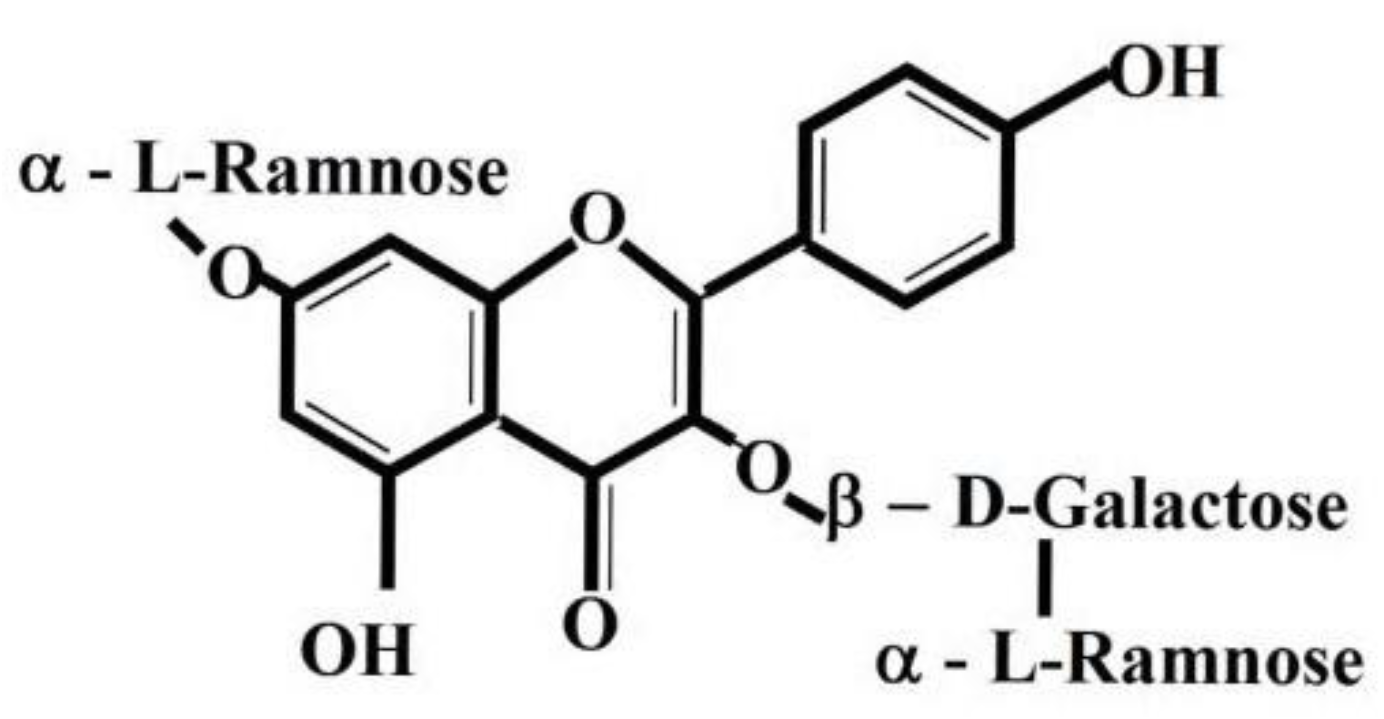
Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Adjuvant Induced Arthritis in LEWIS Rats
2.3. Treatments and Design of Experiment
2.4. Evaluation of Experimental Arthritis
2.5. The Activity of γ-Glutamyltransferase in the Hind Paw Joint and Spleen Tissue
- A—absorbance;
- c—concentration of p-nitroaniline [µg/mL];
- k—coefficient from calibration curve A = k. c.
