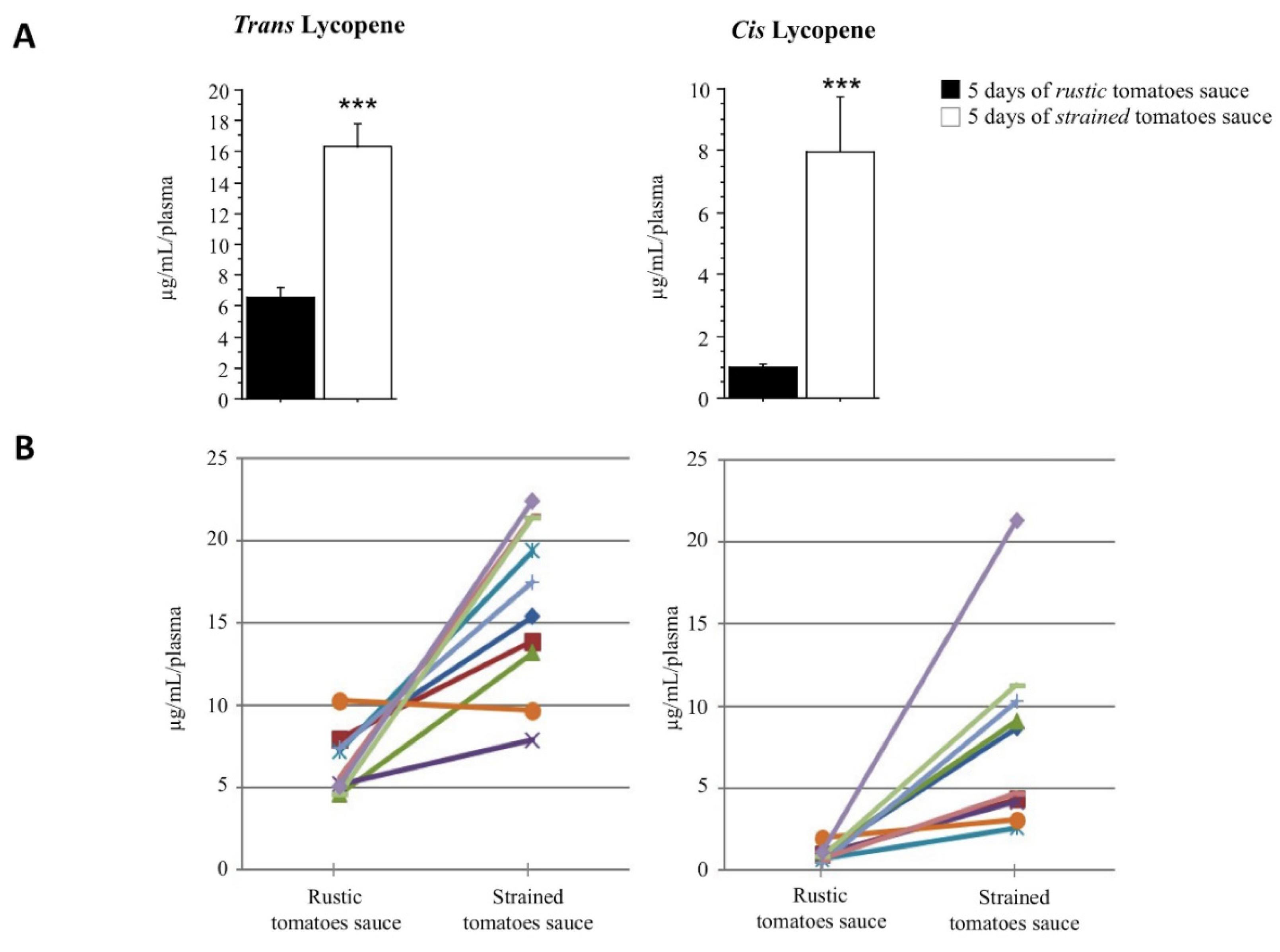
3.2. Lycopene Plasma Content after Tomato Meal Consumption
3.3. Blood Antioxidant Potential Measured after Tomato Meal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Li, T.; Liu, C.; Chen, J.; Zhang, R.; Zhang, Z.; Dai, T.; McClements, D.J. Potential physicochemical basis of Mediterranean diet effect: Ability of emulsified olive oil to increase carotenoid bioaccessibility in raw and cooked tomatoes. Food Res. Int. 2016, 89, 320–329. [Google Scholar] [CrossRef]
- Tommonaro, G.; Speranza, G.; De Prisco, R.; Iodice, C.; Crudele, E.; Abbamondi, G.R.; Nicolaus, B. Antioxidant activity and bioactive compound contents before and after in vitro digestion of new tomato hybrids. J. Sci. Food Agric. 2017, 97, 5241–5246. [Google Scholar] [CrossRef]
- Hidalgo-Mora, J.J.; García-Vigara, A.; Sánchez-Sánchez, M.L.; García-Pérez, M.Á.; Tarín, J.; Cano, A. The Mediterranean diet: A historical perspective on food for health. Maturitas 2020, 132, 65–69. [Google Scholar] [CrossRef]
- Capurso, A.; Crepaldi, G.; Capurso, C. The Mediterranean diet: A pathway to successful aging. Aging Clin. Exp. Res. 2020, 32, 1187–1188. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Sun, D.W. Measurements of lycopene contents in fruit: A review of recent developments in conventional and novel techniques. Crit. Rev. Food Sci. Nutr. 2019, 59, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Dumont, U.; Halimi, C.; Lairon, D.; Page, D.; Sébédio, J.L.; Buisson, C.; Buffière, C.; Rémond, D. Dietary calcium impairs tomato lycopene bioavailability in healthy humans. Br. J. Nutr. 2016, 116, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Egydio, J.A.; Moraes, Â.M.; Rosa, P.T.V. Supercritical fluid extraction of lycopene from tomato juice and characterization of its antioxidation activity. J. Supercrit. Fluids 2010, 54, 159–164. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Del Giudice, R.; Petruk, G.; Raiola, A.; Barone, A.; Monti, D.M.; Rigano, M.M. Carotenoids in fresh and processed tomato (Solanum lycopersicum) fruits protect cells from oxidative stress injury. J. Sci. Food Agric. 2017, 97, 1616–1623. [Google Scholar] [CrossRef]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: Effect of operating conditions and solubility analysis. J. Agric. Food Chem. 2006, 54, 5604–5610. [Google Scholar] [CrossRef]
