Role of Creatine in the Heart: Health and Disease
Abstract
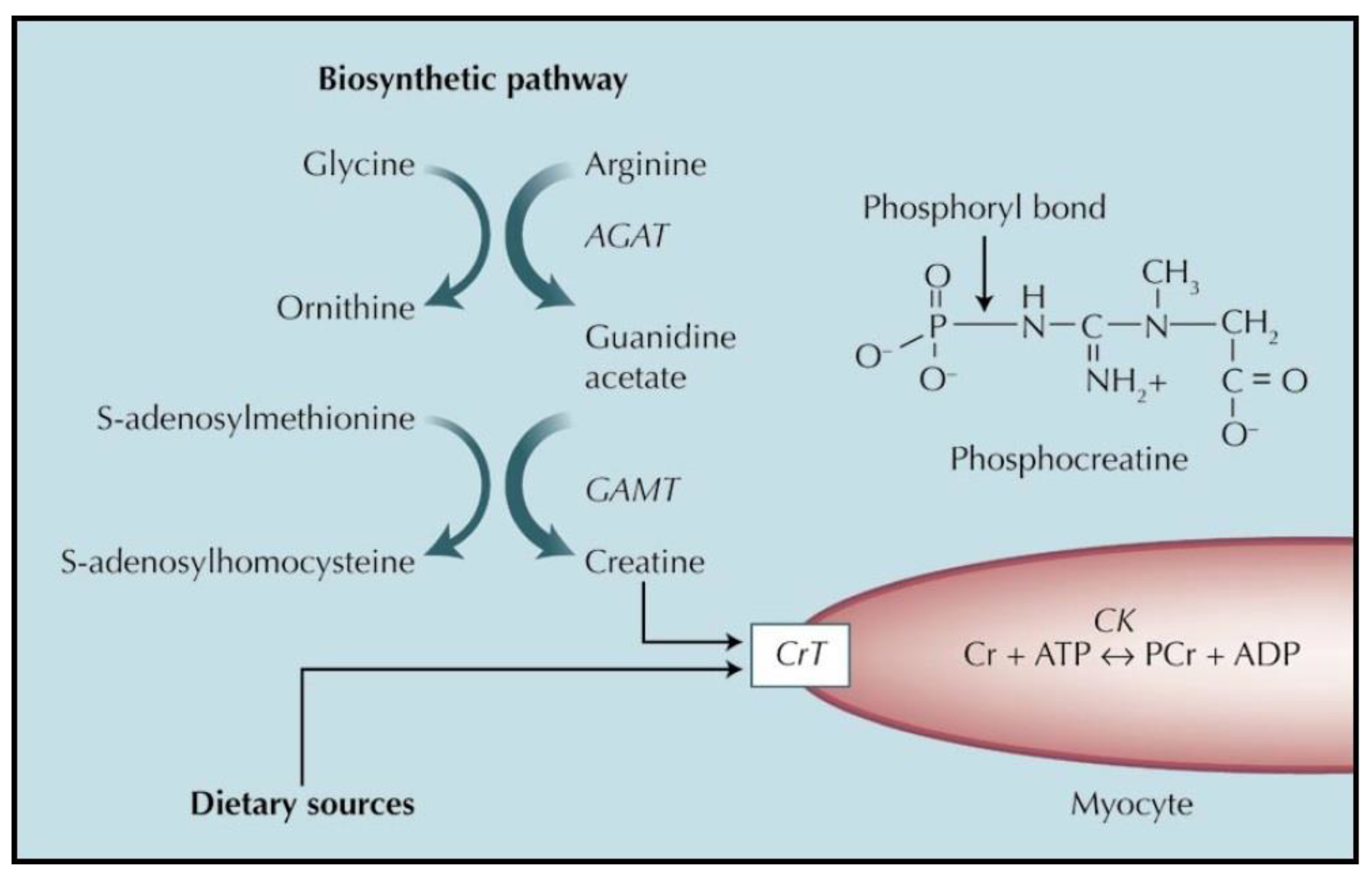
1. Metabolism and Role of Creatine
1.1. Functions of Creatine
1.2. Procurement of Creatine by the Organism, with Specific Reference to the Heart
1.2.1. Endogenous Synthesis of Creatine
1.2.2. Uptake of Creatine from Dietary or Supplement Sources
- Restoration of normal creatine content when it is lower than normal due to lifestyle (e.g., vegetarian or vegan subjects [26]) or to disease (e.g., heart failure, see below).