2.6. Levels of Interleukin 17A in Plasma Samples
3. Results
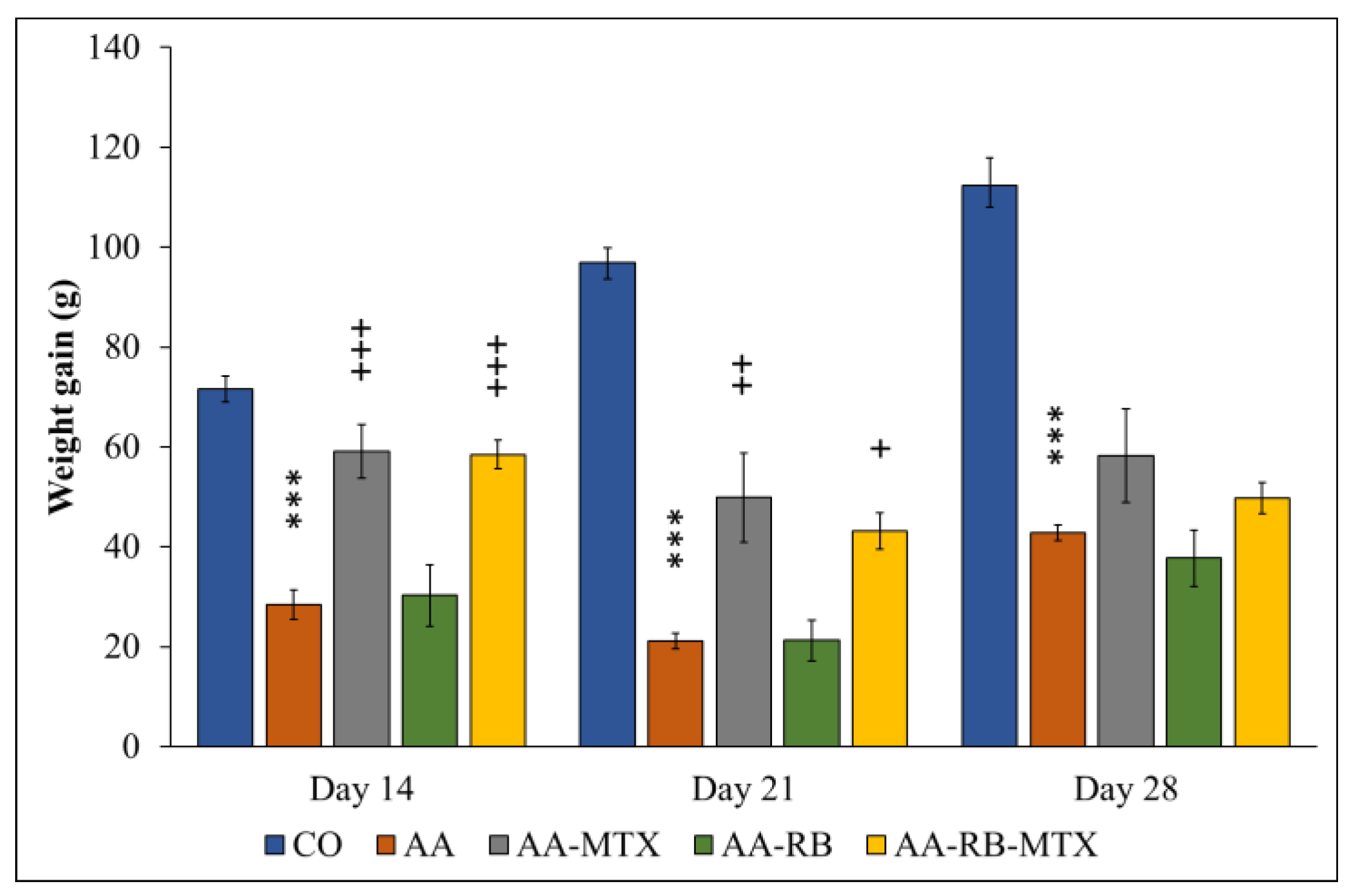
3.1. Change of the Body Weight (CBW)
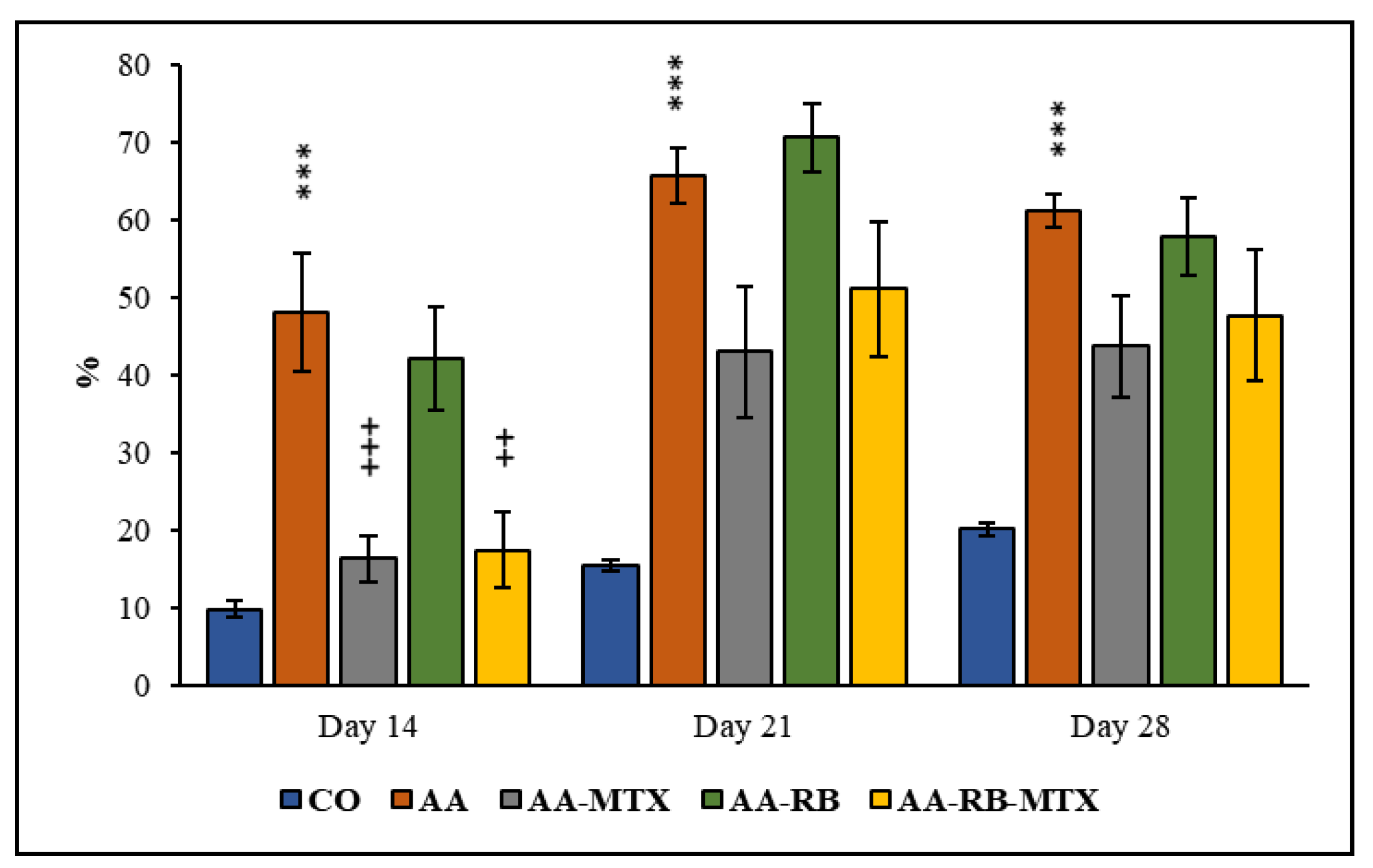
Hind Paw Volume (HPV)
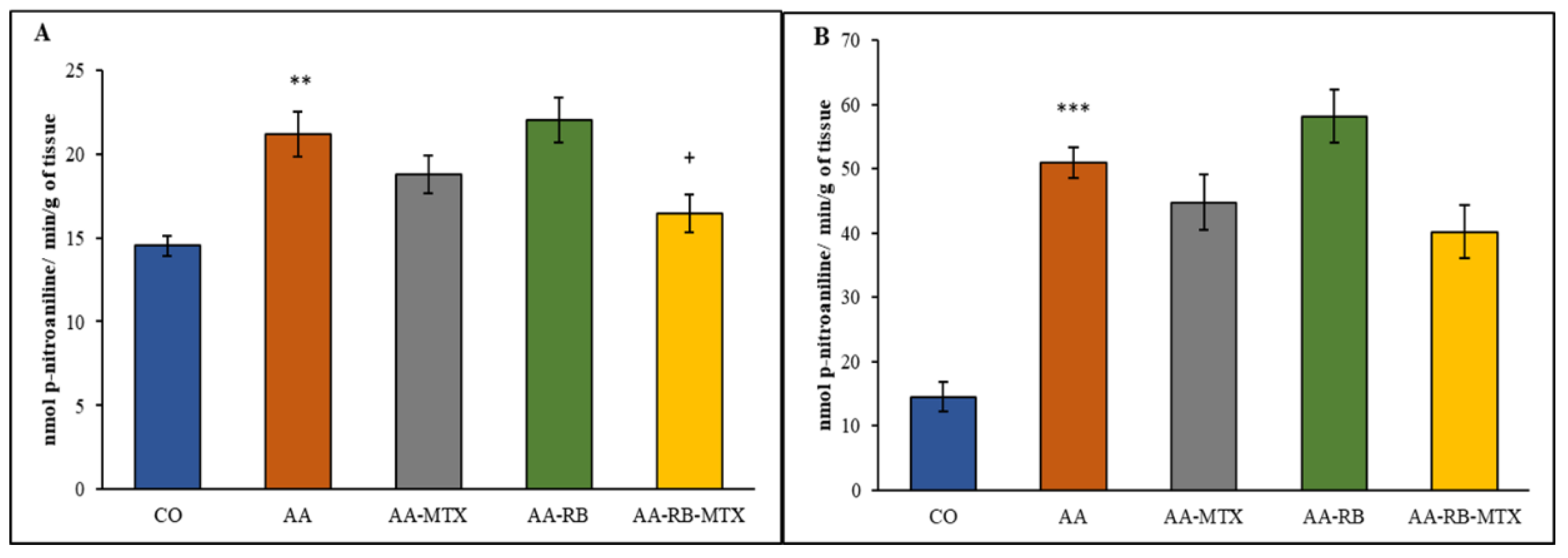
3.2. Activity of Cellular γ-Glutamyl-Transferase in the Hind Paw Joint and Spleen Tissue
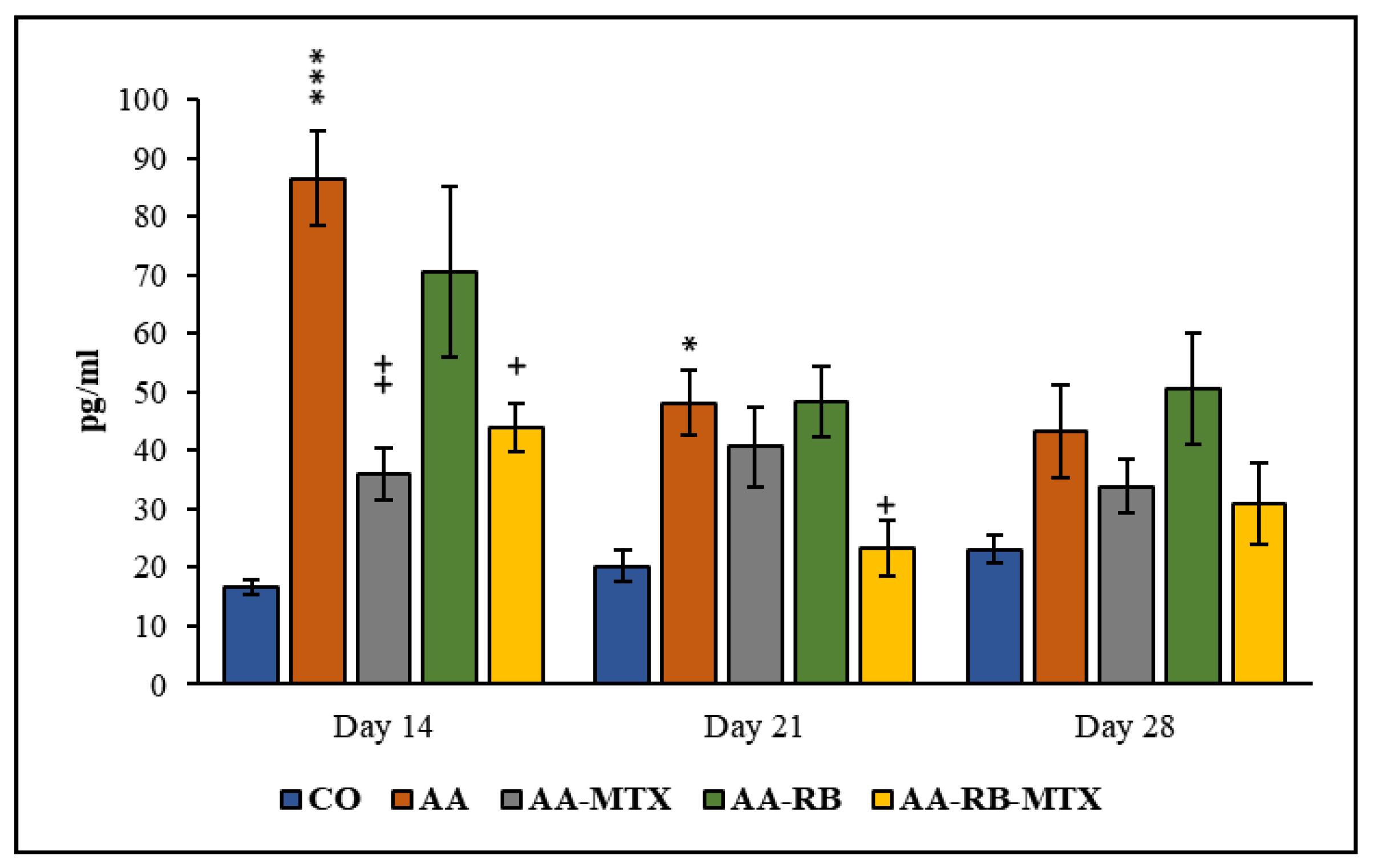
3.3. Interleukin-17A in Blood Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation associated diseases in organs. Oncotarget 2018, 23, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with syntheticand biological disease-modifying antirheumatic drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [PubMed]
- Kesharwani, D.; Paliwal, R.; Satapathy, T.; Das Paul, S. Rheumatiod arthritis: An updated overview of latest therapy and drug delivery. J. Pharmacopunct. 2019, 22, 210–224. [Google Scholar]
- Hazlewood, G.S.; Barnabe, C.; Tomlinson, G.; Marshall, D.; Devoe, D.; Bombardier, C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: Abridged Cochrane systematic review and network meta-analysis. BMJ 2016, 353, i1777. [Google Scholar] [CrossRef]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or negative actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Wu, D.; Yang, L.; Ye, H.; Wang, Q.; Cao, Z.; Tang, K. Exploring the mechanism of flavonoids through systematic bioinformatics analysis. Front. Pharmacol. 2018, 15, 918. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Mondal, S.; Rahaman, S.T. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J. 2020, 8, 91–104. [Google Scholar]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the Genus Astragalus: Phytochemistry and biological activity. Pharmacogn. Rev. 2016, 10, 11–32. [Google Scholar]
- Maresca, M.; Micheli, L.; Cinci, L.; Bilia, A.R.; Ghelardini, C.; Di Cesare Mannelli, L. Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. Pharm. Pharmacol. 2017, 69, 1858–1870. [Google Scholar] [CrossRef]
- Alaniya, M.D.; Sutiashvili, M.G.; Kavtaradze, N.S.; Skhirtladze, A.V. Chemical constituents of Astragalus falcatus. Chem. Nat. Compd. 2017, 53, 1202–1203. [Google Scholar] [CrossRef]
- Kemertelidze, E.; Alania, M.; Sagareishvili, T.; Shalashvili, K.; Kavtaradze, N. Medicinal preparations on the basis of vegetable phenolic compounds. Planta Med. 2009, 75, PD62. [Google Scholar] [CrossRef]
