Dietary Olive Oil Intake Improves Running Endurance with Intramuscular Triacylglycerol Accumulation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Procedures
2.3. Treadmill Endurance Test
2.4. Serum Biochemical Analysis
2.4.1. Glucose, Triacylglycerol (TAG), and Non-Esterified Fatty Acid (NEFA) Analyses
2.4.2. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.5. Muscle TAG Content
2.5.1. Biochemical Determination
2.5.2. Preparation of Muscle Sections and BODIPY 493/503 Staining
2.6. RNA Isolation and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Western Blotting
2.8. Myosin Heavy Chain (MyHC) Isoform Composition
2.9. Statistical Analysis
3. Results
3.1. Olive Oil Intake Improves Treadmill Endurance Capacity in Mice
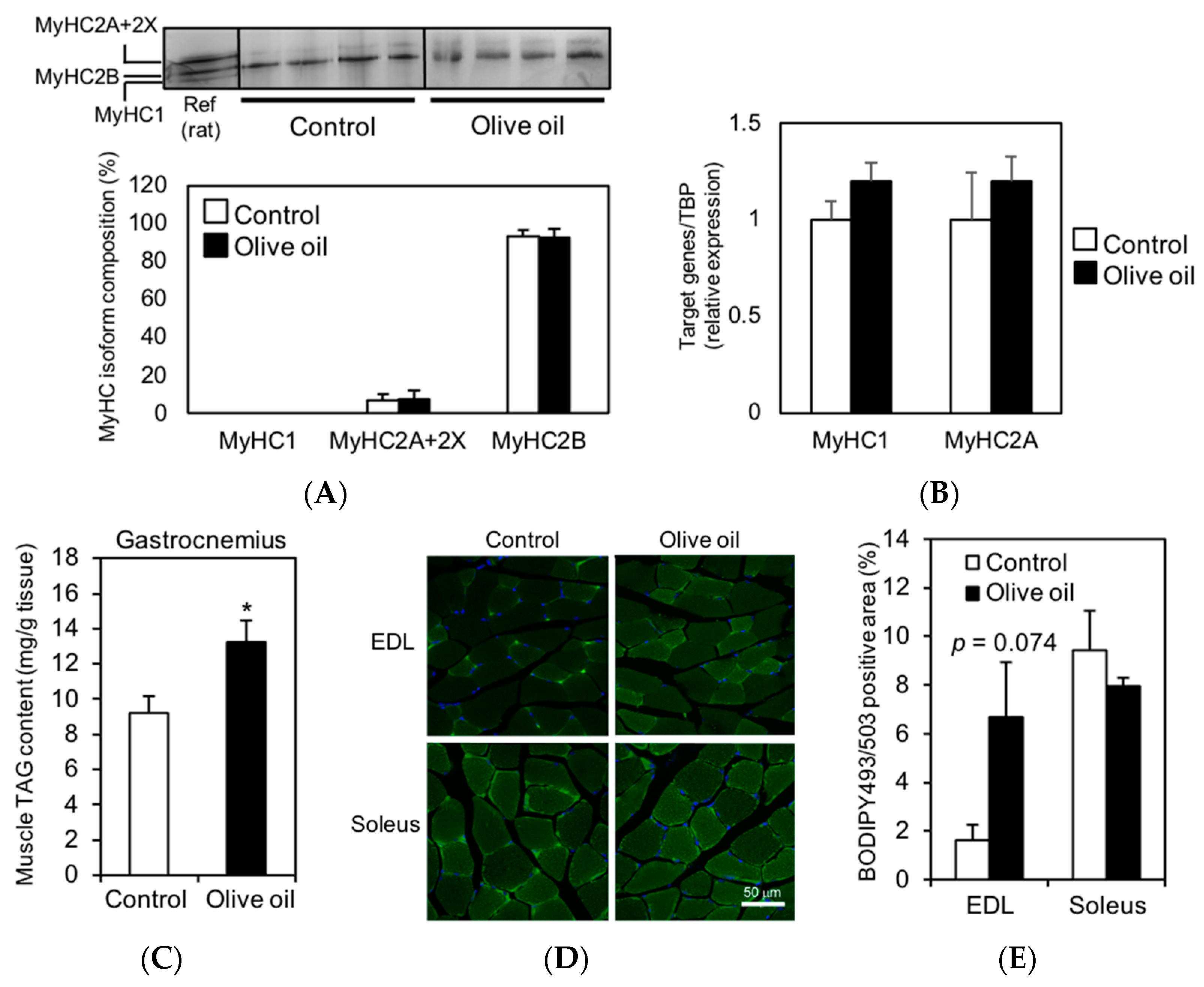
3.2. Olive Oil Intake Increases IMTG Content in Gastrocnemius Muscle
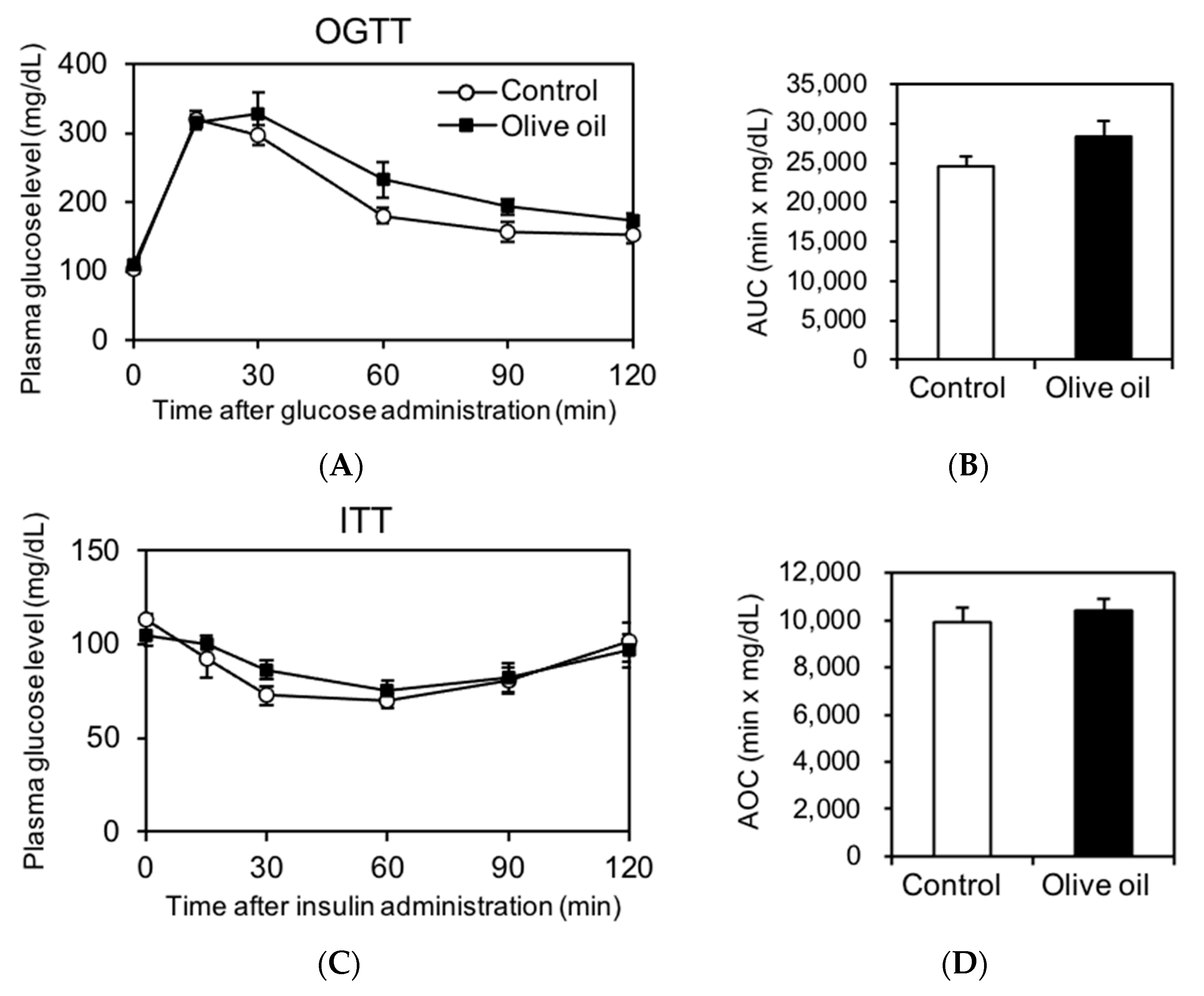
3.3. Olive Oil Intake Induces No Metabolic Impairment in Glucose and Insulin Tolerance
