Management of Childhood Obesity—Time to Shift from Generalized to Personalized Intervention Strategies
Abstract
1. Introduction
2. Weight-Related Behaviors in Children
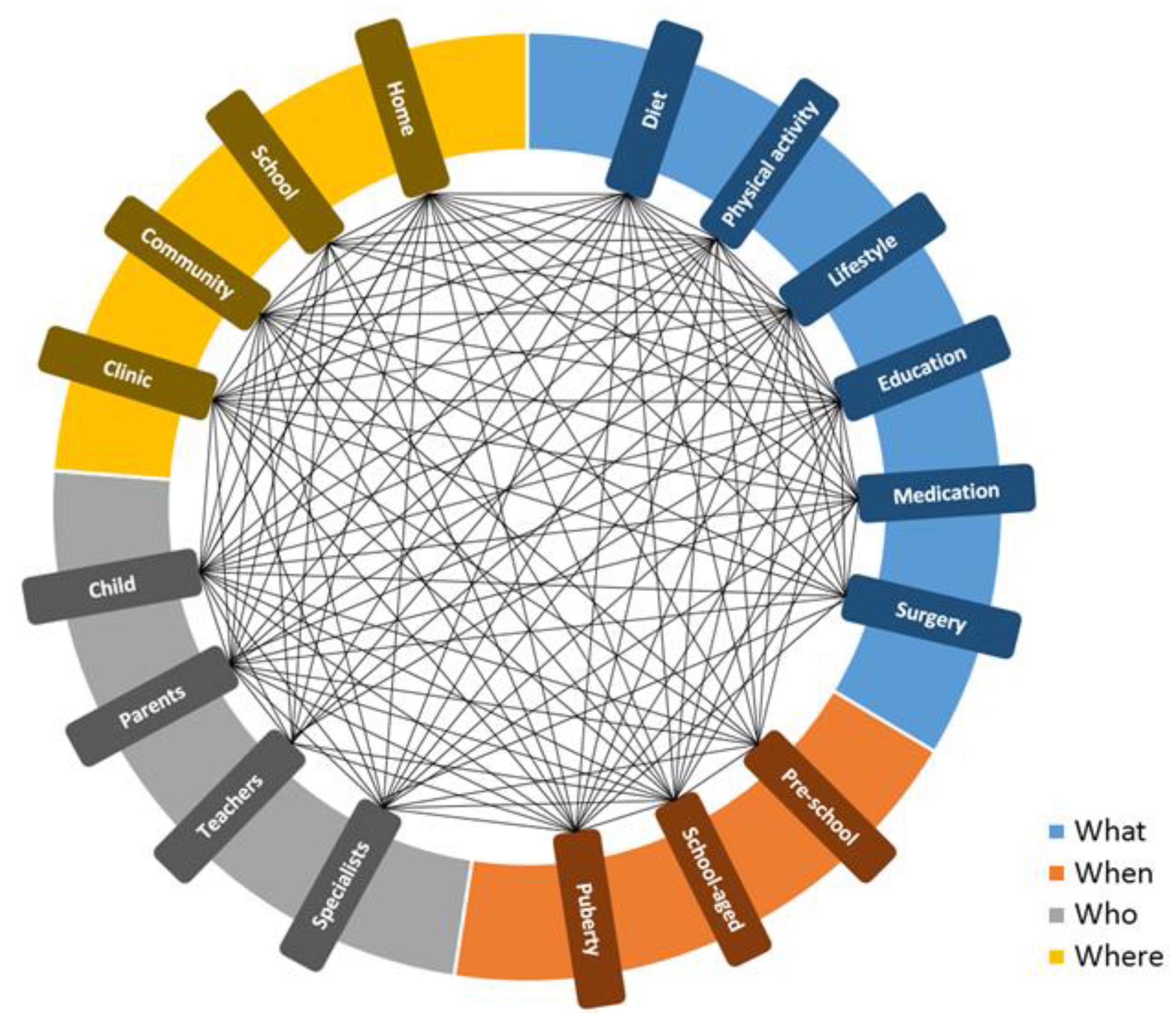
3. Strategies for the Prevention and Treatment of Childhood Obesity
3.1. Intervention Components
3.1.1. Diet
3.1.2. Physical Activity
3.1.3. Lifestyle and Education
3.2. Intervention Settings
3.2.1. Home
3.2.2. School
3.2.3. Community and Clinics
4. Personalized Strategies
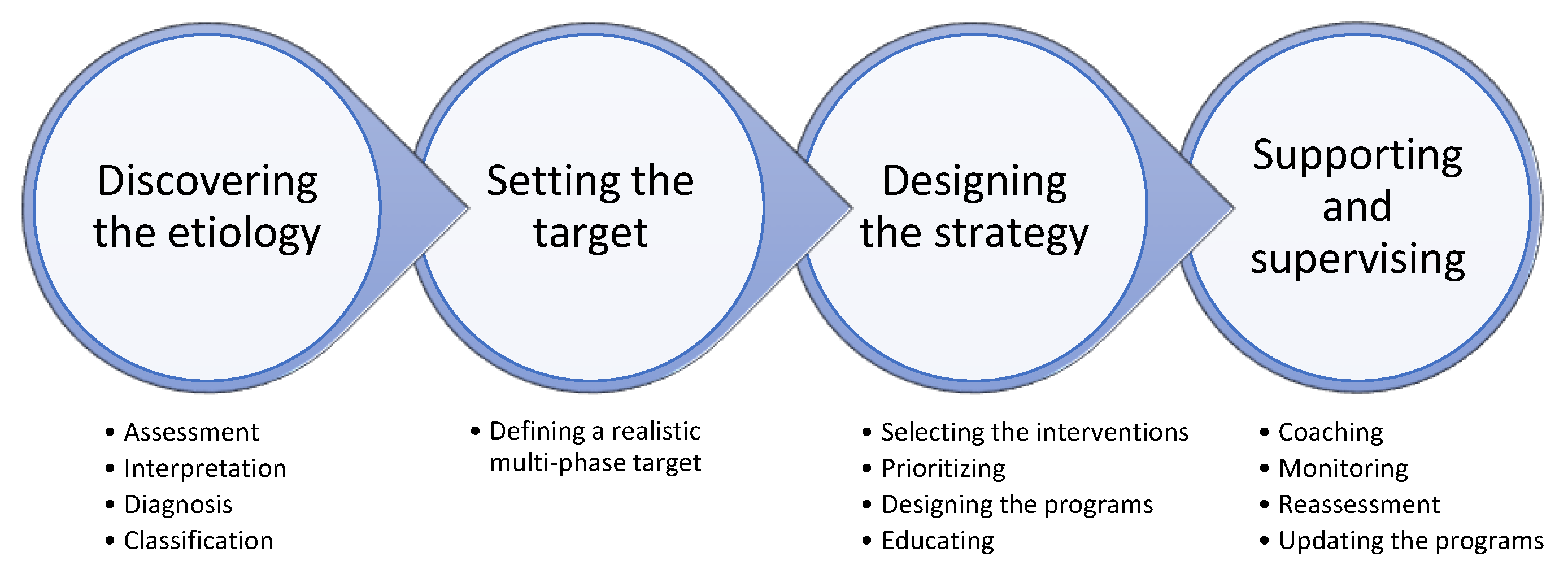
5. EPISTCO Model
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, J.D.; Fu, E.; Kobayashi, M.A. Prevention and management of childhood obesity and its psychological and health comorbidities. Annu. Rev. Clin. Psychol. 2020, 16, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, T.; Motil, K.J.; Moreno, J.P. Multi-etiological perspective on child obesity prevention. Curr. Nutr. Rep. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Gehanno, J.-F.; Gehanno, B.; Schuers, M.; Grosjean, J.; Rollin, L. Analysis of publication trends in childhood obesity research in PubMed since 1945. Child. Obes. 2019, 15, 227–236. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Lobstein, T.; Baur, L.; Uauy, R. IASO International Obesity TaskForce. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 5, 4–104. [Google Scholar] [CrossRef] [PubMed]
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary patterns and childhood obesity risk: A systematic review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.D.; Zuelch, M.L.; Dimitratos, S.M.; Scherr, R.E. Adolescent obesity: Diet quality, psychosocial health, and cardiometabolic risk factors. Nutrients 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.L.; Arenaza, L.; Medrano, M.; García, N.; Cadenas-Sanchez, C.; Ortega, F.B. Associations