Dietary Annatto-Extracted Tocotrienol Reduces Inflammation and Oxidative Stress, and Improves Macronutrient Metabolism in Obese Mice: A Metabolic Profiling Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sample Collection
2.3. Metabolomics Analyses of Serum
2.4. Statistical Analyses
3. Results
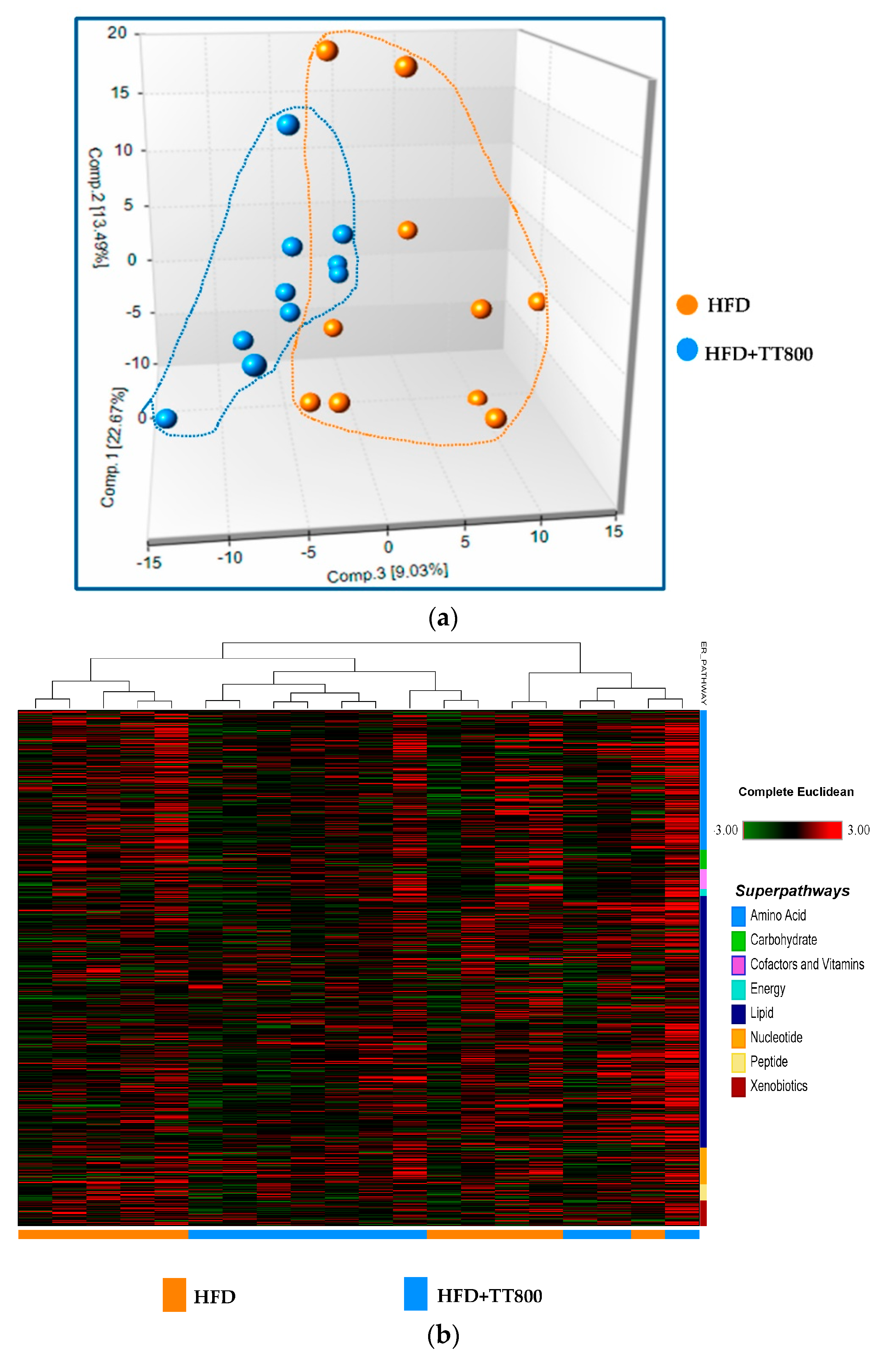
3.1. Metabolite Summary and Significantly Altered Biochemicals
3.2. Effects of TT on Lipid Metabolism
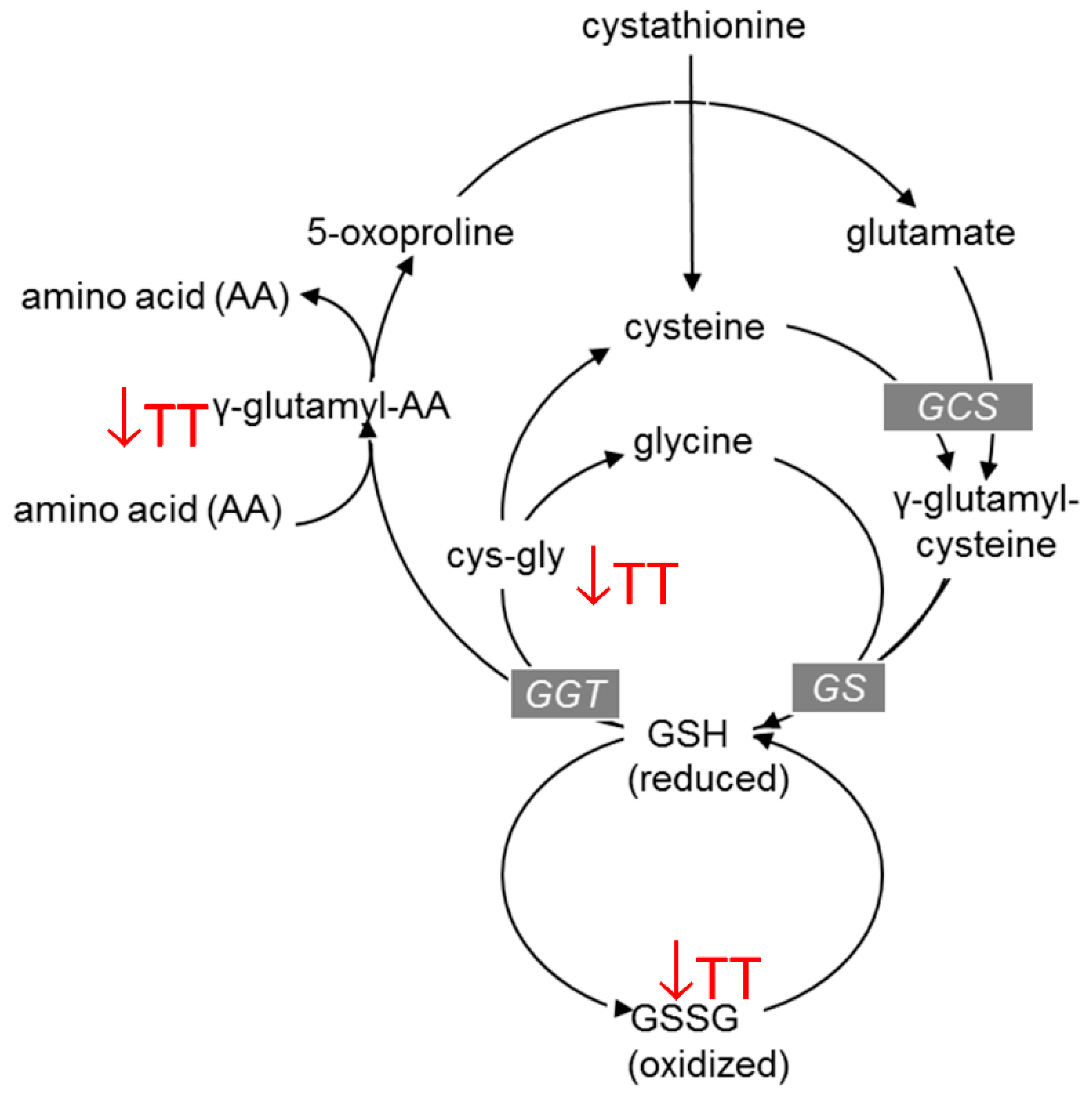
3.3. Effects of TT on Amino Acid Metabolism
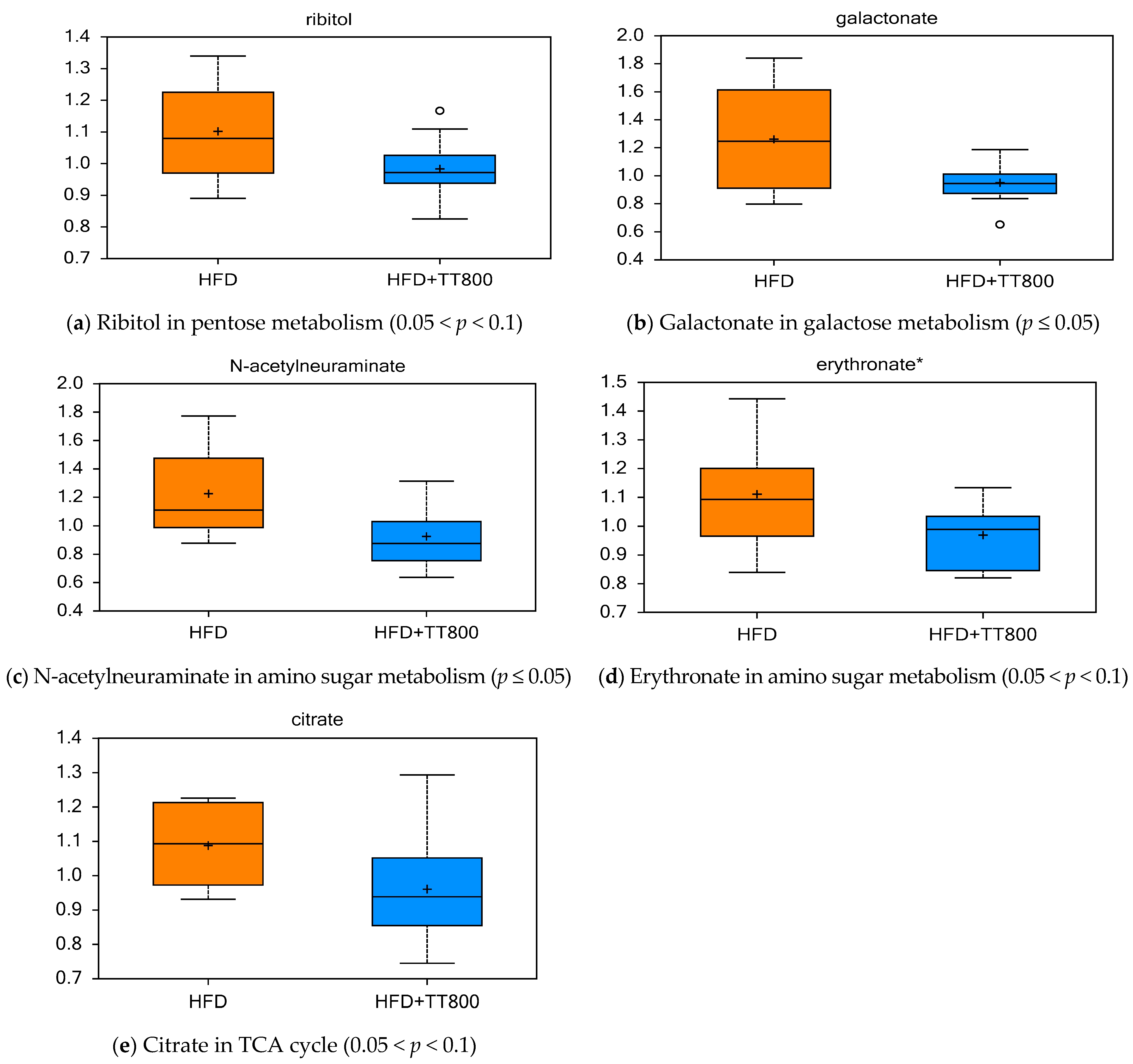
3.4. Effects of TT on Carbohydrate Metabolism and Energy Cycle
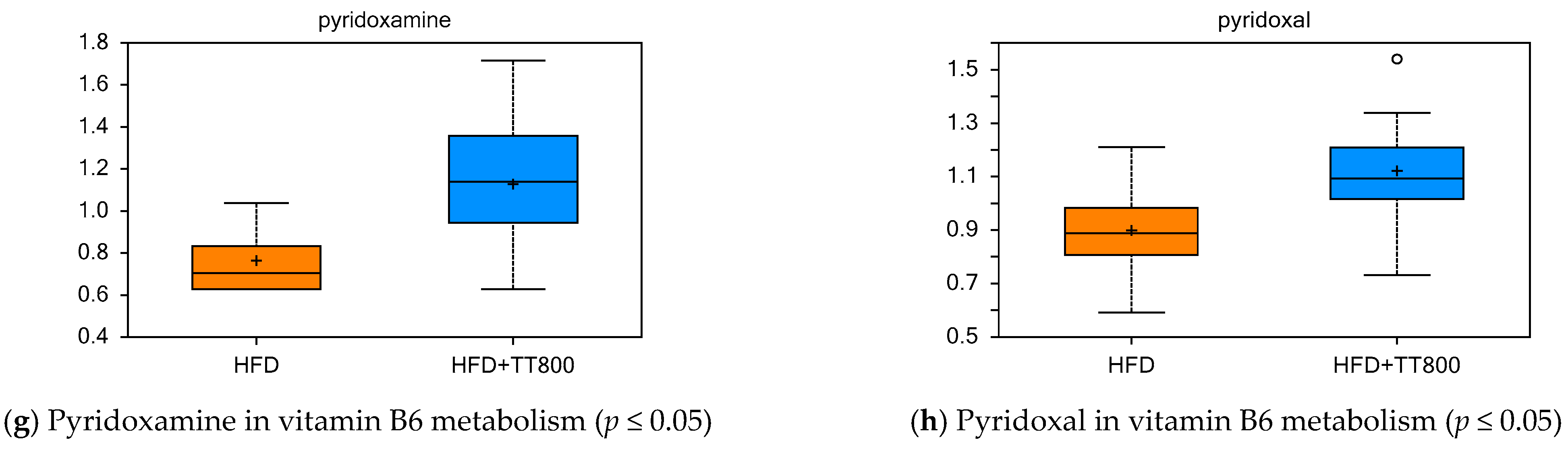
3.5. Effect of TT on Cofactors and Vitamin Metabolism
3.6. Effect of TT on Nucleotide Metabolism
3.7. Effect of TT on Xenobiotics Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalupahana, N.S.; Moustaid-Moussa, N.; Claycombe, K.J. Immunity as a link between obesity and insulin resistance. Mol. Asp. Med. 2012, 33, 26–34. [Google Scholar] [CrossRef]
- Malagón, M.M.; Díaz-Ruiz, A.; Guzmán-Ruiz, R.; Jiménez-Gómez, Y.; Moreno, N.R.; García-Navarro, S.; Vázquez-Martínez, R.; Peinado, J.R. Adipobiology for novel therapeutic approaches in metabolic syndrome. Curr. Vasc. Pharmacol. 2014, 11, 954–967. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–18930. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrition 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Henriksen, E.J. Dysregulation of glycogen synthase kinase-3 in skeletal muscle and the etiology of insulin resistance and type 2 diabetes. Curr. Diabetes Rev. 2010, 6, 285–293. [Google Scholar] [CrossRef]
- Pazdro, R.; Burgess, J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010, 131, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef]