- Increase in energy availability (obtained by increasing phosphocreatine concentration in the tissue) in cases where the balance between energy availability and requirement is limited by decreased energy production (as is the case in hypoxia or ischemia), or by increased demand (e.g., the muscle of athletes during athletic performance).
2. Cardiac Effects of Creatine Supplementation in Healthy Subjects
2.1. Cardiac Effects of Creatine Supplementation in the Normal Heart
2.1.1. In Vitro Studies
2.1.2. In Vivo Studies
2.2. Considerations on the Effects and Safety of Creatine Supplementation in Healthy Subjects
3. Heart Diseases Where Creatine Supplementation May Be Useful
4. Creatine Supplementation in Heart Failure
4.1. Decrease in Creatine in Heart Failure
4.2. Effects of Decreasing Creatine on Cardiac Function
4.3. Effects of Creatine Supplementation in Heart Failure Patients
5. Creatine Supplementation in Heart Ischemia
5.1. Preclinical Studies
5.1.1. Effects of Decreasing Heart Creatine on Vulnerability to Ischemia
5.1.2. Effects of Creatine Supplementation on Ischemic Damage
5.2. Lack of Clinical Studies
5.3. The Use of Creatine Phosphate in Human Myocardial Infarction
6. Creatine Supplementation in Anthracycline Toxicity
6.1. Use and Adverse Effects of Anthracyclines
6.2. Studies Linking Anthracyclines Toxicity and Creatine Metabolism
6.3. Effects of Creatine Supplementation in Animal Models
6.4. Effects of Phosphocreatine
7. Concluding Remarks
8. Scientific Significance and Translational Opportunities
- In healthy hearts, there is currently no demonstration that creatine supplementation may improve cardiac function. However, creatine supplementation is safe, with the possible exception of subjects with renal failure (elevated plasma creatinine), thus fear of adverse events should not prevent willing subjects from trialing creatine supplementation.
- In heart failure, there is a decrease in the creatine content of the myocytes, and such a decrease is highly relevant from the clinical point of view. Moreover, creatine supplementation improves muscle function in these patients. Thus:
- Creatine supplementation should be trialed in heart failure patients, especially when weakness and fatigue are prominent symptoms.
- Further research should correlate, in individual patients, creatine and phosphocreatine content of the myocardium with the clinical benefits obtained from supplementation.
- Further research should be carried out on the effects of creatine supplementation in heart ischemia.