between the adherence to the Mediterranean diet and cardiorespiratory fitness with total and central obesity in preschool children: The PREFIT project. Eur. J. Nutr. 2018, 57, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, L.; La Ruche, G.; Tarantola, A.; Barboza, P.; Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Eurosurveillance 2009, 14, 19309. [Google Scholar] [CrossRef]
- Khan, A.S.; Hichami, A.; Khan, N.A. Obesity and COVID-19: Oro-naso-sensory perception. J. Clin. Med. 2020, 9, 2158. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.-N.; Louwen, F.; Yuan, J. Obesity and COVID-19: Molecular mechanisms linking both pandemics. Int. J. Mol. Sci. 2020, 21, 5793. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Llewellyn, C.H.; Smith, L. The obesity epidemic—Nature via nurture: A narrative review of high-income countries. SAGE Open Med. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xue, Y. Pediatric obesity: Causes, symptoms, prevention and treatment. Exp. Ther. Med. 2016, 11, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.R.; Fox, C.K.; Kelly, A.S. Treatment options for severe obesity in the pediatric population: Current limitations and future opportunities. Obesity 2018, 26, 951–960. [Google Scholar] [CrossRef]
- Sisson, S.B.; Krampe, M.; Anundson, K.; Castle, S. Obesity prevention and obesogenic behavior interventions in child care: A systematic review. Prev. Med. 2016, 87, 57–69. [Google Scholar] [CrossRef]
- Russell, C.G.; Russell, A. A biopsychosocial approach to processes and pathways in the development of overweight and obesity in childhood: Insights from developmental theory and research. Obes. Rev. 2019, 20, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Egan, K.N.; Montaño, Z.; Dawson-McClure, S.; Jake-Schoffman, D.E.; Larson, M.; St. George, S.M. A developmental cascade perspective of paediatric obesity: A conceptual model and scoping review. Health Psychol. Rev. 2018, 12, 271–293. [Google Scholar] [CrossRef]
