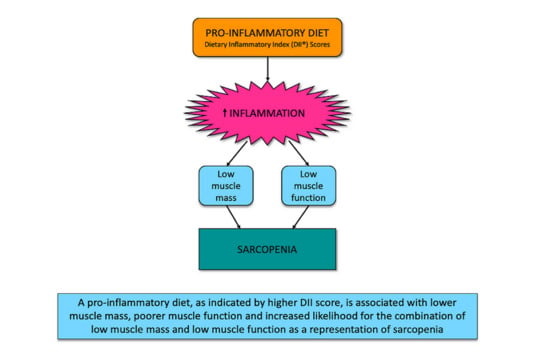
The Dietary Inflammatory Index Is Associated with Low Muscle Mass and Low Muscle Function in Older Australians
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measures
2.3.1. Muscle Mass and Muscle Function
2.3.2. Exposure: Dietary Inflammatory Index (DII)
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Participants
3.2. Characteristics of Participants in the Study Sample
3.3. Dietary Inflammatory Index and Muscle Mass and Muscle Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.-Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Mohebbi, M.; Holloway, K.L.; Brennan-Olsen, S.L.; Hyde, N.K.; Kotowicz, M.A. Musculoskeletal decline and mortality: Prospective data from the Geelong Osteoporosis Study. J. Cachexia Sarcopenia Muscle 2017, 8, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Sui, S.X.; Tembo, M.C.; Holloway Kew, K.L.; Rufus, P.G.; Kotowicz, M.A. Sarcopenic obesity and falls in the elderly. J. Gerontol. Geriatr. Res. 2018, 7, 2–5. [Google Scholar] [CrossRef]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Sui, S.X.; Holloway-Kew, K.L.; Hyde, N.K.; Williams, L.J.; Tembo, M.C.; Leach, S.; Pasco, J.A. Definition-specific prevalence estimates for sarcopenia in an Australian population: The Geelong Osteoporosis Study. JCSM Clin. Rep. 2020, 5, 89–98. [Google Scholar]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Pasco, J.A. Age-related changes in muscle and bone. In Osteosarcopenia: Bone, Muscle and Fat Interactions; Duque, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 45–71. [Google Scholar]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyere, O.; Reginster, J.-Y. The future prevalence of sarcopenia in Europe: A claim for public health action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Pérez-Baos, S.; Prieto-Potin, I.; Román-Blas, J.A.; Sánchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Mediators and patterns of muscle loss in chronic systemic inflammation. Front. Physiol. 2018, 9, 409. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 12. [Google Scholar] [CrossRef]
- Ogawa, S.; Yakabe, M.; Akishita, M. Age-related sarcopenia and its pathophysiological bases. Inflamm. Regen. 2016, 36, 17. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- O’Neil, A.; Shivappa, N.; Jacka, F.N.; Kotowicz, M.A.; Kibbey, K.; Hebert, J.R.; Pasco, J.A. Pro-inflammatory dietary intake as a risk factor for CVD in men: A 5-year longitudinal study. Br. J. Nutr. 2015, 114, 2074–2082. [Google Scholar] [CrossRef]
- Orchard, T.; Yildiz, V.; Steck, S.E.; Hébert, J.R.; Ma, Y.; Cauley, J.A.; Li, W.; Mossavar-Rahmani, Y.; Johnson, K.C.; Sattari, M.; et al. Dietary inflammatory index, bone mineral density and risk of fracture in postmenopausal women: Results from the Women’s Health Initiative. J. Bone Miner. Res. 2017, 32, 1136–1146. [Google Scholar] [CrossRef]
