Non-Celiac Gluten/Wheat Sensitivity: Clinical Characteristics and Microbiota and Mycobiota Composition by Response to the Gluten Challenge Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Issues
2.3. Outcomes
2.4. First Step: Dietary Changes
2.5. Second Step: (DBPC) Crossover Gluten Challenge Test
2.6. Questionnaires
- –
- The Minnesota Leisure Time Physical Activity Questionnaire: the physical activity level was calculated as the product of the duration and frequency of each activity (in h/week), weighted by an estimate of the metabolic equivalent (MET) of the activity and summed for the activities performed [30].
- –
- The Hamilton rating scale for depression: mild depression was defined by scores ranging 8–17, moderate depression with scores ranging 18–24, and serious depression with scores >25 [31].
- –
- The Hamilton rating scale for anxiety: mild anxiety was defined by a score <17, mild-to-moderate by scores ranging 18–24, and moderate-to-severe by scores >25 [32].
- –
- ORTO-15 questionnaire: a 15 multiple-choice item tool investigating the obsessive attitude of the subjects in choosing, buying, preparing, and consuming food they consider to be healthy. A cutoff of 40 discriminates the presence or the absence of orthorexia [33].
- –
- Eating Attitude test (EAT) 26: an abbreviated 26-item version of the EAT-40 relate to attitudes, beliefs, and behaviors concerning food, weight, and body shape. A cutoff score of 20 or above denotes the presence of disturbed eating behavior [34].
- –
- Short-Form (SF)-12 health survey: to measure general health-related quality of life with different domains with scores ranging from 0 to 100 (higher values indicate a better quality of life) [35].
2.7. Fecal Samples
2.8. Fecal DNA Extraction and Amplicon Target Sequencing
2.9. Bioinformatics Analysis
2.10. Accession Numbers
2.11. Statistical Analyses
3. Results
3.1. Characteristics of the Participants
3.2. Response to the DBPC Gluten Challenge Test
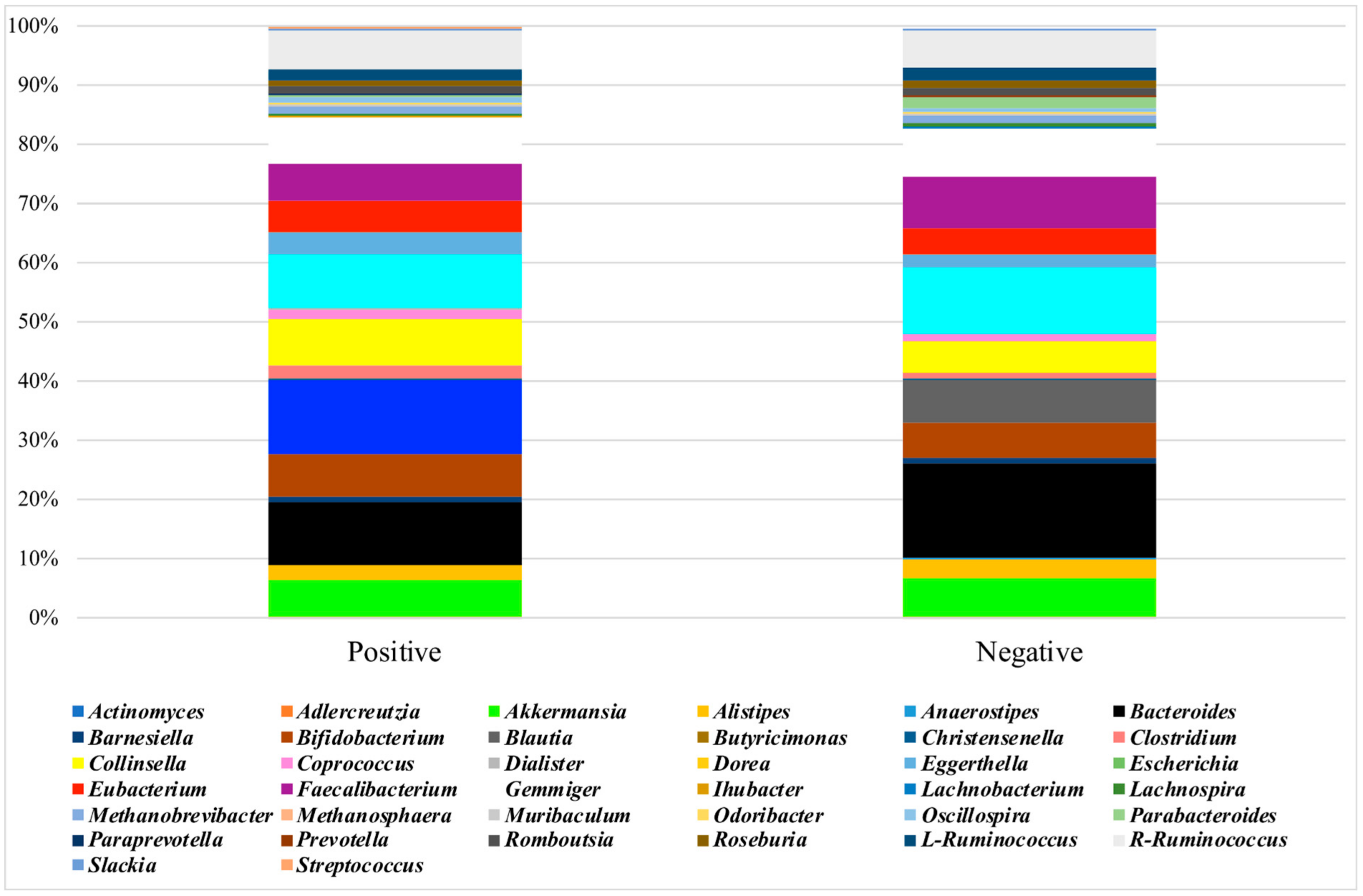
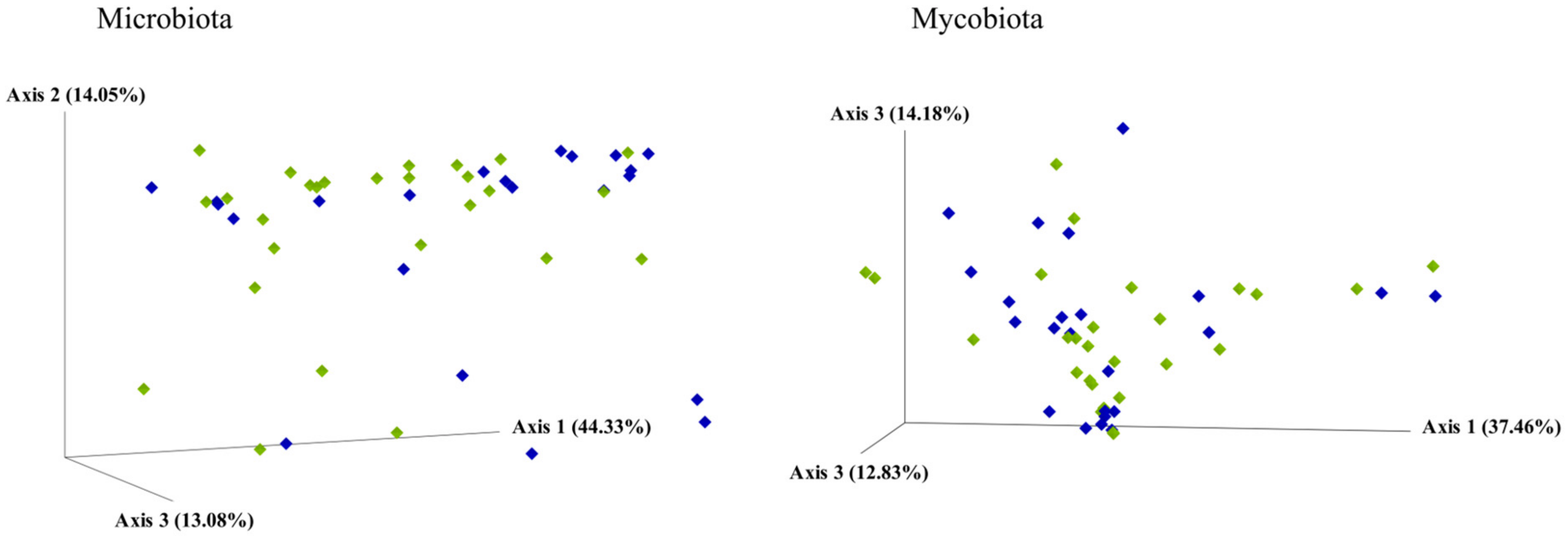
3.3. Analysis of the Microbiota
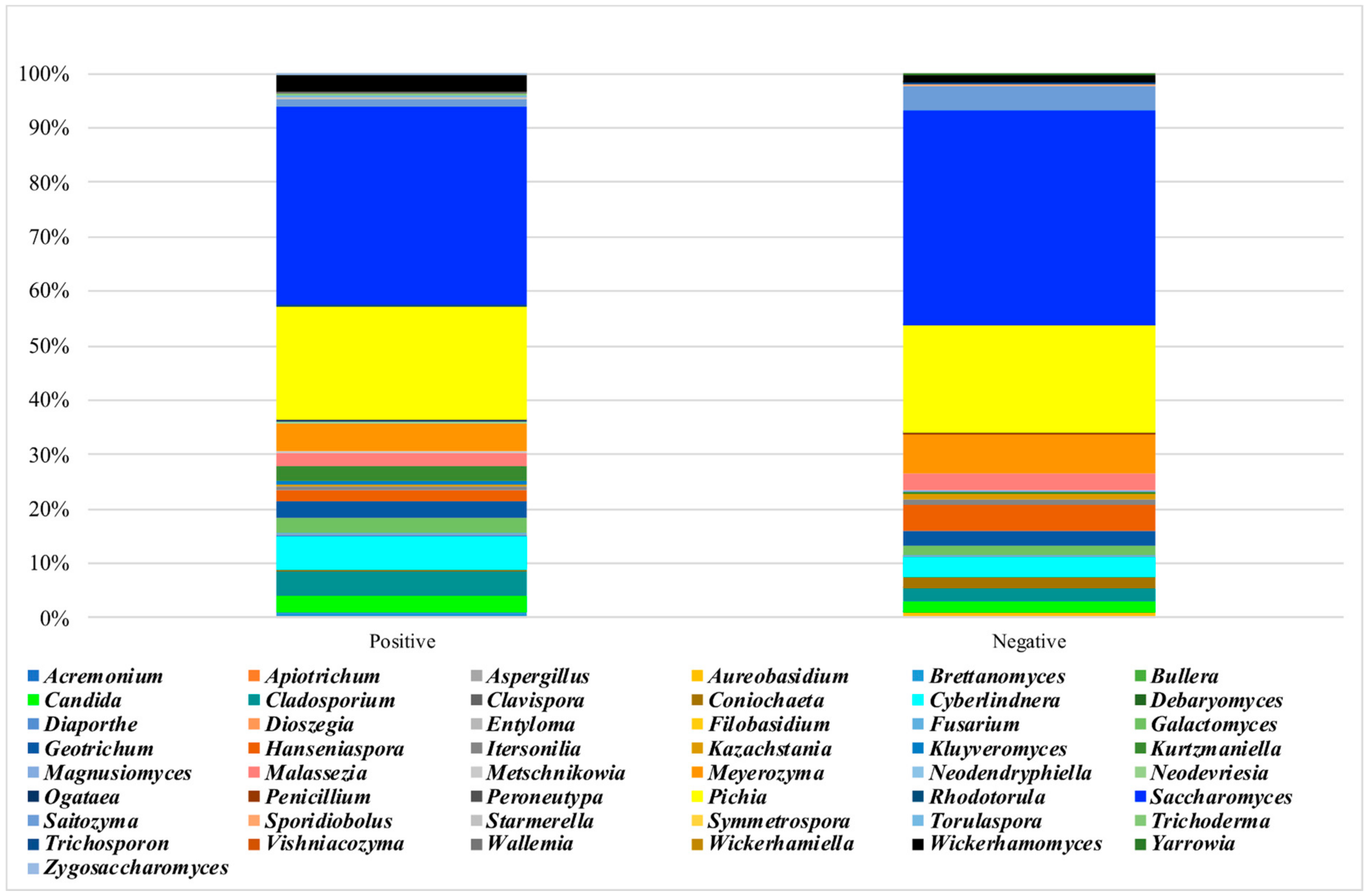
3.4. Analysis of the Mycobiota
4. Discussion
4.1. Gluten Response
4.2. Gluten-Responsive Individuals
4.3. Placebo-Responsive Individuals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mumolo, M.G.; Rettura, F.; Melissari, S.; Costa, F.; Ricchiuti, A.; Ceccarelli, L.; De Bortoli, N.; Marchi, S.; Bellini, M. Is Gluten the Only Culprit for Non-Celiac Gluten/Wheat Sensitivity? Nutrients 2020, 12, 3785. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Cremon, C.; Wrona, D.; Fuschi, D.; Marasco, G.; Stanghellini, V.; Barbara, G. Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients 2020, 12, 3735. [Google Scholar] [CrossRef]