- Rosenbaum, D.L.; White, K.S. Understanding the complexity of biopsychosocial factors in the public health epidemic of overweight and obesity. Health Psychol. Open 2016, 3. [Google Scholar] [CrossRef]
- Drenowatz, C.; Greier, K. The role of energy flux in weight management. Exerc. Med. 2017, 1, 4. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric obesity—Assessment, treatment, and prevention: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Weihrauch-Blüher, S.; Kromeyer-Hauschild, K.; Graf, C.; Widhalm, K.; Korsten-Reck, U.; Jödicke, B.; Markert, J.; Müller, M.J.; Moss, A.; Wabitsch, M.; et al. Current guidelines for obesity prevention in childhood and adolescence. Obes. Facts 2018, 11, 263–276. [Google Scholar] [CrossRef]
- Kim, J.; Lim, H. Nutritional management in childhood obesity. J. Obes. Metab. Syndr. 2019, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Marcos, A.; Wärnberg, J.; Martí, A.; Martin-Matillas, M.; Campoy, C.; Moreno, L.A.; Veiga, O.; Redondo-Figuero, C.; Garagorri, J.M.; et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity 2009, 17, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Tucker, K.L. Dietary quality of the US child and adolescent population: Trends from 1999 to 2012 and associations with the use of federal nutrition assistance programs. Am. J. Clin. Nutr. 2017, 105, 194–202. [Google Scholar] [CrossRef]
- Suskind, D.L. Nutritional deficiencies during normal growth. Pediatr. Clin. N. Am. 2009, 56, 1035–1053. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child. Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/sleep/data_statistics.html (accessed on 18 December 2020).