- Kemertelidze, E.P.; Syrov, V.N.; Alaniya, M.D.; Kavtaradze, N.S.; Khushbaktova, Z.A. Chemical composition and pharmaco-logical activity of the leaves Pueraria hirsuta L. growing in Georgia. Pharm. Chem. J. 2008, 42, 340–343. [Google Scholar] [CrossRef]
- Yahara, S.; Kohjyouma, M.; Kohoda, H. Flavonoid glycosides and saponins from Astragalus shikokianus. Phytochemistry 2000, 53, 469–471. [Google Scholar] [CrossRef]
- Eom, S.H.; Jin, S.J.; Jeong, H.Y.; Song, Y.; Lim, Y.J.; Kim, J.I.; Lee, Y.H.; Kang, H. Kudzu Leaf extract suppresses the production of inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-alpha, and Interleukin-6 via inhibition of JNK, TBK1 and STAT1 in inflammatory macrophages. Int. J. Mol. Sci. 2018, 19, 1536. [Google Scholar] [CrossRef] [PubMed]
- Janeesh, P.A.; Abraham, A. Robinin modulates doxorubicin-induced cardiac apoptosis by TGF-β1 signaling pathway in Sprague Dawley rats. Biomed. Pharmacother. 2014, 68, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Janeesh, P.A.; Sasikala, V.; Dhanya, C.R.; Abraham, A. Robinin modulates TLR/NF-kB signaling pathway in oxidized LDL induced human peripheral blood mono-nuclear cells. Int. Immunopharmacol. 2014, 18, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bevaart, L.; Vervoordeldonk, M.J.; Tak, P.P. Evaluation of therapeutic targets in animal models of arthritis: How does it relate torheumatoid arthritis? Arthritis Rheum. 2010, 62, 2192–2205. [Google Scholar] [CrossRef]
- Bauerova, K.; Ponist, S.; Mihalova, D.; Drafi, F.; Kuncirova, V. Utilization of adjuvant arthritis model for evaluation of new approaches in rheumatoid arthritis therapy focused on regulation of immune processes and oxidative stress. Interdiscip. Toxicol. 2011, 4, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ponist, S.; Gardi, C.; Paskova, L.; Svik, K.; Slovak, L.; Bilka, F.; Tedesco, I.; Bauerova, K.; Russo, L.G. Modulation of methotrexate efficacy by green tea polyphenols in rat adjuvant arthritis. PharmaNutrition 2020, 14, 100228. [Google Scholar] [CrossRef]