- Castillo-Rodal, A.I.; Furuzawa-Carballeda, J.; Peláez-Luna, M.; Castro-Gómez, J.; López-Vidal, Y.; Uscanga, L. More Fuel to the Fire: Some Patients with Non-Celiac Self-Reported Wheat Sensitivity Exhibit Adaptive Immunological Responses in Duodenal Mucosa. BMC Gastroenterol. 2020, 20, 414. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac Wheat Sensitivity: An Immune-Mediated Condition with Systemic Manifestations. Gastroenterol. Clin. N. Am. 2019, 48, 165–182. [Google Scholar] [CrossRef]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of Gut Permeability and Mucosal Immune Gene Expression in Two Gluten-Associated Conditions: Celiac Disease and Gluten Sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Giannone, G.; Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; Iacobucci, R.; Porcasi, R.; Catalano, T.; Geraci, G.; et al. Duodenal and Rectal Mucosa Inflammation in Patients with Non-Celiac Wheat Sensitivity. Clin. Gastroenterol. Hepatol. 2019, 17, 682–690.e3. [Google Scholar] [CrossRef]
- Losurdo, G.; Principi, M.; Iannone, A.; Giangaspero, A.; Piscitelli, D.; Ierardi, E.; Di Leo, A.; Barone, M. Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study. Nutrients 2018, 10, 2001. [Google Scholar] [CrossRef]
- Efthymakis, K.; Clemente, E.; Marchioni, M.; Di Nicola, M.; Neri, M.; Sallese, M. An Exploratory Gene Expression Study of the Intestinal Mucosa of Patients with Non-Celiac Wheat Sensitivity. Int. J. Mol. Sci. 2020, 21, 1969. [Google Scholar] [CrossRef]
- Clemente, E.; Efthymakis, K.; Carletti, E.; Capone, V.; Sperduti, S.; Bologna, G.; Marchisio, M.; Di Nicola, M.; Neri, M.; Sallese, M. An Explorative Study Identifies MiRNA Signatures for the Diagnosis of Non-Celiac Wheat Sensitivity. PLoS ONE 2019, 14, e0226478. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal Cell Damage and Systemic Immune Activation in Individuals Reporting Sensitivity to Wheat in the Absence of Coeliac Disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Rivera-Gutierrez, X.; Cobos-Quevedo, O.D.J.; Grube-Pagola, P.; Meixueiro-Daza, A.; Hernandez-Flores, K.; Cabrera-Jorge, F.J.; Vivanco-Cid, H.; Dowd, S.E.; Remes-Troche, J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients 2018, 10, 1641. [Google Scholar] [CrossRef]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of Low FODMAP and Gluten-Free Diets on Disease Activity and Intestinal Microbiota in Patients with Non-Celiac Gluten Sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Corazza, G.R. Nonceliac Gluten Sensitivity: Sense or Sensibility? Ann. Intern. Med. 2012, 156, 309–311. [Google Scholar] [CrossRef]