- Bacanli, M.; Başaran, N.; Başaran, A.A. Lycopene: Is it beneficial to human health as an antioxidant? | Likopen: Antioksidan olarak ınsan sağliğina faydali mi? Turk. J. Pharm. Sci. 2017, 14, 311–318. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Effects of Carotenoids on Health: Are All the Same? Results from Clinical Trials. Curr. Pharm. Des. 2017, 23, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases—A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Casas, R.; Estruch, R.; Sacanella, E. Influence of bioactive nutrients on the atherosclerotic process: A review. Nutrients 2018, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Inverse association between serum antioxidant levels and inflammatory markers is moderated by adiposity: A report based on a large representative population sample of American adults. Br. J. Nutr. 2018, 120, 1272–1278. [Google Scholar] [CrossRef]
- Rowles, J.L.; Erdman, J.W. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jacobs, E.J.; Newton, C.C.; McCullough, M.L. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Int. J. Cancer 2016, 138, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Prod. Bioprospect. 2020, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Stice, C.P.; Xia, H.; Wang, X.-D. Tomato lycopene prevention of alcoholic fatty liver disease and hepatocellular carcinoma development. Chronic Dis. Transl. Med. 2018, 4, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef]
- Ardawi, M.S.M.; Badawoud, M.H.; Hassan, S.M.; Rouzi, A.A.; Ardawi, J.M.S.; AlNosani, N.M.; Qari, M.H.; Mousa, S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone 2016, 83, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Arokiasamy, J.T. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef]
- Han, G.M.; Liu, P. Higher serum lycopene is associated with reduced prevalence of hypertension in overweight or obese adults. Eur. J. Integr. Med. 2017, 13, 34–40. [Google Scholar] [CrossRef]
- Moran, N.E.; Cichon, M.J.; Riedl, K.M.; Grainger, E.M.; Schwartz, S.J.; Novotny, J.A.; Erdman, J.W.; Clinton, S.K. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am. J. Clin. Nutr. 2015, 102, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Victoria-Campos, C.I.; Ornelas-Paz, J.D.J. Absorption of carotenoids and mechanisms involved in their health-related properties. Subcell. Biochem. 2016, 79, 415–454. [Google Scholar] [CrossRef]
- Yu, J.; Gleize, B.; Zhang, L.; Caris-Veyrat, C.; Renard, C.M.G.C. A D-optimal mixture design of tomato-based sauce formulations: Effects of onion and EVOO on lycopene isomerization and bioaccessibility. Food Funct. 2019, 10, 3589–3602. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Carle, R.; Schweiggert, R. Bioaccessibility of carotenoids from plant and animal foods. J. Sci. Food Agric. 2019, 99, 3220–3239. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Martin-Belloso, O.; Julian McClements, D. Excipient nanoemulsions for improving oral bioavailability of bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef]