- Orlowski, M.; Meister, A. The gamma-glutamyl cycle: A possible transport system for amino acids. Proc. Natl. Acad. Sci. USA 1970, 67, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Ondrejickova, O.; Ziegelhoeffer, A.; Gabauer, I.; Sotnikova, R.; Styk, J.; Gibala, P.; Sedlak, J.; Horakova, L. Evaluation of ischemia-reperfusion injury by malondialdehyde, glutathione and gamma-glutamyltranspeptidase: Lack of specific localeffects in diverse parts of the dog heart following acute coronary occlusion. Cardioscience 1993, 4, 225–230. [Google Scholar] [PubMed]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Schinnerling, K.; Rosas, C.; Soto, L.; Thomas, R.; Aguillón, J.C. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front. Immunol. 2019, 10, 203. [Google Scholar] [CrossRef]
- Bauerova, K.; Acquaviva, A.; Ponist, S.; Gardi, C.; Vecchio, D.; Drafi, F.; Arezzini, B.; Bezakova, L.; Kuncirova, V.; Mihalova, D.; et al. Markers of inflammation and oxidative stress studied in adjuvant-induced arthritis in the rat on systemic and local level affected by pinosylvin and methotrexate and their combination. Autoimmunity 2015, 48, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.L.; Cronstein, B.N. Mechanisms of action of methotrexate. Bull. Hosp. Jt. Dis. 2013, 71, S5–S8. [Google Scholar]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an old drug with new tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Criado, A.B.; Mongey, A.B.; Avouac, J.; Marotte, H.; Mueller, R.B. How to get the most from methotrexate (MTX) treatment for your rheumatoid arthritis patient? MTX in the treat-to-target strategy. J. Clin. Med. 2019, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Tsiklauri, L. Bioavailability of some biologically active compounds from Georgian flora. In Proceedings of the GeoHet-2011 2nd International Conference on Organic Chemistry: Advances in Heterocyclic Chemistry, Tbilisi, Georgia, 25–27 September 2011; p. 61. [Google Scholar]
- Sokolova, V.E.; Vasil’chenko, E.A.; Izmailova, I.K. Ob anaboliziruiushchem deistvii flavonoidov [Anabolic action of flavonoids]. Farmakol. Toksikol. 1978, 41, 323–327. (In Bulgarian) [Google Scholar] [PubMed]
- Vasil’chenko, E.A.; Sokolova, V.E. K voprosu o diureticheskom deĭstvii robinina [Diuretic action of robinin]. Farmakol. Toksikol. 1973, 36, 97–100. (In Bulgarian) [Google Scholar] [PubMed]
- Wang, M.; Li, H.; Wang, Y.; Hao, Y.; Huang, Y.; Wang, X.; Lu, Y.; Du, Y.; Fu, F.; Xin, W.; et al. Anti-rheumatic properties of gentiopicroside are associated with suppression of ROS-NF-κB-NLRP3 axis in fibroblast-like synoviocytes and NF-κB pathway in adjuvant-induced arthritis. Front. Pharmacol. 2020, 11, 515. [Google Scholar] [CrossRef]