- Skodje, G.I.; Minelle, I.H.; Rolfsen, K.L.; Iacovou, M.; Lundin, K.E.A.; Veierød, M.B.; Henriksen, C. Dietary and Symptom Assessment in Adults with Self-Reported Non-Coeliac Gluten Sensitivity. Clin. Nutr. ESPEN 2019, 31, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Brottveit, M.; Vandvik, P.O.; Wojniusz, S.; Løvik, A.; Lundin, K.E.; Boye, B. Absence of Somatization in Non-Coeliac Gluten Sensitivity. Scand. J. Gastroenterol. 2012, 47, 770–777. [Google Scholar] [CrossRef]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R. Study Group for Non-Celiac Gluten Sensitivity. An Italian Prospective Multicenter Survey on Patients Suspected of Having Non-Celiac Gluten Sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients with Self-Reported Non-Celiac Gluten Sensitivity after Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Baschè, R.; Ferraresi, A.; Ricci, C.; Lanzarotto, F.; Marullo, M.; Villanacci, V.; Hidalgo, A.; Lanzini, A. Randomised Clinical Study: Gluten Challenge Induces Symptom Recurrence in Only a Minority of Patients Who Meet Clinical Criteria for Non-Coeliac Gluten Sensitivity. Aliment. Pharmacol. Ther. 2015, 42, 968–976. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small Amounts of Gluten in Subjects with Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients with Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The Histopathology of Coeliac Disease: Time for a Standardized Report Scheme for Pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Magge, S.; Lembo, A. Low-FODMAP Diet for Treatment of Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2012, 8, 739–745. [Google Scholar]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.L.; Jacobs, D.R.; Schucker, B.; Knudsen, J.; Leon, A.S.; Debacker, G. A Questionnaire for the Assessment of Leisure Time Physical Activities. J. Chronic Dis. 1978, 31, 741–755. [Google Scholar] [CrossRef]
- Hamilton, M. Development of a Rating Scale for Primary Depressive Illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. The Assessment of Anxiety States by Rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Marsili, D.; Graziani, M.P.; Imbriale, M.; Cannella, C. Orthorexia Nervosa: Validation of a Diagnosis Questionnaire. Eat. Weight Disord. 2005, 10, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Dotti, A.; Lazzari, R. Validation and Reliability of the Italian EAT-26. Eat. Weight Disord. 1998, 3, 188–194. [Google Scholar] [CrossRef]