- Shen, C.L.; Kaur, G.; Wanders, D.; Sharma, S.; Tomison, M.D.; Ramalingam, L.; Chung, E.; Moustaid-Moussa, N.; Mo, H.; Dufour, J.M. Annatto-extracted tocotrienols improve glucose homeostasis and bone properties in high-fat diet-induced type 2 diabetic mice by decreasing the inflammatory response. Sci. Rep. 2018, 8, 11377. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Viola, V.; Pilolli, F.; Piroddi, M.; Pierpaoli, E.; Orlando, F.; Provinciali, M.; Betti, M.; Mazzini, F.; Galli, F. Why tocotrienols work better: Insights into the in vitro anti-cancer mechanism of vitamin E. Genes Nutr. 2011, 7, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, A.W. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fang, X.; Marshall, M.R.; Chung, S. Regulation of Obesity and Metabolic Complications by Gamma and Delta Tocotrienols. Molecules 2016, 21, 344. [Google Scholar] [CrossRef]
- Chung, E.; Elmassry, M.M.; Kottapalli, P.; Kottapalli, K.R.; Kaur, G.; Dufour, J.M.; Wright, K.; Ramalingam, L.; Moustaid-Moussa, N.; Wang, R.; et al. Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 2020, 77, 97–107. [Google Scholar] [CrossRef]
- Prasad, K. Tocotrienols and Cardiovascular Health. Curr. Pharm. Des. 2011, 17, 2147–2154. [Google Scholar] [CrossRef]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. 2015, 39, 438–446. [Google Scholar] [CrossRef]
- Malavolta, M.; Pierpaoli, E.; Giacconi, R.; Basso, A.; Cardelli, M.; Piacenza, F.; Provinciali, M. Anti-inflammatory Activity of Tocotrienols in Age-related Pathologies: A SASPected Involvement of Cellular Senescence. Biol. Proced. Online 2018, 20, 22. [Google Scholar] [CrossRef]
- Hatano, T.; Saiki, S.; Okuzumi, A.; Mohney, R.P.; Hattori, N. Identification of novel biomarkers for Parkinson’s disease by metabolomic technologies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Evans, A.M.; Bridgewater, B.R.; Liu, Q.; Mitchell, M.W.; Robinson, R.J.; Dai, H.; Stewart, S.J.; DeHaven, C.D.; Miller, L.A.D. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014, 4, 132. [Google Scholar]
- Dehaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010, 2, 9. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Ribel-Madsen, A.; Hellgren, L.I.; Brøns, C.; Ribel-Madsen, R.; Newgard, C.B.; Vaag, A.A. Plasma amino acid levels are elevated in young, healthy low birth weight men exposed to short-term high-fat overfeeding. Physiol. Rep. 2016, 4, e13044. [Google Scholar] [CrossRef]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352. [Google Scholar] [CrossRef]
- Mitsutake, S.; Igarashi, Y. Sphingolipids in Lipid Microdomains and Obesity. Vitam. Horm. 2013, 91, 271–284. [Google Scholar]
- Boini, K.M.; Xia, M.; Koka, S.; Gehr, T.W.B.; Li, P.-L. Sphingolipids in obesity and related complications. Front. Biosci. 2017, 22, 96–116. [Google Scholar]
- Mitsutake, S.; Zama, K.; Yokota, H.; Yoshida, T.; Tanaka, M.; Mitsui, M.; Ikawa, M.; Okabe, M.; Tanaka, Y.; Yamashita, T.; et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 2011, 286, 28544–28555. [Google Scholar] [CrossRef]
- Zhao, L.; Ha, J.-H.; Okla, M.; Chung, S. Activation of autophagy and AMPK by gamma-tocotrienol suppresses the adipogenesis in human adipose derived stem cells. Mol. Nutr. Food Res. 2013, 58, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, A.R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nat. Cell Biol. 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Bordoni, L.; Samulak, J.J.; Sawicka, A.K.; Pelikant-Malecka, I.; Radulska, A.; Lewicki, L.; Kalinowski, L.; Gabbianelli, R.; Olek, R.A. Trimethylamine N-oxide and the reverse cholesterol transport in cardiovascular disease: A cross-sectional study. Sci. Rep. 2020, 10, 18675. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, T.; Huang, Z.-S.; Ying, Y.; Zhang, Y.; Wang, B.; Ye, L.; Zhang, B.; Chen, D.-L.; Chen, J. Relationship between gut microbiota and type 2 diabetic erectile dysfunction in Sprague-Dawley rats. Acta Acad. Med. Wuhan 2017, 37, 523–530. [Google Scholar] [CrossRef]