- Mitigation of anthracyclines toxicity is an unmet clinical need. Thus, treatment of oncological patients with anthracyclines might even now be preceded by an adequate period of creatine supplementation, possibly together with vitamins C and E, to prevent chemotherapy toxicity both to the heart and to the muscle. Moreover, research should be carried out in ample clinical cohorts to definitively determine the usefulness of this supplementation in anthracyclines chemotherapy.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventura-Clapier, R.; Vassort, G. The hypodynamic state of the frog heart. Further evidence for a phosphocreatine—Creatine pathway. J. Physiol. 1980, 76, 583–589. [Google Scholar]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The Creatine kinase system and pleiotropic Effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K.; Harris, R.C. The Creatine Kinase Reaction: A Simple reaction with functional complexity. Amino Acids 2011, 40, 1363–1367. [Google Scholar] [CrossRef]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and Safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, M.; Sarocchi, M.; Adriano, E.; Spallarossa, P. Potential of Creatine or phosphocreatine supplementation in cerebrovascular disease and in ischemic heart disease. Amino Acids 2016, 48, 1955–1967. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.; Greenhaff, P.L. Does Dietary Creatine Supplementation Play a Role in Skeletal Muscle Metabolism and Performance? Am. J. Clin. Nutr. 2000, 72, 607S–617S. [Google Scholar] [CrossRef] [PubMed]
- Hanna-El-Daher, L.; Braissant, O. Creatine synthesis and exchanges between brain cells: What can be learned from human creatine deficiencies and various experimental models? Amino Acids 2016, 48, 1877–1895. [Google Scholar] [CrossRef]
- Koszalka, T.R. Creatine synthesis in the testis. Proc. Soc. Exp. Biol. Med. 1968, 128, 1130–1137. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.H.; Chae, Y.J.; Ogawa, H.; Lee, M.H.; Gerton, G.L. Creatine synthesis and transport systems in the male rat reproductive tract. Biol. Reprod. 1998, 58, 1437–1444. [Google Scholar] [CrossRef][Green Version]
- Lygate, C.A.; Bohl, S.; ten Hove, M.; Faller, K.M.E.; Ostrowski, P.J.; Zervou, S.; Medway, D.J.; Aksentijevic, D.; Sebag-Montefiore, L.; Wallis, J.; et al. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc. Res. 2012, 96, 466–475. [Google Scholar] [CrossRef]
- Zervou, S.; Whittington, H.J.; Russell, A.J.; Lygate, C.A. Augmentation of creatine in the heart. Mini Rev. Med. Chem. 2016, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.E.; Yuen, A.H.Y.; Felkin, L.E.; Smolenski, R.T.; Hall, J.L.; Grindle, S.; Miller, L.W.; Birks, E.J.; Yacoub, M.H.; Barton, P.J.R. Myocardial Expression of the arginine: Glycine Amidinotransferase gene is elevated in heart failure and normalized after recovery: Potential implications for local Creatine synthesis. Circulation 2006, 114 (Suppl. 1), I16–I20. [Google Scholar] [CrossRef]
- Nekhorocheff, J. Degradation and synthesis of creatine in isolated toad heart. C. R. Hebd. Séance Acad. Sci. 1955, 240, 1284–1285. [Google Scholar]
- Fisher, R.B.; Wilhelmi, A.E. The Metabolism of creatine: The conversion of arginine into creatine in the isolated rabbit heart. Biochem. J. 1937, 31, 1136–1156. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.J.; Murphy, R.M. Creatine and the creatine transporter: A review. Mol. Cell Biochem. 2001, 224, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.P.; Wyse, A.T.S. Creatine as a Neuroprotector: An Actor that Can Play Many Parts. Neurotox Res. 2019, 423, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Ingwall, J.S. On the hypothesis that the failing heart is energy starved: Lessons learned from the metabolism of ATP and creatine. Curr. Hypertens. Rep. 2006, 8, 457–464. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef]