- Bauerova, K.; Paulovicova, E.; Mihalova, D.; Drafi, F.; Strosova, M.; Mascia, C.; Biasi, F.; Rovensky, J.; Kucharska, J.; Gvozdjakova, A.; et al. Combined methotrexate and coenzyme Q10 therapy in adjuvant-induced arthritis evaluated using parameters of inflammation and oxidative stress. Acta Biochim. Pol. 2010, 57, 347–354. [Google Scholar] [CrossRef]
- Bauerova, K.; Ponist, S.; Navarova, J.; Dubnickova, M.; Paulovicova, E.; Pajtinka, M.; Kogan, G.; Mihalova, D. Glucomannan in prevention of oxidative stress and inflammation occurring in adjuvant arthritis. Neuroendocrinol. Lett. 2008, 29, 691–696. [Google Scholar]
- Bauerova, K.; Kucharska, J.; Ponist, S.; Gvozdjakova, A. Coenzyme Q10 supplementation in adjuvant arthritis (pre-clinical study). In Mitochondrial Medicine: Mitochondrial Metabolism, Diseases, Diagnosis and Therapy; Gvozdjakova, A., Ed.; Springer: Berlin, Germany, 2008; pp. 340–342. [Google Scholar]
- Bauerova, K.; Paulovicova, E.; Mihalova, D.; Svik, K.; Ponist, S. Study of new ways of supplementary and combinatory therapy of rheumatoid arthritis with immunomodulators. Glucomannan and Imunoglukan in adjuvant arthritis. Toxicol. Ind. Health 2009, 25, 329–335. [Google Scholar] [CrossRef]
- Tsiklauri, L.; Drafi, F.; Ponist, S.; Slovak, L.; Chrastina, M.; Svik, K.; Kemoklidze, Z.; Kemertelidze, E.; Bauerova, K. Study of anti-inflammatory activity of Fatsiphloginum™ (Fatsia japonica) and new purified triterpene-rich extract of saponins (PS-551) in experimental model of arthritis. Physiol. Res. 2019, 68, S75–S85. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Moriwaki, S.; Kawahara-Hanaoka, M.; Uemura, Y.; Serizawa, I.; Miyauchi, M.; Shibata, S.; Kanaya, T.; Takata, T.; Taniguchi, N.; et al. Treatment with anti-gamma-glutamyl transpeptidase antibody attenuates osteolysis in collagen-induced arthritis mice. J. Bone Miner. Res. 2007, 22, 1933–1942. [Google Scholar] [CrossRef]
- Feketeova, L.; Jancova, P.; Moravcova, P.; Janegova, A.; Bauerova, K.; Ponist, S.; Mihalova, D.; Janega, P.; Babal, P. Effect of methotrexate on inflammatory cells redistribution in experimental adjuvant arthritis. Rheumatol. Int. 2012, 32, 3517–3523. [Google Scholar] [CrossRef]