- Xu, Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim. Biophys. Acta 2002, 1582, 81–88. [Google Scholar] [CrossRef]
- Xing, F.; Liu, J.; Mo, Y.; Liu, Z.; Qin, Q.; Wang, J.; Fan, Z.; Long, Y.; Liu, N.; Zhao, K.; et al. Lysophosphatidylcholine up-regulates human endothelial nitric oxide synthase gene transactivity by c-Jun N-terminal kinase signalling pathway. J. Cell. Mol. Med. 2009, 13, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Lim, Y.-M.; Quan, W.; Kim, J.R.; Chung, K.W.; Kang, M.; Kim, S.; Park, S.Y.; Han, J.-S.; Park, S.-Y.; et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J. Lipid Res. 2011, 52, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Yea, K.; Kim, J.; Yoon, J.H.; Kwon, T.; Kim, J.H.; Lee, B.D.; Lee, H.; Lee, S.J.; Kim, J.; Lee, T.G.; et al. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J. Biol. Chem. 2009, 284, 33833–33840. [Google Scholar] [CrossRef]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma Lysophosphatidylcholine Levels Are Reduced in Obesity and Type 2 Diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.J.K. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992, 35, 3595–3606. [Google Scholar] [CrossRef]
- Mo, H.; Jeter, R.; Bachmann, A.; Yount, S.T.; Shen, C.-L.; Yeganehjoo, H. The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins. Front. Pharmacol. 2019, 9, 1515. [Google Scholar] [CrossRef]
- Song, B.L.; DeBose-Boyd, R.A. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J. Biol. Chem. 2006, 281, 25054–25061. [Google Scholar] [CrossRef]
- Zeyda, M.; Stulnig, T.M. Obesity, Inflammation, and Insulin Resistance—A Mini-Review. Gerontology 2009, 55, 379–386. [Google Scholar] [CrossRef]