- Bohn, T. Metabolic Fate of Bioaccessible and Non-bioaccessible Carotenoids. In Food Chemistry, Function and Analysis; RSC Publishing: Cambridge, UK, 2018; ISBN 9781782627081. [Google Scholar]
- Schierle, J.; Bretzel, W.; Bühler, I.; Faccin, N.; Hess, D.; Steiner, K.; Schüep, W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997, 59, 459–465. [Google Scholar] [CrossRef]
- Arranz, S.; Martínez-Huélamo, M.; Vallverdu-Queralt, A.; Valderas-Martinez, P.; Illán, M.; Sacanella, E.; Escribano, E.; Estruch, R.; Lamuela-Raventos, R.M.A. Influence of olive oil on carotenoid absorption from tomato juice and effects on postprandial lipemia. Food Chem. 2015, 168, 203–210. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Prest, M.A.; Parrott, J.S.; Byham-Gray, L. Test-Retest Reliability and Validity of the Nutrition-Specific Quality of Life Questionnaire. J. Ren. Nutr. 2020, 30, 145–153. [Google Scholar] [CrossRef]
- Borel, P.; Tyssandier, V.; Mekki, N.; Grolier, P.; Rochette, Y.; Alexandre-Gouabau, M.C.; Lairon, D.; Azaïs-Braesco, V. Chylomicron β-carotene and retinyl palmitate responses are dramatically diminished when men ingest β-carotene with medium-chain rather than long-chain triglycerides. J. Nutr. 1998, 128, 1361–1367. [Google Scholar] [CrossRef]
- Kajaia, T.; Maskhulia, L.; Chelidze, K.; Akhalkatsi, V.; Mchedlidze, T. Implication of relationship between oxidative stress and antioxidant status in blood serum. Georgian Med. News 2018, 284, 71–76. [Google Scholar]
- Misra, S.; Wahab, M.F.; Patel, D.C.; Armstrong, D.W. The utility of statistical moments in chromatography using trapezoidal and Simpson’s rules of peak integration. J. Sep. Sci. 2019, 42, 1644–1657. [Google Scholar] [CrossRef]
- Gibson, R.S. The role of diet- and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Food Nutr. Bull. 2007, 28, 77–100. [Google Scholar] [CrossRef]
- Melse-Boonstra, A. Bioavailability of Micronutrients From Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Ferreira, A.L.A.; Corrêa, C.R. Lycopene Bioavailability and Its Effects on Health. In Food Quality, Safety and Technology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 63–76. [Google Scholar]
- Santos, C.S.P.; Molina-Garcia, L.; Cunha, S.C.; Casal, S. Fried potatoes: Impact of prolonged frying in monounsaturated oils. Food Chem. 2018, 243, 192–201. [Google Scholar] [CrossRef]
- Zhubi-Bakija, F.; Bajraktari, G.; Bytyçi, I.; Mikhailidis, D.P.; Henein, M.Y.; Latkovskis, G.; Rexhaj, Z.; Zhubi, E.; Banach, M.; Alnouri, F.; et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP). Clin. Nutr. 2020, 40, 255–276. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef] [PubMed]
- Redfern, C.P.F. Vitamin A and its natural derivatives. Methods Enzymol. 2020, 637, 1–25. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]