- Rambabu, K.; Ansari, A.A.; Shaafie, I.A.; Chelvam, A.P.; Ziu, M. Gamma-glutamyltranspeptidase in synovial fluid, serum, and urine of patients with rheumatoid arthritis. Biochem. Med. Metab. Biol. 1990, 43, 183–192. [Google Scholar] [CrossRef]
- Drafi, F.; Bauerova, K.; Kuncirova, V.; Ponist, S.; Mihalova, D.; Fedorova, T.; Harmatha, J.; Nosal, R. Pharmacological influence on processes of adjuvant arthritis: Effect of the combination of an antioxidant active substance with methotrexate. Interdiscip. Toxicol. 2012, 5, 84–91. [Google Scholar] [CrossRef]
- Bauerova, K.; Ponist, S.; Ondrejickova, O.; Komendova, D.; Mihalova, D. Association between tissue gamma-glutamyl-transferase and clinical markers of adjuvant arthritisin Lewis rats. Neuro Endocrinol. Lett. 2006, 27, 172–175. [Google Scholar] [PubMed]
- Chen, Q.J.; Niu, X.H.; Li, N.N. Exploring the natural chemiome to target interleukin-6 receptor (IL-6R) cytokines: An atomic scale investigation for novel rheumatoid arthritis drug discovery. Braz. J. Pharm. Sci. 2017, 53, e17256. [Google Scholar] [CrossRef]
- Neumann, E.; Lefèvre, S.; Zimmermann, B.; Gay, S.; Müller-Ladner, U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 2010, 16, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Larionova, R.V.; Brooks, W.H.; Bettacchioli, E.; Renaudineau, Y. Toll-like receptors, infections, and rheumatoid arthritis. Clin. Rev. Allerg. Immunol. 2020, 58, 172–181. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoon, B.Y.; Kim, J.I.; Heo, Y.M.; Woo, Y.J.; Park, S.H.; Kim, H.Y.; Kim, S.I.; Cho, M.L. Interleukin-17 increases the expression of Toll-like receptor 3 via the STAT3 pathway in rheumatoid arthritis fibroblast-like synoviocytes. Immunology 2014, 141, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 2020, 22, 16. [Google Scholar] [CrossRef]
- Kugyelka, R.; Kohl, Z.; Olasz, K.; Mikecz, K.; Rauch, T.A.; Glant, T.T.; Boldizsar, F. Enigma of IL-17 and Th17 cells in rheumatoid arthritis and in autoimmune animal models of arthritis. Mediat. Inflamm. 2016, 2016, 6145810. [Google Scholar] [CrossRef]
- Nasef, N.; Elnagdy, M.; Younis, W.; Badr, R.; El-bussiouni, S.; Akef, A.; Rashwan, M. T helper 17 cells and interleukin-17 in patients with rheumatoid arthritis. Int. J. Clin. Rheumatol. 2019, 14, 113–119. [Google Scholar]
- Robert, M.; Miossec, P. IL-17 in rheumatoid arthritis and precision medicine: From synovitis expression to circulating bioactive levels. Front. Med. 2019, 5, 364. [Google Scholar] [CrossRef]