- Linna, M.S.; Ahotupa, M.; Kukkonen-Harjula, K.; Fogelholm, M.; Vasankari, T.J. Co-existence of insulin resistance and high concentrations of circulating oxidized LDL lipids. Ann. Med. 2015, 47, 394–398. [Google Scholar] [CrossRef]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Meaney, M.P.; Dew, D.A.; Pappan, K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R68–R74. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef]
- Ramanathan, N.; Tan, E.; Loh, L.J.; Soh, B.S.; Yap, W.N. Tocotrienol is a cardioprotective agent against ageing-associated cardiovascular disease and its associated morbidities. Nutr. Metab. 2018, 15, 6. [Google Scholar] [CrossRef]



| Ingredient | HFD | HFD+TT800 |
|---|---|---|
| Casein, 80 Mesh | 267.1 | 267.1 |
| L-Cystine | 4 | 4 |
| Maltodextrin 10 | 166.9 | 166.9 |
| Sucrose | 91.9 | 91.9 |
| Cellulose, BW 200 | 66.8 | 66.8 |
| Soybean oil a | 33.38 | 33.38 |
| Lard | 327.2 | 327.2 |
| Mineral mix b, S10026B | 13.4 | 13.4 |
| Dicalcium phosphate | 17.4 | 17.4 |
| Calcium carbonate | 7.3 | 7.3 |
| Potassium citrate, 1 H2O | 22 | 22 |
| Vitamin mix c, V13401 (without E) | 13.4 | 13.4 |
| Vitamin E acetate (500 IU/gm) | 0.1 | 0.1 |
| Choline bitartrate | 2.7 | 2.7 |
| Tocotrienol d | 0 | 0.56 |
| Sub Pathway | Metabolite Name | HFD+TT800/HFD |
|---|---|---|
| Fatty acid branched | 13-methylmyristate | 0.80 |
| Medium chain fatty acid | heptanoate (C7:0) | 1.13 |
| pelargonate (C9:0) | 0.84 | |
| 10-undecenoate (C11:1n1) | 0.69 | |
| Fatty acid monohydroxy | 2-hydroxyoctanoate | 0.84 |
| 9-HODE + 13-HODE | 0.71 | |
| 14-HDoHE/17-HDoHE | 0.82 | |
| Fatty acid, dicarboxylate | 2-hydroxyadipate | 0.69 |
| suberate (octanedioate) | 0.81 | |
| azelate (nonanedioate) | 0.79 | |
| sebacate (decanedioate) | 0.77 | |
| Undecanedioate | 0.81 | |
| 1,11-undecanedicarboxylate | 0.75 | |