- Barnett, T.A.; Kelly, A.S.; Young, D.R.; Perry, C.K.; Pratt, C.A. Edwards, N.M.; Rao, G.; Vos, M.B. Sedentary behaviors in today’s youth: Approaches to the prevention and management of childhood obesity: A scientific statement from the American Heart Association. Circulation 2018, 138, e142–e159. [Google Scholar] [CrossRef] [PubMed]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2016, 12, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Gillman, M.W.; Peña, M.M.; Redline, S.; Rifas-Shiman, S.L. Chronic sleep curtailment and adiposity. Pediatrics 2014, 133, 1013–1022. [Google Scholar] [CrossRef]
- Miller, A.L.; Lumeng, J.C.; LeBourgeois, M.K. Sleep patterns and obesity in childhood. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 41–47. [Google Scholar] [CrossRef]
- Guan, H.; Okely, A.D.; Aguilar-Farias, N.; Del Pozo Cruz, B.; Draper, C.E.; El Hamdouchi, A.; Florindo, A.A.; Jáuregui, A.; Katzmarzyk, P.T.; Kontsevaya, A.; et al. Promoting healthy movement behaviours among children during the COVID-19 pandemic. Lancet Child Adolesc. Health 2020, 4, 416–418. [Google Scholar] [CrossRef]
- Robinson, T.N.; Banda, J.A.; Hale, L.; Lu, A.S.; Fleming-Milici, F.; Calvert, S.L.; Wartella, E. Screen media exposure and obesity in children and adolescents. Pediatrics 2017, 140, S97–S101. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Guan, S. Screen time and sleep among school-aged children and adolescents: A systematic literature review. Sleep Med. Rev. 2015, 21, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Atkin, A.J.; Sharp, S.J.; Corder, K.; van Sluijs, E.M.; ICAD Collaborators. Prevalence and correlates of screen time in youth: An international perspective. Am. J. Prev. Med. 2014, 47, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; George, M.; Russell, M.; Odgers, C. Young adolescents’ digital technology use and mental health symptoms: Little evidence of longitudinal or daily linkages. Clin. Psychol. Sci. 2019, 7, 1416–1433. [Google Scholar] [CrossRef] [PubMed]
- Rideout, V. Measuring time spent with media: The common sense census of media use by US 8- to 18-year-olds. J. Child. Media 2016, 10, 138–144. [Google Scholar] [CrossRef]
- Weaver, R.G.; Armstrong, B.; Hunt, E.; Beets, M.W.; Brazendale, K.; Dugger, R.; Turner-McGrievy, G.; Pate, R.R.; Maydeu-Olivares, A.; Saelens, B.; et al. The impact of summer vacation on children’s obesogenic behaviors and body mass index: A natural experiment. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 153. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef]
- Brown, T.; Moore, T.H.; Hooper, L.; Gao, Y.; Zayegh, A.; Ijaz, S.; Elwenspoek, M.; Foxen, S.C.; Magee, L.; O’Malley, C.; et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2019, 7, CD001871. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.; Wu, Y.; Wilson, R.F.; Weston, C.; Fawole, O.; Bleich, S.N.; Cheskin, L.J.; Showell, N.N.; Lau, B.D.; et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 547–565. [Google Scholar] [CrossRef]
- Chao, A.M.; Wadden, T.A.; Berkowitz, R.I. The safety of pharmacologic treatment for pediatric obesity. Expert Opin. Drug Saf. 2018, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.E.; Mitsopoulou, D.; Katsilambros, N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan nutrition for mothers and children: Practical tools for healthcare providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef]
- Wirnitzer, K.C. Vegan diet in sports and exercise—health benefits and advantages to athletes and physically active people: A narrative review. Int. J. Sports Exerc. Med. 2020, 6, 165. [Google Scholar] [CrossRef]
- Kaisari, P.; Yannakoulia, M.; Panagiotakos, D.B. Eating frequency and overweight and obesity in children and adolescents: A meta-analysis. Pediatrics 2013, 131, 958–967. [Google Scholar] [CrossRef]
- Barnard, N.; Kahleova, H.; Levin, S. The Use of Plant-Based Diets for Obesity Treatment. Int. J. Dis. Reversal Prev. 2019, 1, 12. [Google Scholar]
- Greger, M. A whole food plant-based diet is effective for weight loss: The evidence. Am. J. Lifestyle Med. 2020, 14, 500–510. [Google Scholar] [CrossRef]