- Hu, D.; Tjon, E.C.; Andersson, K.M.; Molica, G.M.; Pham, M.C.; Healy, B.; Murugaiyan, G.; Pochet, N.; Kuchroo, V.K.; Bokarewa, M.I.; et al. Aberrant expression of USF2 in refractory rheumatoid arthritis and its regulation of proinflammatory cytokines in Th17 cells. Proc. Natl. Acad. Sci. USA 2020, 117, 30639–30648. [Google Scholar] [CrossRef]
- Dai, Q.; Li, Y.; Wang, M.; Li, Y.; Li, J. TlR2 and TlR4 are involved in the treatment of rheumatoid arthritis synovial fibroblasts with a medicated serum of asarinin through inhibition of Th1/Th17 cytokines. Exp. Ther. Med. 2020, 19, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Codreanu, C.; Popescu, C.C.; Mogoșan, C.D.; Enache, L.; Manda, G.; Berghea, F.; Groșeanu, L.; Predețeanu, D. Targeting Interleukin 17 in the treatment of rheumatoid arthritis. Farmacia 2018, 66, 390–398. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Zhang, S.; Yin, L.; Ma, L.; He, D.; Shen, J. Methotrexate attenuates the Th17/IL-17 levels in peripheral blood mononuclear cells from healthy individuals and RA patients. Rheumatol. Int. 2012, 32, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Lin, L.; Li, J.; Zhu, H.; He, Y.; Liu, Y.; Zeng, K.; Zhang, X. Effects of methotrexate on the expression of Toll like receptor (TLR)2 and TLR4 in human peripheral blood CD14+ mononuclear cells from patients with psoriasis vulgaris. Chin. J. Dermatol. 2009, 42, 760–762. [Google Scholar]
- Toh, M.L.; Gonzales, G.; Koenders, M.I.; Tournadre, A.; Boyle, D.; Lubberts, E.; Zhou, Y.; Firestein, G.S.; van den Berg, W.B.; Miossec, P. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS ONE 2010, 5, e13416. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; Fadel, N.A.; Abdel-Naby, D.H.; El-Rehim, H.A.A.; Zaki, H.F.; Kenawy, S.A. Potential anti-inflammatory action of resveratrol and piperine in adjuvant-induced arthritis: Effect on pro-inflammatory cytokines and oxidative stress biomarkers. Egypt. Rheumatol. 2020, 42, 71–77. [Google Scholar] [CrossRef]
- Khan, M.A.; Sarwar, A.H.; Rahat, R.; Ahmed, R.S.; Umar, S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2020, 85, 106642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, L.; Lu, M.; Wang, J.; Li, Y.T.; Gao, W.L.; Yin, Z.S. Evodiamine attenuates adjuvant-induced arthritis in rats by inhibiting synovial inflammation and restoring the Th17/Treg balance. J. Pharm. Pharmacol. 2020, 72, 798–806. [Google Scholar] [CrossRef]