| Trans-Lycopene (µg/g) | Cis-Lycopene (µg/g) | β-Carotene (µg/g) | ||||
|---|---|---|---|---|---|---|
| Shape | Rustic Tomatoes | Strained Tomatoes | Rustic Tomatoes | Strained Tomatoes | Rustic Tomatoes | Strained Tomatoes |
| Puree | 249.6 ± 28.4 | 308.7 ± 27.3 | 16.6 ± 1.5 | 36.3 ± 2.4 | 7.6 ± 0.6 | 8.3 ± 1.1 |
| Boiled puree | 336.6 ± 28.6 | 324.8 ± 24.6 | 34.6 ± 2.0 | 40.3 ± 3.2 | 9.4 ± 0.9 | 10.5 ± 0.5 |
| Sauce | 321.8 ± 23.5 | 501.6 ± 14.5 | 32.2 ± 3.5 | 45.3 ± 3.2 | 14.1 ± 1.5 | 30.2 ± 2.8 |
| Trans-Lycopene (µg/g) | Cis-Lycopene (µg/g) | β-Carotene (µg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooking Time (min) | Rustic Tomatoes | Strained Tomatoes | Rustic Tomatoes | Strained Tomatoes | Rustic Tomatoes | Strained Tomatoes | ||||||
| After Boiling | − evo | + evo | − evo | + evo | − evo | + evo | − evo | + evo | − evo | + evo | − evo | + evo |
| 10 | 250.4 ± 15.9 | 316.6 ± 13.2 | 319.0 ± 22.0 | 468.8 ± 22.3 | 24.1 ± 1.1 | 28.4 ± 2.8 | 18.3 ± 0.5 | 32.2 ± 0.7 | 9.0 ± 1.0 | 12.4 ± 0.6 | 8.7 ± 0.3 | 20.4 ± 1.7 |
| 20 | 273.7 ± 16.5 | 324.5 ± 50.5 | 339.0 ± 29.2 | 489.6 ± 55.6 | 27.2 ± 2.0 | 30.1 ± 2.0 | 21.0 ± 1.8 | 35.8 ± 2.9 | 10.7 ± 0.8 | 15.4 ± 1.2 | 9.2 ± 0.3 | 25.0 ± 2.3 |
| 30 | 301.5 ± 38.6 | 330.7 ± 35.4 | 364.6 ± 29.1 | 525.7 ± 34.5 | 30.4 ± 0.9 | 34.8 ± 2.5 | 27.2 ± 2.4 | 40.6 ± 1.9 | 13.6 ± 1.4 | 18.4 ± 2.5 | 10.1 ± 0.6 | 34.9 ± 2.4 |
| 40 | 319.2 ± 13.4 | 343.3 ±27.7 | 410.3 ± 32.9 | 547.2 ± 51.6 | 36.6 ± 3.4 | 38.1 ± 4.4 | 32.6 ± 4.2 | 49.5 ± 4.6 | 15.0 ± 1.0 | 25.7 ± 1.1 | 12.4 ± 0.9 | 39.9 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitucci, D.; Amoresano, A.; Nunziato, M.; Muoio, S.; Alfieri, A.; Oriani, G.; Scalfi, L.; Frusciante, L.; Rigano, M.M.; Pucci, P.; et al. Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study. Nutrients 2021, 13, 1336. https://doi.org/10.3390/nu13041336
Vitucci D, Amoresano A, Nunziato M, Muoio S, Alfieri A, Oriani G, Scalfi L, Frusciante L, Rigano MM, Pucci P, et al. Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study. Nutrients. 2021; 13(4):1336. https://doi.org/10.3390/nu13041336
Chicago/Turabian StyleVitucci, Daniela, Angela Amoresano, Marcella Nunziato, Simona Muoio, Andreina Alfieri, Giovannangelo Oriani, Luca Scalfi, Luigi Frusciante, Maria Manuela Rigano, Piero Pucci, and et al. 2021. "Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study" Nutrients 13, no. 4: 1336. https://doi.org/10.3390/nu13041336
APA StyleVitucci, D., Amoresano, A., Nunziato, M., Muoio, S., Alfieri, A., Oriani, G., Scalfi, L., Frusciante, L., Rigano, M. M., Pucci, P., Fontana, L., Buono, P., & Salvatore, F. (2021). Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study. Nutrients, 13(4), 1336. https://doi.org/10.3390/nu13041336