| dodecanedioate | 0.81 | |
| tetradecanedioate | 0.80 | |
| hexadecanedioate | 0.85 | |
| Octadecanedioate | 0.79 | |
| Eicosanoid | Thromboxane B2 | 0.45 |
| 12-HHTE | 0.72 | |
| 12-HHTrE | 0.47 | |
| Endocannabinoid | oleoyl ethanolamide | 1.07 |
| palmitoyl ethanolamide | 1.09 | |
| linoleoyl ethanolamide | 1.11 | |
| Fatty acid acyl glycine | Valerylglycine | 0.61 |
| Hexanoylglycine | 0.51 | |
| heptanoyl glycine | 0.53 | |
| 3,4-methylene heptanoylglycine | 0.63 | |
| N-octanoylglycine | 0.57 | |
| N-palmitoylglycine | 0.93 | |
| Fatty acid acyl carnitine | hexanoylcarnitine (C6) | 0.71 |
| decanoylcarnitine (C10) | 1.29 | |
| lignoceroylcarnitine (C24) | 1.18 | |
| Carnitine | Carnitine | 1.22 |
| Sub Pathway | Metabolite Name | HFD+TT800/HFD |
|---|---|---|
| Sphingolipid Metabolism | palmitoyl dihydrosphingomyelin (d18:0/16:0) | 1.08 |
| palmitoyl sphingomyelin (d18:1/16:0) | 0.31 | |
| behenoyl sphingomyelin (d18:1/22:0) | 1.32 | |
| sphingomyelin (d17:1/16:0, d18:1/15:0, d16:1/17:0) | 1.19 | |
| sphingomyelin (d18:2/16:0, d18:1/16:1) | 1.11 | |
| sphingomyelin (d18:1/18:1, d18:2/18:0) | 1.26 | |
| sphingomyelin (d18:1/20:1, d18:2/20:0) | 1.24 | |
| sphingomyelin (d18:1/24:1, d18:2/24:0) | 1.14 | |
| sphingomyelin (d18:2/21:0, d16:2/23:0) | 0.78 | |
| sphingomyelin (d18:2/24:2) | 1.21 | |
| sphingomyelin (d18:1/22:2, d18:2/22:1, d16:1/24:2) | 1.21 | |
| sphingomyelin (d18:2/18:1) | 1.24 | |
| sphingomyelin (d18:1/19:0, d19:1/18:0) | 0.72 | |
| Phospholipid Metabolism | glycerophosphorylcholine (GPC) | 1.25 |
| glycerophosphoinositol (GPI) | 1.20 | |
| trimethylamine N-oxide (TMAO) | 0.58 | |
| Phosphatidylcholine (PC) | 1-palmitoyl-2-palmitoleoyl-GPC (16:0/16:1) | 1.15 |
| 1-palmitoyl-2-oleoyl-GPC (16:0/18:1) | 1.12 | |
| 1,2-dilinoleoyl-GPC (18:2/18:2) | 1.24 | |
| Lysophospholipid | 1-lignoceroyl-GPC (24:0) | 1.39 |
| 1-stearoyl-GPE (18:0) | 1.17 | |