- Tran, E.; Dale, H.F.; Jensen, C.; Lied, G.A. Effects of plant-based diets on weight status: A systematic review. Diabetes Metab. Syndr. Obes. 2020, 13, 3433–3448. [Google Scholar] [CrossRef]
- Sabaté, J.; Wien, M. Vegetarian diets and childhood obesity prevention. Am. J. Clin. Nutr. 2010, 91, 1525S–1529S. [Google Scholar] [CrossRef]
- Bailey, C.J.; Drummond, M.J.; Ward, P.R. Food literacy programmes in secondary schools: A systematic literature review and narrative synthesis of quantitative and qualitative evidence. Public Health Nutr. 2019, 22, 2891–2913. [Google Scholar] [CrossRef]
- Dey, M.; Kashyap, P.C. A diet for healthy weight: Why reaching a consensus seems difficult. Nutrients 2020, 12, 2997. [Google Scholar] [CrossRef]
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and diet in the prevention of chronic diseases in future generations. Int. J. Mol. Sci. 2020, 21, 2633. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.A.; Sainsbury, A. Strategies to improve adherence to dietary weight loss interventions in research and real-World settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Lambert. M.; Mathieu. M.E.; Tremblay, M.S.; O’ Loughlin, J.; Tremblay, A. Physical activity vs. sedentary time: Independent associations with adiposity in children. Pediatr. Obes. 2012, 7, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Melby, C.L.; Paris, H.L.; Sayer, R.D.; Bell, C.; Hill, J.O. Increasing energy flux to maintain diet-induced weight loss. Nutrients 2019, 11, 2533. [Google Scholar] [CrossRef]
- Hand, G.A.; Shook, R.P.; Hill, J.O.; Giacobbi, P.R.; Blair, S.N. Energy flux: Staying in energy balance at a high level is necessary to prevent weight gain for most people. Expert Rev. Endocrinol. Metab. 2015, 10, 599–605. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Thang, C.; Whitley, M.; Izadpanah, N.; DeUgarte, D.; Slusser, W. Barriers and comorbidities from a pediatric multidisciplinary tertiary care obesity program. J. Child. Obes. 2017, 2, 2. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Carraça, E.V.; Markland, D.; Silva, M.N.; Ryan, R.M. Exercise, physical activity, and self-determination theory: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 78. [Google Scholar] [CrossRef]
- Woods, J.A.; Hutchinson, N.T.; Powers, S.K.; Robertsd, W.O.; Gomez-Cabrera, M.C.; Radak, Z.; Berkes, I.; Boros, A.; Boldogh, I.; Leeuwenburgh, C.; et al. The COVID-19 pandemic and physical activity. Sports Med. Health Sci. 2020, 2, 55–64. [Google Scholar] [CrossRef]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef]
- Natale, R.A.; Messiah, S.E.; Asfour, L.S.; Uhlhorn, S.B.; Englebert, N.E.; Arheart, K.L. Obesity prevention program in childcare centers: Two-year follow-up. Am. J. Health Promot. 2017, 31, 502–510. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, G.A.; Cook, L.; Spruijt-Metz, D.; Black, D.S. Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obes. Rev. 2014, 15, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Haubenreiser, M.; Johnson, M.; Nordby, K.; Aggarwal, S.; Myer, S.; Thomas, C. Mindfulness approaches and weight loss, weight maintenance, and weight regain. Curr. Obes. Rep. 2018, 7, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.P.I.; Claassen, M.A.; Klein, O. The time is ripe: Thinking about the future reduces unhealthy eating in those with a higher BMI. Foods 2020, 9, 1391. [Google Scholar] [CrossRef]
- Paulson, S.; Davidson, R.; Jha, A.; Kabat-Zinn, J. Becoming conscious: The science of mindfulness. Ann. N. Y. Acad. Sci. 2013, 1303, 87–104. [Google Scholar] [CrossRef]
- Black, D.S.; Fernando, R. Mindfulness Training and classroom behavior among lower-income and ethnic minority elementary school children. J. Child Fam. Stud. 2014, 23, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.N.; Hawley, N.L.; Wing, R.R. Development of a behavioral sleep intervention as a novel approach for pediatric obesity in school-aged children. Pediatr. Clin. N. Am. 2016, 63, 511–523. [Google Scholar] [CrossRef]
- Hart, C.N.; Carskadon, M.A.; Considine, R.V.; Fava, J.L.; Lawton, J.; Raynor, H.A.; Jelalian, E.; Owens, J.; Wing, R. Changes in children’s sleep duration on food intake, weight, and leptin. Pediatrics 2013, 132, e1473–e1480. [Google Scholar] [CrossRef]