- Shivappa, N.; Stubbs, B.; Hébert, J.R.; Cesari, M.; Schofield, P.; Soysal, P.; Maggi, S.; Veronese, N. The relationship between the dietary inflammatory index and incident frailty: A longitudinal cohort study. J. Am. Med. Dir. Assoc. 2018, 19, 77–82. [Google Scholar] [CrossRef]
- Laclaustra, M.; Rodriguez-Artalejo, F.; Guallar-Castillon, P.; Banegas, J.R.; Graciani, A.; Garcia-Esquinas, E.; Lopez-Garcia, E. The inflammatory potential of diet is related to incident frailty and slow walking in older adults. Clin. Nutr. 2020, 39, 185–191. [Google Scholar] [CrossRef]
- Sardo Molmenti, C.L.; Steck, S.E.; Thomson, C.A.; Hibler, E.A.; Yang, J.; Shivappa, N.; Greenlee, H.; Wirth, M.D.; Neugut, A.I.; Jacobs, E.T.; et al. Dietary inflammatory index and risk of colorectal adenoma recurrence: A pooled analysis. Nutr. Cancer 2017, 69, 238–247. [Google Scholar] [CrossRef]
- Bagheri, A.; Soltani, S.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr. J. 2020, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Mohebbi, M.; Collier, F.; Loughman, A.; Shivappa, N.; Hébert, J.R.; Pasco, J.A.; Jacka, F.N. Diet quality and a traditional dietary pattern predict lean mass in Australian women: Longitudinal data from the Geelong Osteoporosis Study. Prev. Med. Rep. 2021, 21. [Google Scholar] [CrossRef]
- Cervo, M.M.; Shivappa, N.; Hebert, J.R.; Oddy, W.H.; Winzenberg, T.; Balogun, S.; Wu, F.; Ebeling, P.; Aitken, D.; Jones, G.; et al. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin. Nutr. 2020, 39, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Na, W.; Sohn, C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Nicholson, G.C.; Kotowicz, M.A. Cohort profile: Geelong Osteoporosis Study. Int. J. Epidemiol. 2012, 41, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and appendicular lean mass reference ranges for Australian men and women: The Geelong Osteoporosis Study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Giles, C.G.; Ireland, P.D. Dietary Questionnaire for Epidemiological Studies (Version 2); The Cancer Council Victoria: Melbourne, Australia, 1996. [Google Scholar]
- Xinying, P.X.; Noakes, M.; Keogh, J. Can a food frequency questionnaire be used to capture dietary intake data in a 4 week clinical intervention trial? Asia Pac. J. Clin. Nutr. 2004, 13, 318–323. [Google Scholar] [CrossRef]
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef]
- Wirth, M.D.; Shivappa, N.; Davis, L.; Hurley, T.G.; Ortaglia, A.; Drayton, R.; Blair, S.N.; Hébert, J.R. Construct validation of the dietary inflammatory index among African Americans. J. Nutr. Health Aging 2017, 21, 487–491. [Google Scholar] [CrossRef]
- Kotemori, A.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shivappa, N.; Hebert, J.R.; Ishihara, J.; Inoue, M.; Tsugane, S. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: A cross-sectional study of the JPHC-FFQ validation study. Nutrition 2020, 69, 110569. [Google Scholar] [CrossRef]