| Plasmalogen | 1-(1-enyl-palmitoyl)-2-palmitoyl-GPC (P-16:0/16:0) | 1.23 |
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2) | 1.17 | |
| Monoacylglycerol | 1-dihomo-linolenylglycerol (20:3) | 1.66 |
| Diacylglycerol | palmitoyl-linoleoyl-glycerol (16:0/18:2) | 0.40 |
| Ceramides | glycosyl ceramide (d18:1/20:0, d16:1/22:0) | 1.21 |
| Mevalonate Metabolism | Mevalonate | 0.84 |
| Primary Bile Acid Metabolism | beta-muricholate | 0.69 |
| Sub Pathway | Biochemical Name | HFD+TT800/HFD |
|---|---|---|
| Glycine, Serine, and Threonine Metabolism | N-acetylglycine | 0.75 |
| Serine | 1.13 | |
| Alanine and Aspartate Metabolism | Alanine | 1.17 |
| Glutamate Metabolism | Pyroglutamine | 1.15 |
| Histidine Metabolism | Histidine | 1.06 |
| N-acetyl-3-methylhistidine | 0.68 | |
| Imidazole propionate | 0.35 | |
| 1-Methylhistamine | 1.28 | |
| Lysine Metabolism | N2-acetyllysine | 0.76 |
| N2,N6-diacetyllysine | 0.83 | |
| N6,N6,N6-trimethyllysine | 0.76 | |
| 2-Aminoadipate | 0.84 | |
| Phenylalanine Metabolism | Phenylpyruvate | 1.28 |
| Tyrosine Metabolism | Tyrosine | 1.12 |
| 2-Hydroxyphenylacetate | 1.29 | |
| Tryptophan Metabolism | Kynurenate | 0.70 |
| Xanthurenate | 0.43 | |
| Serotonin | 1.39 | |
| Indoleacetate | 1.31 | |
| Leucine, Isoleucine, and Valine Metabolism | 3-Methylglutaconate | 2.00 |
| Alpha-hydroxyisovalerate | 0.88 | |
| Ethylmalonate | 0.84 | |
| 3-Methyl-2-oxobutyrate | 1.35 | |
| Methionine, Cysteine, SAM, and Taurine Metabolism | S-methylcysteine | 1.42 |
| N-acetyltaurine | 0.77 | |
| Urea cycle, Arginine, and Proline Metabolism | 2-Oxoarginine | 0.81 |
| N-monomethylarginine | 1.22 | |
| Creatine Metabolism | Creatine phosphate | 1.86 |
| Guanidino and Acetamido Metabolism | 4-Guanidinobutanoate | 0.80 |
| Glutathione Metabolism | 2-Hydroxybutyrate/2-Hydroxyisobutyrate | 0.75 |