- Shirley, K.; Rutfield, R.; Hall, N.; Fedor, N.; McCaughey, V.K.; Zajac, K. Combinations of obesity prevention strategies in US elementary schools: A critical review. J. Prim. Prev. 2015, 36, 1–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ho, M.; Garnett, S.P.; Baur, L.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics 2012, 130, e1647–e1671. [Google Scholar] [CrossRef] [PubMed]
- Ickes, M.J.; McMullen, J.; Haider, T.; Sharma, M. Global school-based childhood obesity interventions: A review. Int. J. Environ. Res. Public Health 2014, 11, 8940–8961. [Google Scholar] [CrossRef]
- Bleich, S.N.; Segal, J.; Wu, Y.; Wilson, R.; Wang, Y. Systematic review of community-based childhood obesity prevention studies. Pediatrics 2013, 132, e201–e210. [Google Scholar] [CrossRef]
- Ayine, P.; Selvaraju, V.; Venkatapoorna, C.M.K.; Geetha, T. Parental feeding practices in relation to maternal education and childhood obesity. Nutrients 2020, 12, 1033. [Google Scholar] [CrossRef]
- Pamungkas, R.A.; Chamroonsawasdi, K. Home-based interventions to treat and prevent childhood obesity: A systematic review and meta-analysis. Behav. Sci. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Mazzeschi, C.; Pazzagli, C.; Laghezza, L.; Battistini, D.; Reginato, E.; Perrone, C.; Ranucci, C.; Fatone, C.; Pippi, R.; Giaimo, M.D.; et al. Description of the EUROBIS program: A combination of an Epode community-based and a clinical care intervention to improve the lifestyles of children and adolescents with overweight or obesity. Biomed. Res. Int. 2014, 2014, 546262. [Google Scholar] [CrossRef] [PubMed]
- Janicke, D.M.; Sallinen, B.J.; Perri, M.G.; Lutes, L.D.; Silverstein, J.H.; Brumback, B. Comparison of program costs for parent-only and family-based interventions for pediatric obesity in medically underserved rural settings. J. Rural Health 2009, 25, 326–330. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Nematy, M.; Vatanparast, H.; Khadem-Rezaiyan, M.; Emadzadeh, M. Impact of parent engagement in childhood obesity prevention interventions on anthropometric indices among preschool children: A systematic review. Child. Obes. 2020, 16, 3–19. [Google Scholar] [CrossRef]
- An, R.; Xiang, X.; Xu, N.; Shen, J. Influence of grandparental child care on childhood obesity: A systematic review and meta-analysis. Child. Obes. 2020, 16, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Benjamins, M.R.; Whitman, S. A culturally appropriate school wellness initiative: Results of a 2-year pilot intervention in 2 Jewish schools. J. Sch. Health 2010, 80, 378–386. [Google Scholar] [CrossRef]
- Bogart, L.M.; Elliott, M.N.; Cowgill, B.O.; Klein, D.J.; Hawes-Dawson, J.; Uyeda, K.; Schuster, M.A. Two-year BMI outcomes from a school-based intervention for nutrition and exercise: A randomized trial. Pediatrics 2016, 137, e20152493. [Google Scholar] [CrossRef]
- Khambalia, A.Z.; Dickinson, S.; Hardy, L.L.; Gill, T.; Baur, L.A. A synthesis of existing systematic reviews and meta-analyses of school-based behavioural interventions for controlling and preventing obesity. Obes. Rev. 2012, 13, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Pyle, S.A.; Sharkey, J.; Yetter, G.; Felix, E.; Furlong, M.J.; Poston, W.S.C. Fighting an epidemic: The role of schools in reducing childhood obesity. Psychol. Sch. 2006, 43, 361–376. [Google Scholar] [CrossRef]
- Karacabeyli, D.; Allender, S.; Pinkney, S.; Amed, S. Evaluation of complex community-based childhood obesity prevention interventions. Obes. Rev. 2018, 19, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.M.; Le Bodo, Y.; Jebb, S.A.; Seidell, J.C.; Summerbell, C.; Richard, D.; De Henauw, S.; Moreno, L.A.; Romon, M.; Visscher, T.L.; et al. EPODE approach for childhood obesity prevention: Methods, progress and international development. Obes. Rev. 2012, 13, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Economos, C.D.; Hammond, R.A. Designing effective and sustainable multifaceted interventions for obesity prevention and healthy communities. Obesity 2017, 25, 1155–1156. [Google Scholar] [CrossRef]
- Mitchell, T.B.; Amaro, C.M.; Steele, R.G. Pediatric weight management interventions in primary care settings: A meta-analysis. Health Psychol. 2016, 37, 704–713. [Google Scholar] [CrossRef]