- Amakye, W.K.; Zhang, Z.; Wei, Y.; Shivappa, N.; Hebert, J.R.; Wang, J.; Su, Y.; Mao, L. The relationship between dietary inflammatory index (DII) and muscle mass and strength in Chinese children aged 6–9 years. Asia Pac. J. Clin. Nutr. 2018, 27, 1315–1324. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Jennings, A.; Steves, C.J.; Skinner, J.; Cassidy, A.; MacGregor, A.J.; Welch, A.A. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef]
- Hashemi, R.; Motlagh, A.D.; Heshmat, R.; Esmaillzadeh, A.; Payab, M.; Yousefinia, M.; Siassi, F.; Pasalar, P.; Baygi, F. Diet and its relationship to sarcopenia in community dwelling iranian elderly: A cross sectional study. Nutrition 2015, 31, 97–104. [Google Scholar] [CrossRef]
- Kim, D.; Park, Y. Association between the dietary inflammatory index and risk of frailty in older individuals with poor nutritional status. Nutrients 2018, 10, 1363. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, C.-O.; Lim, H. Quality of diet and level of physical performance related to inflammatory markers in community-dwelling frail, elderly people. Nutrition 2017, 38, 48–53. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Fanelli Kuczmarski, M.; Mason, M.A.; Beydoun, M.A.; Allegro, D.; Zonderman, A.B.; Evans, M.K. Dietary patterns and sarcopenia in an urban African American and white population in the United States. J. Nutr. Gerontol. Geriatr. 2013, 32, 291–316. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J. Am. Med. Dir. Assoc. 2016, 17, 336–342. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Mendonça, N.; Sayer, A.A.; Hill, T.R.; Davies, K.; Siervo, M.; Mathers, J.C.; Jagger, C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: The Newcastle 85+ study. Clin. Nutr. 2020, 39, 166–173. [Google Scholar] [CrossRef]
- Steck, S.; Shivappa, N.; Tabung, F.; Harmon, B.; Wirth, M.; Hurley, T.; Hebert, J. The dietary inflammatory index: A new tool for assessing diet quality based on inflammatory potential. Digest 2014, 49, 1–9. [Google Scholar]
- Zhang, Z.; Cao, W.; Shivappa, N.; Hebert, J.R.; Li, B.; He, J.; Tang, X.; Liang, Y.; Chen, Y. Association between diet inflammatory index and osteoporotic hip fracture in elderly Chinese population. J. Am. Med. Dir. Assoc. 2017, 18, 671–677. [Google Scholar] [CrossRef]
- Resciniti, N.V.; Lohman, M.C.; Wirth, M.D.; Shivappa, N.; Hebert, J.R. Dietary inflammatory index, pre-frailty and frailty among older US adults: Evidence from the National Health and Nutrition Examination Survey, 2007-2014. J. Nutr. Health Aging 2019, 23, 323–329. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Hébert, J.R.; Hurley, T.G.; Steck, S.E.; Miller, D.R.; Tabung, F.K.; Peterson, K.E.; Kushi, L.H.; Frongillo, E.A. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv. Nutr. 2014, 5, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.J.; Collerton, J.; Davies, K.; Foster, E.; Jagger, C.; Stamp, E.; Mathers, J.C.; Kirkwood, T. Nutrition in advanced age: Dietary assessment in the Newcastle 85+ study. Eur. J. Nutr. 2009, 63, S6–S18. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The dietary inflammatory index (DII)—Lessons learned, improvements made, and future directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Wynder, E.L.; Hebert, J.R. Homogeneity in nutritional exposure: An impediment in cancer epidemiology. J. Natl. Cancer Inst. 1987, 79, 605–607. [Google Scholar]