- Harris, R.C.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by Creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef]
- Ipsiroglu, O.S.; Stromberger, C.; Ilas, J.; Höger, H.; Mühl, A.; Stöckler-Ipsiroglu, S. Changes of tissue creatine concentrations upon oral supplementation of Creatine-monohydrate in various animal species. Life Sci. 2001, 69, 1805–1815. [Google Scholar] [CrossRef]
- Dechent, P.; Pouwels, P.J.W.; Wilken, B.; Hanefeld, F.; Frahm, J. Increase of total creatine in human brain after oral supplementation of Creatine-monohydrate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R698–R704. [Google Scholar] [CrossRef]
- Sweeney, H.L. The Importance of the Creatine Kinase Reaction: The Concept of Metabolic Capacitance. Med. Sci. Sports Exerc. 1994, 26, 30–36. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-based supplements for the enhancement of athletic performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14. [Google Scholar] [CrossRef]
- Blancquaert, L.; Baguet, A.; Bex, T.; Volkaert, A.; Everaert, I.; Delanghe, J.; Petrovic, M.; Vervaet, C.; De Henauw, S.; Constantin-Teodosiu, D.; et al. Changing to a vegetarian diet reduces the body creatine pool in omnivorous women, but appears not to affect carnitine and carnosine homeostasis: A randomised trial. Br. J. Nutr. 2018, 119, 759–770. [Google Scholar] [CrossRef]
- Guimbal, C.; Kilimann, M.W. A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney. CDNA cloning and functional expression. J. Biol. Chem. 1993, 268, 8418–8421. [Google Scholar] [CrossRef]
- Fischer, A.; Ten Hove, M.; Sebag-Montefiore, L.; Wagner, H.; Clarke, K.; Watkins, H.; Lygate, C.A.; Neubauer, S. Changes in creatine transporter function during cardiac maturation in the rat. BMC Dev. Biol. 2010, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.A.; Rosenshtraukh, L.V.; Undrovinas, A.I.; Smirnov, V.N.; Chazov, E.I. Studies of energy transport in heart cells. intracellular creatine content as a regulatory factor of frog heart energetics and force of contraction. Biochem. Med. 1976, 16, 21–36. [Google Scholar] [CrossRef]
- Rosenshtraukh, L.V.; Saks, V.A.; Undrovinas, A.I.; Chazov, E.I.; Smirnov, V.N.; Sharov, V.G. Studies of energy transport in heart cells. The effect of creatine phosphate on the frog ventricular contractile force and action potential duration. Biochem. Med. 1978, 19, 148–164. [Google Scholar] [CrossRef]
- Santacruz, L.; Arciniegas, A.J.L.; Darrabie, M.; Mantilla, J.G.; Baron, R.M.; Bowles, D.E.; Mishra, R.; Jacobs, D.O. Hypoxia decreases creatine uptake in cardiomyocytes, while creatine supplementation enhances HIF activation. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Kilian, G.; Jana, A.K.; Grant, G.D.; Milne, P.J. The Effects of creatine on the retrogradely perfused isolated rat heart. J. Pharm. 2002, 54, 105–109. [Google Scholar] [CrossRef]
- Horn, M.; Frantz, S.; Remkes, H.; Laser, A.; Urban, B.; Mettenleiter, A.; Schnackerz, K.; Neubauer, S. Effects of chronic dietary Creatine feeding on cardiac energy metabolism and on creatine content in heart, skeletal muscle, brain, liver and kidney. J. Mol. Cell Cardiol. 1998, 30, 277–284. [Google Scholar] [CrossRef]
- Neubauer, S.; Remkes, H.; Spindler, M.; Horn, M.; Wiesmann, F.; Prestle, J.; Walzel, B.; Ertl, G.; Hasenfuss, G.; Wallimann, T. Downregulation of the Na(+)-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation 1999, 100, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Nascimben, L.; Ingwall, J.S.; Pauletto, P.; Friedrich, J.; Gwathmey, J.K.; Saks, V.; Pessina, A.C.; Allen, P.D. Creatine kinase system in failing and nonfailing human myocardium. Circulation 1996, 94, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Remkes, H.; Dienesch, C.; Hu, K.; Ertl, G.; Neubauer, S. Chronic high-dose creatine feeding does not attenuate left ventricular remodeling in rat hearts post-myocardial infarction. Cardiovasc. Res. 1999, 43, 117–124. [Google Scholar] [CrossRef][Green Version]