| Sub Pathway | Biochemical Name | HFD+TT800/HFD |
|---|---|---|
| Purine metabolism, xanthine/Inosine containing | Hypoxanthine | 0.23 |
| Xanthine | 0.41 | |
| Xanthosine | 0.53 | |
| 2′-Deoxyinosine | 0.15 | |
| Allantoin | 0.85 | |
| Purine metabolism, Adenine containing | Adenosine-3′,5′-cyclic monophosphate (cAMP) | 1.55 |
| Purine metabolism, Guanine containing | 7-Methylguanine | 0.74 |
| Purine Metabolism, Orotate containing | Dihydroorotate | 1.55 |
| Pyrimidine Metabolism, Uracil containing | Uridine | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-L.; Ramamoorthy, S.; Kaur, G.; Dufour, J.M.; Wang, R.; Mo, H.; Watkins, B.A. Dietary Annatto-Extracted Tocotrienol Reduces Inflammation and Oxidative Stress, and Improves Macronutrient Metabolism in Obese Mice: A Metabolic Profiling Study. Nutrients 2021, 13, 1267. https://doi.org/10.3390/nu13041267
Shen C-L, Ramamoorthy S, Kaur G, Dufour JM, Wang R, Mo H, Watkins BA. Dietary Annatto-Extracted Tocotrienol Reduces Inflammation and Oxidative Stress, and Improves Macronutrient Metabolism in Obese Mice: A Metabolic Profiling Study. Nutrients. 2021; 13(4):1267. https://doi.org/10.3390/nu13041267
Chicago/Turabian StyleShen, Chwan-Li, Sivapriya Ramamoorthy, Gurvinder Kaur, Jannette M. Dufour, Rui Wang, Huanbiao Mo, and Bruce A. Watkins. 2021. "Dietary Annatto-Extracted Tocotrienol Reduces Inflammation and Oxidative Stress, and Improves Macronutrient Metabolism in Obese Mice: A Metabolic Profiling Study" Nutrients 13, no. 4: 1267. https://doi.org/10.3390/nu13041267
APA StyleShen, C.-L., Ramamoorthy, S., Kaur, G., Dufour, J. M., Wang, R., Mo, H., & Watkins, B. A. (2021). Dietary Annatto-Extracted Tocotrienol Reduces Inflammation and Oxidative Stress, and Improves Macronutrient Metabolism in Obese Mice: A Metabolic Profiling Study. Nutrients, 13(4), 1267. https://doi.org/10.3390/nu13041267