| Characteristics | Total (n = 809) | Females (n = 278) | Males (n = 531) | p Value |
|---|---|---|---|---|
| DII score | 0.6 (−0.3, 1.3) | 0.8 (−0.2, 1.5) | 0.4 (−0.4, 1.2) | 0.003 |
| Age (yr) | 66.4 (72.4, 78.8) | 70.6 (65.0, 75.3) | 74.0 (67.0, 81.3) | <0.001 |
| Height (cm) | 167.9 ± 8.7 | 159.9 ± 6.0 | 172.1 ± 6.6 | <0.001 |
| Weight (kg) | 78.2 ± 13.7 | 73.5 ± 14.6 | 80.6 ± 12.5 | <0.001 |
| BMI (kg/m2) | 27.7 ± 4.5 | 28.8 ± 5.6 | 27.2 ± 3.8 | <0.001 |
| Body fat (%) | 32.0 ± 10.1 | 42.1 ± 8.0 | 26.7 ± 6.4 | <0.001 |
| ALM/h2 (kg/m2) | 7.7 ± 1.1 | 6.6 ± 0.8 | 8.2 ± 0.9 | <0.001 |
| TUG (s) | 8.9 (7.6, 10.3) | 9.1 (7.8, 10.8) | 8.6 (7.6, 10.1) | 0.02 |
| Mobility level (active) * | 533 (66.2) | 161 (58.8) | 372 (70.0) | 0.001 |
| ALM/h2 cutpoint (below) † | 257 (31.8) | 74 (26.6) | 183 (34.5) | 0.02 |
| TUG >10 s (yes) | 183 (22.6) | 76 (27.3) | 107 (20.1) | 0.02 |
| Low ALM/h2 and TUG > 10 s (yes) | 82 (10.1) | 24 (8.6) | 58 (10.9) | 0.31 |
| Nutrient | Total (n = 809) | Females (n = 278) | Males (n = 531) | p Value | |||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| Energy (kJ) | 6927.9 | (5593.3, 8632.0) | 5991.5 | (4784.0, 7438.5) | 7353.2 | (6195.3, 9230.9) | <0.001 |
| Protein (g) | 78.2 | (63.0, 96.4) | 70.0 | (55.1, 88.5) | 83.1 | (67.6, 100.0) | <0.001 |
| Carbohydrate (g) | 186.7 | (146.7, 234.6) | 154.0 | (119.8, 194.2) | 205.0 | (164.0, 251.1) | <0.001 |
| Total fat (g) | 66.8 | (51.0, 85.4) | 57.5 | (45.4, 72.5) | 71.3 | (56.4, 92.3) | <0.001 |
| Saturated fats (g) | 26.4 | (19.6, 34.6) | 23.4 | (17.7, 30.5) | 28.1 | (21.0, 36.4) | <0.001 |
| Polyunsaturated fatty acids (g) | 10.6 | (7.2, 14.9) | 8.7 | (6.1, 12.1) | 12.1 | (8.3, 16.2) | <0.001 |
| Monounsaturated fatty acids (g) | 23.2 | (17.6, 30.1) | 20.1 | (15.4, 25.5) | 24.6 | (19.2, 32.0) | <0.001 |
| Sugar (g) | 86.4 | (67.3, 111.8) | 74.1 | (55.1, 95.4) | 93.3 | (74.0, 120.8) | <0.001 |
| Starch (g) | 97.4 | (73.0,126.2) | 78.8 | (60.2, 99.8) | 109.3 | (85.0, 138.6) | <0.001 |
| Fibre (g) | 20.5 | (16.4, 26.3) | 18.8 | (14.6, 23.4) | 21.5 | (16.9, 27.8) | <0.001 |
| Alcohol (g) | 5.6 | (0.3, 21.9) | 2.1 | (0.0, 12.7) | 10.1 | (0.9, 26.7) | <0.001 |
| Beta-carotene (μg) | 2358.2 | (1680.8, 3323.5) | 2246.8 | (1624.0, 3089.6) | 2440.9 | (1710.6, 3539.8) | 0.02 |
| Calcium (mg) | 849.0 | (672.3, 1061.7) | 848.8 | (648.6, 1113.3) | 849.1 | (683.0, 1047.1) | 0.67 |
| Cholesterol (mg) | 239.2 | (182.0, 316.3) | 221.8 | (167.5, 297.9) | 249.6 | (190.1, 322.0) | 0.001 |
| Folate (μg) | 255.0 | (197.9, 321.1) | 227.3 | (185.7, 291.7) | 271.2 | (214.8, 335.6) | <0.001 |