- Hoffman, J.; Frerichs, L.; Story, M.; Jones, J.; Gaskin, K.; Apple, A.; Skinner, A.; Armstrong, S. An integrated clinic-community partnership for child obesity treatment: A randomized pilot trial. Pediatrics 2018, 141, e20171444. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Robbins, L.B.; Wen, F. Interventions to prevent and manage overweight or obesity in preschool children: A systematic review. Int. J. Nurs. Stud. 2016, 53, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Janicke, D.M.; Steele, R.G.; Gayes, L.A.; Lim, C.S.; Clifford, L.M.; Schneider, E.M.; Carmody, J.K.; Westen, S. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. J. Pediatr. Psychol. 2014, 39, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.P.; O’Connor, E.A.; Williams, S.B.; Beil, T.L.; Lutz, K.W. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics 2010, 125, e396–e418. [Google Scholar] [CrossRef] [PubMed]
- McGowan, B.M. A practical guide to engaging individuals with obesity. Obes. Facts 2016, 9, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Hampl, S.; Paves, H.; Laubscher, K.; Eneli, I. Patient engagement and attrition in pediatric obesity clinics and programs: Results and recommendations. Pediatrics 2011, 128, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Vercammen, K.A.; Zatz, L.Y.; Frelier, J.M.; Ebbeling, C.B.; Peeters, A. Interventions to prevent global childhood overweight and obesity: A systematic review. Lancet Diabetes Endocrinol. 2018, 6, 332–346. [Google Scholar] [CrossRef]
- Haddad, J.; Ullah, S.; Bell, L.; Leslie, E.; Magarey, A. the influence of home and school environments on children’s diet and physical activity, and body mass index: A structural equation modelling approach. Matern. Child. Health J. 2018, 22, 364–375. [Google Scholar] [CrossRef]
- Kothandan, S.K. School based interventions versus family based interventions in the treatment of childhood obesity-a systematic review. Arch. Public Health 2014, 72, 3. [Google Scholar] [CrossRef]
- Ward, D.S.; Welker, E.; Choate, A.; Henderson, K.E.; Lott, M.; Tovar, A.; Wilson, A.; Sallis, J.F. Strength of obesity prevention interventions in early care and education settings: A systematic review. Prev. Med. 2017, 95, S37–S52. [Google Scholar] [CrossRef]
- Mauro, M.; Taylor, V.; Wharton, S.; Sharma, A.M. Barriers to obesity treatment. Eur. J. Intern. Med. 2008, 19, 173–180. [Google Scholar] [CrossRef]
- Byrne, S.; Cooper, Z.; Fairburn, C. Weight maintenance and relapse in obesity: A qualitative study. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 955–962. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Bridging the Evidence Gap in Obesity Prevention: A Framework to Inform Decision Making; The National Academies Press: Washington, DC, USA, 2010; pp. 227–267. [Google Scholar] [CrossRef]
- Accelerating Progress in Obesity Prevention. Available online: https://www.ncbi.nlm.nih.gov/books/NBK201141/ (accessed on 18 December 2020).
- Barrea, L.; Annunziata, G.; Bordoni, L.; Muscogiuri, G.; Colao, A.; Savastano, S.; OPERA Group. Nutrigenetics-personalized nutrition in obesity and cardiovascular diseases. Int. J. Obes. Suppl. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, T.; Holzapfel, C. A scientific perspective of personalised gene-based dietary recommendations for weight management. Nutrients 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.M.; Gance-Cleveland, B.; Hassink, S.; Johnson, R.; Paradis, G.; Resnicow, K. Recommendations for prevention of childhood obesity. Pediatrics 2007, 120, S229–S253. [Google Scholar] [CrossRef]
- Belfrage, A.S.V.; Grotmol, K.S.; Tyssen, R.; Moum, T.; Finset, A.; Isaksson Rø, K.; Lien, L. Factors influencing doctors’ counselling on patients’ lifestyle habits: A cohort study. BJGP Open 2018, 2, bjgpopen18X101607. [Google Scholar] [CrossRef]
- Smith, A.W.; Borowski, L.A.; Liu, B.; Galuska, D.A.; Signore, C.; Klabunde, C.; Huang, T.T.; Krebs-Smith, S.M.; Frank, E.; Pronk, N.; et al. primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am. J. Prev. Med. 2011, 41, 33–42. [Google Scholar] [CrossRef]