- del Favero, S.; Roschel, H.; Artioli, G.; Ugrinowitsch, C.; Tricoli, V.; Costa, A.; Barroso, R.; Negrelli, A.L.; Otaduy, M.C.; da Costa Leite, C.; et al. Creatine but not Betaine supplementation increases muscle Phosphorylcreatine content and strength performance. Amino Acids 2012, 42, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Op’t Eijnde, B.; Jijakli, H.; Hespel, P.; Malaisse, W.J. Creatine supplementation increases soleus muscle creatine content and lowers the insulinogenic index in an animal model of inherited Type 2 Diabetes. Int. J. Mol. Med. 2006, 17, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.; Chan, S.; Monfared, M.; Wallimann, T.; Clarke, K.; Neubauer, S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E399–E406. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Ten Hove, M.; Schneider, J.E.; Wu, C.O.; Sebag-Montefiore, L.; Aponte, A.M.; Lygate, C.A.; Wallis, J.; Clarke, K.; Watkins, H.; et al. Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity. J. Mol. Cell Cardiol. 2010, 48, 582–590. [Google Scholar] [CrossRef]
- Wallis, J.; Lygate, C.A.; Fischer, A.; ten Hove, M.; Schneider, J.E.; Sebag-Montefiore, L.; Dawson, D.; Hulbert, K.; Zhang, W.; Zhang, M.H.; et al. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: Insights from creatine transporter-overexpressing transgenic mice. Circulation 2005, 112, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, L.; Hernandez, A.; Nienaber, J.; Mishra, R.; Pinilla, M.; Burchette, J.; Mao, L.; Rockman, H.A.; Jacobs, D.O. Normal cardiac function in mice with supraphysiological cardiac creatine levels. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H373–H381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zervou, S.; Yin, X.; Nabeebaccus, A.A.; O’Brien, B.A.; Cross, R.L.; McAndrew, D.J.; Atkinson, R.A.; Eykyn, T.R.; Mayr, M.; Neubauer, S.; et al. Proteomic and metabolomic changes driven by elevating myocardial creatine suggest novel metabolic feedback mechanisms. Amino Acids 2016, 48, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Mert, K.; Ilgüy, S.; Dural, M.; Mert, G.; Ozakin, E. Effects of creatine supplementation on cardiac autonomic functions in bodybuilders. Pacing Clin. Electrophysiol. 2017, 40. [Google Scholar] [CrossRef]
- Nanchen, D. Resting heart rate: What is normal? Heart 2018, 104, 1048–1049. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. Heart. J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Feinstein, M.B. Effects of experimental congestive heart failure, ouabain, and asphyxia on the high-energy phosphate and creatine content of the guinea pig heart. Circ. Res. 1962, 10, 333–346. [Google Scholar] [CrossRef]
- Fox, A.C.; Wikler, N.S.; Reed, G.E. High energy phosphate compounds in the myocardium during experimental congestive heart failure. purine and pyrimidine nucleotides, creatine, and creatine phosphate in normal and in failing hearts. J. Clin. Investig. 1965, 44, 202–218. [Google Scholar] [CrossRef]
- Shen, W.; Asai, K.; Uechi, M.; Mathier, M.A.; Shannon, R.P.; Vatner, S.F.; Ingwall, J.S. Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs: A compensatory role for the parallel loss of creatine. Circulation 1999, 100, 2113–2118. [Google Scholar] [CrossRef]