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Petersen, S.; Paice, C.; Stradling, J. A Shorter Form Health Survey: Can the SF-12 Replicate Results from the SF-36 in Longitudinal Studies? J. Public Health Med. 1997, 19, 179–186. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Ferrocino, I.; Rantsiou, K.; Cocolin, L. Metataxonomic Comparison between Internal Transcribed Spacer and 26S Ribosomal Large Subunit (LSU) RDNA Gene. Int. J. Food Microbiol. 2019, 290, 132–140. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Ajamian, M.; Rosella, G.; Newnham, E.D.; Biesiekierski, J.R.; Muir, J.G.; Gibson, P.R. Effect of Gluten Ingestion and FODMAP Restriction on Intestinal Epithelial Integrity in Patients with Irritable Bowel Syndrome and Self-Reported Non-Coeliac Gluten Sensitivity. Mol. Nutr. Food Res. 2020, 65, e1901275. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Duncanson, K.; Jones, M.P.; Walker, M.M.; Keely, S.; Talley, N.J. Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients 2020, 12, 1947. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkhani, B.; Fanaeian, M.M.; Farahvash, M.J.; Aletaha, N.; Alborzi, F.; Elli, L.; Shahbazkhani, A.; Zebardast, J.; Rostami-Nejad, M. Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: A Randomized Double-Blind Placebo Controlled Trial. Sci. Rep. 2020, 10, 2401. [Google Scholar] [CrossRef]
- Kodraliu, G.; Mosconi, P.; Groth, N.; Carmosino, G.; Perilli, A.; Gianicolo, E.A.; Rossi, C.; Apolone, G. Subjective Health Status Assessment: Evaluation of the Italian Version of the SF-12 Health Survey. Results from the MiOS Project. J. Epidemiol. Biostat. 2001, 6, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Bascuñán, K.A.; Vecchi, M.; Doneda, L.; Bardella, M.T.; Lombardo, V.; Scricciolo, A.; Branchi, F.; Elli, L. Exposure to Different Amounts of Dietary Gluten in Patients with Non-Celiac Gluten Sensitivity (NCGS): An Exploratory Study. Nutrients 2019, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised Clinical Trial: Gluten May Cause Depression in Subjects with Non-Coeliac Gluten Sensitivity—An Exploratory Clinical Study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef]