- Lobelo, F.; de Quevedo, I.G. The evidence in support of physicians and health care providers as physical activity role models. Am. J. Lifestyle Med. 2016, 10, 36–52. [Google Scholar] [CrossRef]
- Bray, M.S.; Loos, R.J.; McCaffery, J.M.; Ling, C.; Franks, P.W.; Weinstock, G.M.; Snyder, M.P.; Vassy, J.L.; Agurs-Collins, T.; Conference Working Group. NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity 2016, 24, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Swan, W.I.; Vivanti, A.; Hakel-Smith, N.A.; Hotson, B.; Orrevall, Y.; Trostler, N.; Beck Howarter, K.; Papoutsakis, C. Nutrition care process and model update: Toward realizing people-centered care and outcomes management. J. Acad. Nutr. Diet. 2017, 117, 2003–2014. [Google Scholar] [CrossRef]
- Thompson, K.L.; Davidson, P.; Swan, W.I.; Hand, R.K.; Rising, C.; Dunn, A.V.; Lewis, N.; Murphy, W.J. Nutrition care process chains: The "missing link" between research and evidence-based practice. J. Acad. Nutr. Diet. 2015, 115, 1491–1498. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.M.; Jang, H.B.; Lee, H.J.; Park, S.I.; Park, K.H.; Lim, H. Evidence-based nutritional intervention protocol for korean moderate-severe obese children and adolescents. Clin. Nutr. Res. 2019, 8, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.L.; De Bourdeaudhuij, I.; Maes, L.; Haerens, L.; Grammatikaki, E.; Widhalm, K.; Kwak, L.; Plada, M.; Moreno, L.A.; Zampelas, A.; et al. Moderators of the effectiveness of a web-based tailored intervention promoting physical activity in adolescents: The HELENA Activ-O-Meter. J. Sch. Health 2014, 84, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Ghanvatkar, S.; Kankanhalli, A.; Rajan, V. User models for personalized physical activity interventions: Scoping review. JMIR mHealth uHealth 2019, 7, e11098. [Google Scholar] [CrossRef]
- Faulkner, M.S.; Michaliszyn, S.F.; Hepworth, J.T.; Wheeler, M.D. Personalized exercise for adolescents with diabetes or obesity. Biol. Res. Nurs. 2014, 16, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Heerman, W.J.; Teeters, L.; Sommer, E.C.; Burgess, L.E.; Escarfuller, J.; Van Wyk, C.; Barkin, S.L.; Duhon, A.A.; Cole, J.; Samuels, L.R.; et al. Competency-based approaches to community health: A randomized controlled trial to reduce childhood obesity among latino preschool-aged children. Child. Obes. 2019, 15, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Koulouglioti, C.; Cole, R.; McQuillan, B.; Moskow, M.; Kueppers, J.; Pigeon, W. Feasibility of an individualized, home-based obesity prevention program for preschool-age children. Child. Health Care 2013, 42, 134–152. [Google Scholar] [CrossRef]
| Questionnaire | Laboratory Tests | Field Tests | |
|---|---|---|---|
| 1. Individual and parental information | * | ||
| 2. Self-reported targets | * | ||
| 3. Facilities/limitations in personal environment | * | ||
| 4. Energy balance status with a short history | * | * | |
| 5. Lifestyle behaviors with a short history | * | ||
| 6. Body composition status with a short history | * | * | |
| 7. Clinical status with a short history | * | * | |
| 8. Biochemistry status with a short history | * | ||
| 9. Physical fitness status with a short history | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motevalli, M.; Drenowatz, C.; Tanous, D.R.; Khan, N.A.; Wirnitzer, K. Management of Childhood Obesity—Time to Shift from Generalized to Personalized Intervention Strategies. Nutrients 2021, 13, 1200. https://doi.org/10.3390/nu13041200
Motevalli M, Drenowatz C, Tanous DR, Khan NA, Wirnitzer K. Management of Childhood Obesity—Time to Shift from Generalized to Personalized Intervention Strategies. Nutrients. 2021; 13(4):1200. https://doi.org/10.3390/nu13041200
Chicago/Turabian StyleMotevalli, Mohamad, Clemens Drenowatz, Derrick R. Tanous, Naim Akhtar Khan, and Katharina Wirnitzer. 2021. "Management of Childhood Obesity—Time to Shift from Generalized to Personalized Intervention Strategies" Nutrients 13, no. 4: 1200. https://doi.org/10.3390/nu13041200
APA StyleMotevalli, M., Drenowatz, C., Tanous, D. R., Khan, N. A., & Wirnitzer, K. (2021). Management of Childhood Obesity—Time to Shift from Generalized to Personalized Intervention Strategies. Nutrients, 13(4), 1200. https://doi.org/10.3390/nu13041200