- Ten Hove, M.; Chan, S.; Lygate, C.; Monfared, M.; Boehm, E.; Hulbert, K.; Watkins, H.; Clarke, K.; Neubauer, S. Mechanisms of Creatine Depletion in Chronically Failing Rat Heart. J. Mol. Cell Cardiol. 2005, 38, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.L.; Castro, P.; Meneses, L.; Chalhub, M.; Verdejo, H.; Greig, D.; Gabrielli, L.; Chiong, M.; Concepción, R.; Mellado, R.; et al. Myocardial Lipids and Creatine Measured by Magnetic Resonance Spectroscopy among Patients with Heart Failure. Rev. Méd. Chile 2010, 138, 1475–1479. [Google Scholar] [CrossRef][Green Version]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial Phosphocreatine-to-ATP Ratio Is a Predictor of Mortality in Patients with Dilated Cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Nakae, I.; Mitsunami, K.; Matsuo, S.; Matsumoto, T.; Morikawa, S.; Inubushi, T.; Koh, T.; Horie, M. Assessment of Myocardial Creatine Concentration in Dysfunctional Human Heart by Proton Magnetic Resonance Spectroscopy. Magn. Reason. Med. Sci. 2004, 3, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Nakae, I.; Mitsunami, K.; Matsuo, S.; Inubushi, T.; Morikawa, S.; Tsutamoto, T.; Koh, T.; Horie, M. Myocardial Creatine Concentration in Various Nonischemic Heart Diseases Assessed by 1H Magnetic Resonance Spectroscopy. Circ. J. 2005, 69, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S. The Failing Heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Ten Hove, M.; Lygate, C.A.; Fischer, A.; Schneider, J.E.; Sang, A.E.; Hulbert, K.; Sebag-Montefiore, L.; Watkins, H.; Clarke, K.; Isbrandt, D.; et al. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient Guanidinoacetate-N-methyltransferase–knockout mice. Circulation 2005, 111, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Kapelko, V.I.; Saks, V.A.; Novikova, N.A.; Golikov, M.A.; Kupriyanov, V.V.; Popovich, M.I. Adaptation of Cardiac Contractile Function to Conditions of Chronic Energy Deficiency. J. Mol. Cell Cardiol. 1989, 21 (Suppl. 1), 79–83. [Google Scholar] [CrossRef]
- Field, M.L. Creatine Supplementation in congestive heart failure. Cardiovasc. Res. 1996, 31, 174–176. [Google Scholar] [CrossRef]
- Faller, K.M.E.; Medway, D.J.; Aksentijevic, D.; Sebag-Montefiore, L.; Schneider, J.E.; Lygate, C.A.; Neubauer, S. Ribose supplementation alone or with elevated creatine does not preserve high energy nucleotides or cardiac function in the failing mouse heart. PLoS ONE 2013, 8, e66461. [Google Scholar] [CrossRef]
- Ahmed, M.; Anderson, S.D.; Schofield, R.S. Coenzyme Q10 and creatine in heart failure: Micronutrients, macrobenefit? Clin. Cardiol. 2011, 34, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Fattirolli, F.; Guarducci, L.; Cellai, T.; Baldasseroni, S.; Tarantini, F.; Di Bari, M.; Masotti, G.; Marchionni, N. Coenzyme Q10 Terclatrate and creatine in chronic heart failure: A randomized, placebo-controlled, double-blind study. Clin. Cardiol. 2011, 34, 211–217. [Google Scholar] [CrossRef]
- Carvalho, A.P.P.F.; Rassi, S.; Fontana, K.E.; Correa, K.S.; Feitosa, R.H.F. Influence of creatine supplementation on the functional capacity of patients with heart failure. Arq. Bras. Cardiol. 2012, 99, 623–629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gordon, A.; Hultman, E.; Kaijser, L.; Kristjansson, S.; Rolf, C.J.; Nyquist, O.; Sylvén, C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc. Res. 1995, 30, 413–418. [Google Scholar] [CrossRef]
- Andrews, R.; Greenhaff, P.; Curtis, S.; Perry, A.; Cowley, A.J. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur. Heart J. 1998, 19, 617–622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaufelberger, M.; Swedberg, K. Is Creatine supplementation helpful for patients with chronic heart failure? Eur. Heart J. 1998, 19, 533–534. [Google Scholar] [CrossRef]
- Kuethe, F.; Krack, A.; Richartz, B.M.; Figulla, H.R. Creatine supplementation improves muscle strength in patients with congestive heart failure. Pharmazie 2006, 61, 218–222. [Google Scholar] [PubMed]
- Perasso, L.; Spallarossa, P.; Gandolfo, C.; Ruggeri, P.; Balestrino, M. Therapeutic use of creatine in brain or heart ischemia: Available data and future perspectives. Med. Res. Rev. 2013, 33, 336–363. [Google Scholar] [CrossRef] [PubMed]