- Busby, E.; Bold, J.; Fellows, L.; Rostami, K. Mood Disorders and Gluten: It’s Not All in Your Mind! A Systematic Review with Meta-Analysis. Nutrients 2018, 10, 1708. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-Celiac Wheat Sensitivity Diagnosed by Double-Blind Placebo-Controlled Challenge: Exploring a New Clinical Entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef]
- Masaebi, F.; Azizmohammad Looha, M.; Rostami-Nejad, M.; Pourhoseingholi, M.A.; Mohseni, N.; Samasca, G.; Lupan, I.; Rezaei-Tavirani, M.; Zali, M.R. The Predictive Value of Serum Cytokines for Distinguishing Celiac Disease from Non-Celiac Gluten Sensitivity and Healthy Subjects. Iran. Biomed. J. 2020, 24, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Brottveit, M.; Beitnes, A.-C.R.; Tollefsen, S.; Bratlie, J.E.; Jahnsen, F.L.; Johansen, F.-E.; Sollid, L.M.; Lundin, K.E.A. Mucosal Cytokine Response after Short-Term Gluten Challenge in Celiac Disease and Non-Celiac Gluten Sensitivity. Am. J. Gastroenterol. 2013, 108, 842–850. [Google Scholar] [CrossRef]
- Zanini, B.; Villanacci, V.; Marullo, M.; Cadei, M.; Lanzarotto, F.; Bozzola, A.; Ricci, C. Duodenal Histological Features in Suspected Non-Celiac Gluten Sensitivity: New Insights into a Still Undefined Condition. Virchows Arch. 2018, 473, 229–234. [Google Scholar] [CrossRef]
- Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; D’Alcamo, A.; Carroccio, A. Body Mass Index and Associated Clinical Variables in Patients with Non-Celiac Wheat Sensitivity. Nutrients 2019, 11, 1220. [Google Scholar] [CrossRef] [PubMed]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of Gliadin on Permeability of Intestinal Biopsy Explants from Celiac Disease Patients and Patients with Non-Celiac Gluten Sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Villatoro, M.; Vaquero, L.; Pastor, J.; Giménez, M.J.; Ozuna, C.V.; Sánchez-León, S.; García-Molina, M.D.; Segura, V.; Comino, I.; et al. The Dietary Intervention of Transgenic Low-Gliadin Wheat Bread in Patients with Non-Celiac Gluten Sensitivity (NCGS) Showed No Differences with Gluten Free Diet (GFD) but Provides Better Gut Microbiota Profile. Nutrients 2018, 10, 1964. [Google Scholar] [CrossRef]
- Zhong, L.; Shanahan, E.R.; Raj, A.; Koloski, N.A.; Fletcher, L.; Morrison, M.; Walker, M.M.; Talley, N.J.; Holtmann, G. Dyspepsia and the Microbiome: Time to Focus on the Small Intestine. Gut 2017, 66, 1168–1169. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Zhang, H.; Lee, Y.-K.; Zhai, Q.; Chen, W. Roles of Intestinal Bacteroides in Human Health and Diseases. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Orho-Melander, M.; Ohlsson, B. Self-Reported Bowel Symptoms Are Associated with Differences in Overall Gut Microbiota Composition and Enrichment of Blautia in a Population-Based Cohort. J. Gastroenterol. Hepatol. 2021, 36, 174–180. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and Deep Molecular Analysis of Microbiota Signatures in Fecal Samples from Patients with Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; De Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Sendid, B.; Hoarau, G.; Colombel, J.-F.; Poulain, D.; Ghannoum, M.A. Mycobiota in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The Gut Mycobiota: Insights into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the Gut: A Multiperspective Review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef] [PubMed]
- Kabwe, M.H.; Vikram, S.; Mulaudzi, K.; Jansson, J.K.; Makhalanyane, T.P. The Gut Mycobiota of Rural and Urban Individuals Is Shaped by Geography. BMC Microbiol. 2020, 20, 257. [Google Scholar] [CrossRef]
- Huseyin, C.E.; O’Toole, P.W.; Cotter, P.D.; Scanlan, P.D. Forgotten Fungi-the Gut Mycobiome in Human Health and Disease. FEMS Microbiol. Rev. 2017, 41, 479–511. [Google Scholar] [CrossRef]
- Botschuijver, S.; Roeselers, G.; Levin, E.; Jonkers, D.M.; Welting, O.; Heinsbroek, S.E.M.; de Weerd, H.H.; Boekhout, T.; Fornai, M.; Masclee, A.A.; et al. Intestinal Fungal Dysbiosis Is Associated with Visceral Hypersensitivity in Patients with Irritable Bowel Syndrome and Rats. Gastroenterology 2017, 153, 1026–1039. [Google Scholar] [CrossRef]
- Lang, S.; Duan, Y.; Liu, J.; Torralba, M.G.; Kuelbs, C.; Ventura-Cots, M.; Abraldes, J.G.; Bosques-Padilla, F.; Verna, E.C.; Brown, R.S.; et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients with Alcoholic Hepatitis. Hepatology 2020, 71, 522–538. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2015, 18, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. Allergens to Wheat and Related Cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat Amylase Trypsin Inhibitors Drive Intestinal Inflammation via Activation of Toll-like Receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Leccioli, V.; Oliveri, M.; Romeo, M.; Berretta, M.; Rossi, P. A New Proposal for the Pathogenic Mechanism of Non-Coeliac/Non-Allergic Gluten/Wheat Sensitivity: Piecing Together the Puzzle of Recent Scientific Evidence. Nutrients 2017, 9, 1203. [Google Scholar] [CrossRef]


| All c | Positive c (NCGS) | Negative c | P | |
|---|---|---|---|---|
| Number | 50 | 27 | 23 | |
| Age (years) | 42.2 ± 13.6 | 41.3 ± 14.8 | 43.3 ± 12.1 | 0.60 |