| Iron (mg) | 11.7 | (9.0, 14.7) | 10.3 | (7.7, 13.3) | 12.4 | (9.6, 16.0) | <0.001 |
| Magnesium (mg) | 270.2 | (217.3, 336.3) | 247.8 | (197.0, 310.4) | 282.6 | (227.6, 346.2) | <0.001 |
| Niacin (mg) | 33.7 | (26.7, 42.1) | 30.4 | (22.8, 37.9) | 35.9 | (28.5, 44.4) | <0.001 |
| Phosphorus (mg) | 1393.7 | (1100.6, 1708.0) | 1281.7 | (1025.5, 1616.7) | 1440.1 | (1150.8, 1732.3) | 0.001 |
| Potassium (mg) | 2684.6 | (2196.3, 3270.8) | 2451.4 | (1979.5, 3015.7) | 2830.4 | (2300.8, 3379.9) | <0.001 |
| Retinol (μg) | 965.8 | (609.7, 966.7) | 686.7 | (532.0, 858.4) | 810.4 | (641.3, 1020.1) | <0.001 |
| Riboflavin (mg) | 2.1 | (1.7, 2.7) | 2.0 | (1.6, 2.5) | 2.2 | (1.7, 2.8) | 0.002 |
| Sodium (mg) | 2163.1 | (1688.1, 2767.4) | 1840.5 | (1459.1, 2306.2) | 2317.1 | (1875.9, 2976.4) | <0.001 |
| Thiamine (mg) | 1.4 | (1.1, 1.8) | 1.2 | (1.0, 1.5) | 1.5 | (1.2, 2.0) | <0.001 |
| Vitamin C (mg) | 102.1 | (71.6, 148.5) | 92.1 | (67.8, 125.7) | 107.0 | (75.7, 160.6) | 0.001 |
| Vitamin E (mg) | 5.9 | (4.6, 7.6) | 5.4 | (4.0, 7.0) | 6.2 | (4.8, 8.0) | <0.001 |
| Zinc (mg) | 10.2 | (8.1, 12.8) | 9.1 | (7.1, 11.6) | 10.7 | (8.8, 13.1) | <0.001 |
| Outcome Variable | Model 1 * | Model 2 † | Model 3 ‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p Value | β | SE | p Value | β | SE | p Value | |
| ALM/h2 (kg/m2) | −0.13 | 0.04 | <0.001 | −0.05 | 0.02 | 0.036 | −0.05 | 0.02 | 0.028 |
| ln(TUG) (s) | 0.03 | 0.01 | <0.001 | 0.02 | 0.01 | 0.028 | 0.02 | 0.01 | 0.035 |
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||||
| Low ALM/h2 and TUG > 10 s (yes) | 1.34 | 1.08, 1.67 | 0.007 | 1.33 | 1.05, 1.69 | 0.015 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gojanovic, M.; Holloway-Kew, K.L.; Hyde, N.K.; Mohebbi, M.; Shivappa, N.; Hebert, J.R.; O’Neil, A.; Pasco, J.A. The Dietary Inflammatory Index Is Associated with Low Muscle Mass and Low Muscle Function in Older Australians. Nutrients 2021, 13, 1166. https://doi.org/10.3390/nu13041166
Gojanovic M, Holloway-Kew KL, Hyde NK, Mohebbi M, Shivappa N, Hebert JR, O’Neil A, Pasco JA. The Dietary Inflammatory Index Is Associated with Low Muscle Mass and Low Muscle Function in Older Australians. Nutrients. 2021; 13(4):1166. https://doi.org/10.3390/nu13041166
Chicago/Turabian StyleGojanovic, Marlene, Kara L. Holloway-Kew, Natalie K. Hyde, Mohammadreza Mohebbi, Nitin Shivappa, James R. Hebert, Adrienne O’Neil, and Julie A. Pasco. 2021. "The Dietary Inflammatory Index Is Associated with Low Muscle Mass and Low Muscle Function in Older Australians" Nutrients 13, no. 4: 1166. https://doi.org/10.3390/nu13041166
APA StyleGojanovic, M., Holloway-Kew, K. L., Hyde, N. K., Mohebbi, M., Shivappa, N., Hebert, J. R., O’Neil, A., & Pasco, J. A. (2021). The Dietary Inflammatory Index Is Associated with Low Muscle Mass and Low Muscle Function in Older Australians. Nutrients, 13(4), 1166. https://doi.org/10.3390/nu13041166