- Lygate, C.A.; Aksentijevic, D.; Dawson, D.; ten Hove, M.; Phillips, D.; de Bono, J.P.; Medway, D.J.; Sebag-Montefiore, L.; Hunyor, I.; Channon, K.M.; et al. Living without creatine: Unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice. Circ. Res. 2013, 112, 945–955. [Google Scholar] [CrossRef]
- Kan, H.E.; Renema, W.K.J.; Isbrandt, D.; Heerschap, A. Phosphorylated guanidinoacetate partly compensates for the lack of phosphocreatine in skeletal muscle of mice lacking guanidinoacetate methyltransferase. J. Physiol. 2004, 560, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Remkes, H.; Strömer, H.; Dienesch, C.; Neubauer, S. Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation 2001, 104, 1844–1849. [Google Scholar] [CrossRef]
- Webster, I.; Toit, E.F.D.; Huisamen, B.; Lochner, A. The effect of creatine supplementation on myocardial function, mitochondrial respiration and susceptibility to ischaemia/reperfusion injury in sedentary and exercised rats. Acta Physiol. 2012, 206, 6–19. [Google Scholar] [CrossRef]
- Horjus, D.L.; Oudman, I.; van Montfrans, G.A.; Brewster, L.M. Creatine and creatine analogues in hypertension and cardiovascular disease. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Perasso, L.; Lunardi, G.L.; Risso, F.; Pohvozcheva, A.V.; Leko, M.V.; Gandolfo, C.; Florio, T.; Cupello, A.; Burov, S.V.; Balestrino, M. Protective effects of some creatine derivatives in brain tissue anoxia. Neurochem. Res. 2008, 33, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Soboll, S.; Conrad, A.; Eistert, A.; Herick, K.; Krämer, R. Uptake of Creatine Phosphate into heart mitochondria: A leak in the creatine shuttle. Biochim. Biophys. Acta 1997, 1320, 27–33. [Google Scholar] [CrossRef][Green Version]
- Preobrazhenskiĭ, A.N.; Dzhavadov, S.A.; Saks, V.A. Possible mechanism of the protective effect of phosphocreatine on the ischemic myocardium. Biokhimiia 1986, 51, 675–683. [Google Scholar] [PubMed]
- Saks, V.A.; Dzhaliashvili, I.V.; Konorev, E.A.; Strumia, E. Molecular and cellular aspects of the cardioprotective mechanism of phosphocreatine. Biokhimiia 1992, 57, 1763–1784. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Epand, R.F.; Meiler, F.; Zandomeneghi, G.; Neumann, D.; Widmer, H.R.; Meier, B.H.; Epand, R.M.; Saks, V.; Wallimann, T.; et al. Phosphocreatine interacts with phospholipids, affects membrane properties and exerts membrane-protective Effects. PLoS ONE 2012, 7, e43178. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, E.; Dobrovolsky, A.; Rogoza, A.; Sorokin, E.; Ageeva, N.; Markova, L.; Titaeva, E.; Anuchin, V.; Karpov, Y.; Saks, V. The effect of exogenous phosphocreatine on maximal walking distance, blood rheology, platelet aggregation, and fibrinolysis in patients with intermittent claudication. Int. Angiol. 1994, 13, 59–64. [Google Scholar]
- Saks, V.A.; Strumia, E. Phosphocreatine: Molecular and cellular aspects of the mechanism of cardioprotective action. Curr. Ther. Res. 1993, 53, 565–598. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Rivankar, S. An Overview of doxorubicin formulations in cancer therapy. J. Cancer Res. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Saleem, T.; Kasi, A. Daunorubicin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Berthiaume, J.M.; Wallace, K.B. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol. Toxicol. 2007, 23, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.S.; Lenihan, D.J.; Ky, B. Cancer therapy-induced cardiotoxicity: Basic mechanisms and potential cardioprotective therapies. J. Am. Heart Assoc. 2014, 3, e000665. [Google Scholar] [CrossRef] [PubMed]
- DeAtley, S.M.; Aksenov, M.Y.; Aksenova, M.V.; Jordan, B.; Carney, J.M.; Butterfield, D.A. Adriamycin-induced changes of creatine kinase activity in vivo and in cardiomyocyte culture. Toxicology 1999, 134, 51–62. [Google Scholar] [CrossRef]