| Male/Female | 7/43 | 4/23 | 3/20 | 0.86 |
| Smoking (actual or past) (%) | 16.0 | 11.1 | 21.7 | 0.24 |
| Education level | ||||
| Primary school (%) | 16.0 | 19.2 | 13.0 | |
| Secondary school (%) | 36.0 | 26.9 | 43.5 | |
| University degree (%) | 48.0 | 53.9 | 43.5 | 0.31 |
| Weight (kg) | 64.0 ± 13.6 | 62.0 ± 9.7 | 66.3 ± 17.0 | 0.27 |
| BMI (kg/m2) a | 23.7 ± 5.1 | 23.0 ± 3.2 | 24.6 ± 6.7 | 0.29 |
| Waist circumference (cm) | 85.5 ± 12.1 | 84.1 ± 8.6 | 87.0 ± 1.3 | 0.40 |
| Systolic blood pressure (mmHg) | 114.5 ± 13.4 | 115.4 ± 12.0 | 113.5 ± 15.2 | 0.61 |
| Diastolic blood pressure (mmHg) | 77.0 ± 8.1 | 76.1 ± 7.2 | 78.0 ± 9.1 | 0.40 |
| METS (h/week) b | 38.8 (43.0) | 35.0 (64.3) | 39.2 (28.3) | 0.97 d |
| Depression | ||||
| Score | 9.1 ± 5.7 | 7.8 ± 4.5 | 10.6 ± 6.7 | 0.09 |
| Absent (%) | 54.0 | 59.3 | 47.8 | |
| Mild (%) | 34.0 | 37.0 | 30.4 | |
| Moderate (%) | 10.0 | 3.7 | 17.4 | |
| Severe (%) | 2.0 | 0 | 4.4 | 0.27 |
| Anxiety | ||||
| Score | 23.3 ± 7.7 | 22.7 ± 6.6 | 24.1 ± 8.8 | 0.52 |
| Mild (%) | 24.0 | 22.2 | 26.1 | |
| Mild to Moderate (%) | 32.0 | 37.0 | 26.1 | |
| Moderate to Severe (%) | 44.0 | 40.7 | 47.8 | 0.71 |
| Orthorexia | ||||
| Score | 35.4 ± 5.4 | 35.9 ± 6.0 | 34.8 ± 4.7 | 0.51 |
| Increased risk (%) | 44.0 | 37.0 | 52.2 | 0.28 |
| Eating disorders | ||||
| Score | 4.5 (8.0) | 3 (6.0) | 6 (9.0) | 0.014 d |
| Increased risk (%) | 8.0 | 0 | 17.4 | 0.024 |
| Quality of life | ||||
| Total score | 35.4 ± 6.0 | 37.5 ± 4.3 | 33.0 ± 6.8 | 0.007 |
| Physical health score | 16.0 ± 2.7 | 16.7 ± 2.1 | 15.3 ± 3.2 | 0.07 |
| Mental health score | 19.4 ± 3.9 | 20.8 ± 3.3 | 17.7 ± 4.0 | 0.004 |
| Positive (NCGS), n = 27 | Negative, n = 23 | |||
|---|---|---|---|---|
| After Gluten | After Placebo | After Gluten | After Placebo | |
| Total score, median (IQR) | 17.0 (7.0) | 2.0 (5.1) | 5.4 (6.1) | 14.0 (8.7) |
| Gastrointestinal symptoms, median (IQR) | 14.9 (9.0) | 2.0 (5.0) | 4.0 (7.4) | 10.7 (7.9) |
| Abdominal pain, n (%) | 11 (40.1) | 5 (18.5) | 5 (21.7) | 6 (26.1) |
| Bloating, n (%) | 9 (33.3) | 6 (22.2) | 8 (34.8) | 12 (52.2) |
| Meteorism, n (%) | 7 (25.9) | 7 (25.9) | 5 (21.7) | 10 (43.5) |
| Increased stool frequency, n (%) | 7 (25.9) | 2 (7.4) | 3 (13.0) | 2 (8.7) |
| Abdominal distention, n (%) | 3 (11.1) | 1 (3.7) | 9 (39.1) | 11 (47.8) |
| Extra-gastrointestinal symptoms, median (IQR) | 0.0 (4.5) | 0.0 (0.6) | 1.0 (4.0) | 2.7 (7.0) |
| Lesions of the oral cavity, n (%) | 4 (14.8) | 2 (7.4) | 0 (0.0) | 1 (4.3) |
| Dermatitis, n (%) | 5 (18.5) | 1 (3.7) | 4 (17.4) | 4 (17.4) |