- Griffiths, L.A.; Smith, G.E. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972, 128, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Najmanova, I.; Pourova, J.; Mladenka, P. A mixture of phenolic metabolites of quercetin can decrease elevated blood pressure of spontaneously hypertensive rats even in low doses. Nutrients 2020, 12, 213. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.H. Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef]
- Tang, X.L.; Liu, J.; Dong, W.; Li, P.; Li, L.; Hou, J.C.; Zheng, Y.Q.; Lin, C.R.; Ren, J.G. Protective effect of kaempferol on LPS plus ATP-induced inflammatory response in cardiac fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef]
| Group | Treatment and Active Substance | Posology |
|---|---|---|
| Group 1: Healthy controls (CO) | vehiculum | 0.5 mL |
| Group 2: AA untreated | vehiculum | 0.5 mL |
| Group 3: AA + treatment | Methotrexate (MTX) | 0.3 mg/kg of b.w. twice a week |
| Group 4: AA + treatment | Robinin (RB) | 50 mg/kg of b.w. daily |
| Group 5: AA + treatment | Robinin+MTX (RB-MTX) | 50 mg/kg + 0.3 mg/kg of b.w. twice a week |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiklauri, L.; Švík, K.; Chrastina, M.; Poništ, S.; Dráfi, F.; Slovák, L.; Alania, M.; Kemertelidze, E.; Bauerova, K. Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis. Nutrients 2021, 13, 1268. https://doi.org/10.3390/nu13041268
Tsiklauri L, Švík K, Chrastina M, Poništ S, Dráfi F, Slovák L, Alania M, Kemertelidze E, Bauerova K. Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis. Nutrients. 2021; 13(4):1268. https://doi.org/10.3390/nu13041268
Chicago/Turabian StyleTsiklauri, Lia, Karol Švík, Martin Chrastina, Silvester Poništ, František Dráfi, Lukáš Slovák, Mery Alania, Ether Kemertelidze, and Katarina Bauerova. 2021. "Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis" Nutrients 13, no. 4: 1268. https://doi.org/10.3390/nu13041268
APA StyleTsiklauri, L., Švík, K., Chrastina, M., Poništ, S., Dráfi, F., Slovák, L., Alania, M., Kemertelidze, E., & Bauerova, K. (2021). Bioflavonoid Robinin from Astragalus falcatus Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis. Nutrients, 13(4), 1268. https://doi.org/10.3390/nu13041268