- Darrabie, M.D.; Arciniegas, A.J.L.; Mantilla, J.G.; Mishra, R.; Vera, M.P.; Santacruz, L.; Jacobs, D.O. Exposing cardiomyocytes to subclinical concentrations of doxorubicin rapidly reduces their creatine transport. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H539–H548. [Google Scholar] [CrossRef] [PubMed]
- Tokarska-Schlattner, M.; Wallimann, T.; Schlattner, U. Multiple interference of anthracyclines with mitochondrial creatine kinases: Preferential damage of the cardiac isoenzyme and its implications for drug cardiotoxicity. Mol. Pharm. 2002, 61, 516–523. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Dolder, M.; Gerber, I.; Speer, O.; Wallimann, T.; Schlattner, U. Reduced creatine-stimulated respiration in doxorubicin challenged mitochondria: Particular sensitivity of the heart. Biochim. Biophys. Acta 2007, 1767, 1276–1284. [Google Scholar] [CrossRef]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef]
- Gupta, A.; Rohlfsen, C.; Leppo, M.K.; Chacko, V.P.; Wang, Y.; Steenbergen, C.; Weiss, R.G. Creatine kinase-overexpression improves myocardial energetics, contractile dysfunction and survival in murine doxorubicin cardiotoxicity. PLoS ONE 2013, 8, e74675. [Google Scholar] [CrossRef]
- Aksentijević, D.; Zervou, S.; Faller, K.M.E.; McAndrew, D.J.; Schneider, J.E.; Neubauer, S.; Lygate, C.A. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS ONE 2014, 9, e109021. [Google Scholar] [CrossRef]
- Santos, R.V.T.; Batista, M.L.; Caperuto, E.C.; Costa Rosa, L.F. Chronic supplementation of creatine and vitamins C and E increases survival and improves biochemical parameters after doxorubicin treatment in rats. Clin. Exp. Pharm. Physiol. 2007, 34, 1294–1299. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. Vitam. Horm. 2007, 76, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, L.; Darrabie, M.D.; Mantilla, J.G.; Mishra, R.; Feger, B.J.; Jacobs, D.O. Creatine supplementation reduces doxorubicin-induced cardiomyocellular injury. Cardiovasc. Toxicol. 2015, 15, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Bredahl, E.C.; Hydock, D.S. Creatine supplementation and doxorubicin-induced skeletal muscle dysfunction: An ex vivo investigation. Nutr. Cancer 2017, 69, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Torok, Z.A.; Busekrus, R.B.; Hydock, D.S. Effects of creatine supplementation on muscle fatigue in rats receiving doxorubicin treatment. Nutr. Cancer 2020, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.U.; Zhi-Hui, H.E.; Li-Ying, X.; Xiao-Tong, S.; Jie, L.; Jing-Yi, F.; Yu, W.; Ming, Z. Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2016, 32, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Guo, X.; Fu, Y.; Long, J.; Wei, C.-X.; Zhao, M. Creatine phosphate disodium salt protects against dox-induced cardiotoxicity by increasing calumenin. Med. Mol. Morphol. 2018, 51, 96–101. [Google Scholar] [CrossRef]
- Parve, S.; Aliakberova, G.I.; Gylmanov, A.A.; Abdulganieva, D.I. Role of exogenous phosphocreatine in chemotherapy-induced cardiomyopathy. Rev. Cardiovasc. Med. 2017, 18, 82–87. [Google Scholar]
- Cheuk, D.K.L.; Sieswerda, E.; van Dalen, E.C.; Postma, A.; Kremer, L.C.M. medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016, CD008011. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. https://doi.org/10.3390/nu13041215
Balestrino M. Role of Creatine in the Heart: Health and Disease. Nutrients. 2021; 13(4):1215. https://doi.org/10.3390/nu13041215
Chicago/Turabian StyleBalestrino, Maurizio. 2021. "Role of Creatine in the Heart: Health and Disease" Nutrients 13, no. 4: 1215. https://doi.org/10.3390/nu13041215
APA StyleBalestrino, M. (2021). Role of Creatine in the Heart: Health and Disease. Nutrients, 13(4), 1215. https://doi.org/10.3390/nu13041215