| Headache, n (%) | 4 (14.8) | 2 (7.4) | 4 (17.4) | 5 (21.7) |
| Pain in the limbs, n (%) | 0 (0.0) | 1 (3.7) | 1 (4.3) | 3 (13.0) |
| Crude | Adjusted a | |
|---|---|---|
| OR; 95% CI, p | OR; 95% CI, p | |
| EAT-26 score | 0.87; 0.77–0.98, 0.022 | 0.87; 0.76–0.98, 0.021 |
| Mental health score | 1.28; 1.05–1.56, 0.011 | 1.30; 1.06–1.59, 0.009 |
| Gut microbiota | ||
| Bacteroides | 0.94; 0.88–1.00, 0.048 | 0.94; 0.88–1.00, 0.050 |
| Blautia | 1.20; 1.04–1.38, 0.008 | 1.23; 1.05–1.44, 0.009 |
| Dorea | 0.93; 0.84–1.04, 0.18 | 0.93; 0.83–1.03, 0.17 |
| Parabacteroides | 0.35; 0.16–0.76, 0.007 | 0.34; 0.15–0.76, 0.007 |
| Actinomycesb | 1.10; 0.94–1.27, 0.227 | 1.11; 0.95–1.31, 0.182 |
| Streptococcusc | Inf., p = 0.002 | Inf., p = 0.004 |
| Gut mycobiota | ||
| Debaryomycesb | 0.61; 0.22–1.71, 0.34 | 0.58; 0.19–1.83, 0.36 |
| Kluyveromyces | 3.05; 0.49–19.0, 0.22 | 2.89; 0.48–17.5, 0.25 |
| Rhodotorulab | 1.10; 0.96–1.27, 0.17 | 1.13; 0.96–1.34, 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponzo, V.; Ferrocino, I.; Goitre, I.; Pellegrini, M.; Bruno, M.; Astegiano, M.; Cadario, G.; Castellana, E.; Bioletto, F.; Corvaglia, M.R.; et al. Non-Celiac Gluten/Wheat Sensitivity: Clinical Characteristics and Microbiota and Mycobiota Composition by Response to the Gluten Challenge Test. Nutrients 2021, 13, 1260. https://doi.org/10.3390/nu13041260
Ponzo V, Ferrocino I, Goitre I, Pellegrini M, Bruno M, Astegiano M, Cadario G, Castellana E, Bioletto F, Corvaglia MR, et al. Non-Celiac Gluten/Wheat Sensitivity: Clinical Characteristics and Microbiota and Mycobiota Composition by Response to the Gluten Challenge Test. Nutrients. 2021; 13(4):1260. https://doi.org/10.3390/nu13041260
Chicago/Turabian StylePonzo, Valentina, Ilario Ferrocino, Ilaria Goitre, Marianna Pellegrini, Mauro Bruno, Marco Astegiano, Gianni Cadario, Eleonora Castellana, Fabio Bioletto, Maria Rita Corvaglia, and et al. 2021. "Non-Celiac Gluten/Wheat Sensitivity: Clinical Characteristics and Microbiota and Mycobiota Composition by Response to the Gluten Challenge Test" Nutrients 13, no. 4: 1260. https://doi.org/10.3390/nu13041260
APA StylePonzo, V., Ferrocino, I., Goitre, I., Pellegrini, M., Bruno, M., Astegiano, M., Cadario, G., Castellana, E., Bioletto, F., Corvaglia, M. R., Malfa, P., Cocolin, L., Ghigo, E., & Bo, S. (2021). Non-Celiac Gluten/Wheat Sensitivity: Clinical Characteristics and Microbiota and Mycobiota Composition by Response to the Gluten Challenge Test. Nutrients, 13(4), 1260. https://doi.org/10.3390/nu13041260