3.4. Olive Oil Intake Upregulates DGAT1 Expression Levels in Skeletal Muscle
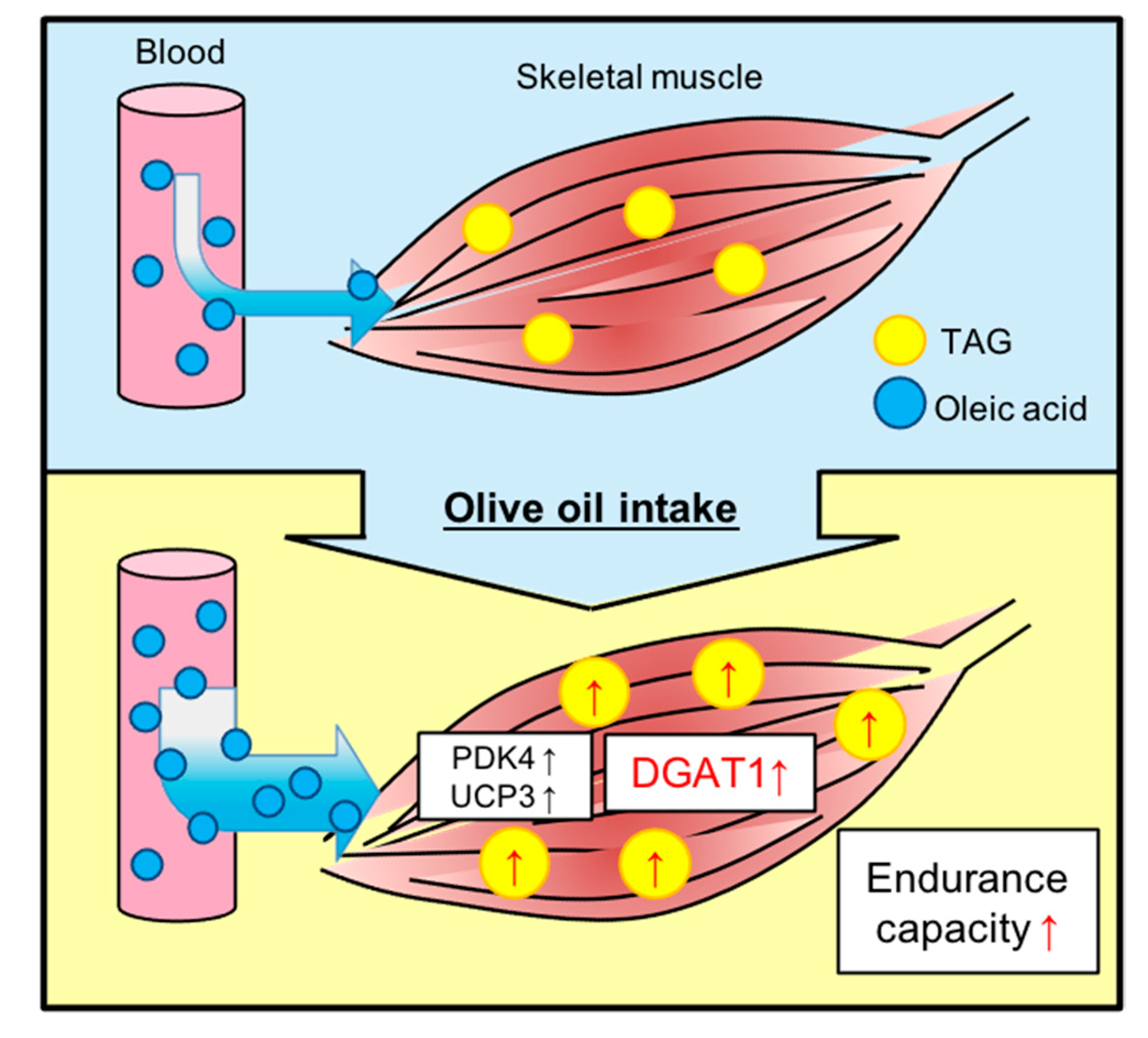
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schiaffino, S.; Reggiani, C. Fiber types in Mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Van Loon, L.J.C. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J. Appl. Physiol. 2004, 97, 1170–1187. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.M.; Shaw, C.S. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J. Physiol. 2013, 591, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Portillo, M.P.; Picó, C.; Teresa Macarulla, M.; Palou, A. Olive oil feeding up-regulates uncoupling protein genes in rat brown adipose tissue and skeletal muscle. Am. J. Clin. Nutr. 2002, 75, 213–220. [Google Scholar] [CrossRef]
- Mizunoya, W.; Iwamoto, Y.; Shirouchi, B.; Sato, M.; Komiya, Y.; Razin, F.R.; Tatsumi, R.; Sato, Y.; Nakamura, M.; Ikeuchi, Y. Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G. A Simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Komiya, Y.; Sawano, S.; Mashima, D.; Ichitsubo, R.; Nakamura, M.; Tatsumi, R.; Ikeuchi, Y.; Mizunoya, W. Mouse soleus (slow) muscle shows greater intramyocellular lipid droplet accumulation than EDL (fast) muscle: Fiber type-specific analysis. J. Muscle Res. Cell Motil. 2017, 38. [Google Scholar] [CrossRef]
- Komiya, Y.; Mizunoya, W.; Kajiwara, K.; Yokoyama, I.; Ogasawara, H.; Arihara, K. Correlation between skeletal muscle fiber type and responses of a taste sensing system in various beef samples. Anim. Sci. J. 2020, 91, 1–7. [Google Scholar] [CrossRef]
- Goodpaster, B.H. CrossTalk proposal: Intramuscular lipid accumulation causes insulin resistance. J. Physiol. 2020, 598, 3803–3806. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Greimel, P.; Hirabayashi, Y. GPRC5B-Mediated Sphingomyelin Synthase 2 Phosphorylation Plays a Critical Role in Insulin Resistance. iScience 2018, 8, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Sawano, S.; Komiya, Y.; Ichitsubo, R.; Ohkawa, Y.; Nakamura, M.; Tatsumi, R.; Ikeuchi, Y.; Mizunoya, W. A one-step immunostaining method to visualize rodent muscle fiber type within a single specimen. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Cree, M.G.; Paddon-Jones, D.; Newcomer, B.R.; Ronsen, O.; Aarsland, A.; Wolfe, R.R.; Ferrando, A. Twenty-eight-day bed rest with hypercortisolemia induces peripheral insulin resistance and increases intramuscular triglycerides. Metabolism 2010, 59, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Badin, P.M.; Vila, I.K.; Louche, K.; Mairal, A.; Marques, M.A.; Bourlier, V.; Tavernier, G.; Langin, D.; Moro, C. High-fat diet-mediated lipotoxicity and insulin resistance is related to impaired lipase expression in mouse skeletal muscle. Endocrinology 2013, 154, 1444–1453. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Tokimitsu, I.; Hase, T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1550–1556. [Google Scholar] [CrossRef]
- Fan, W.; Waizenegger, W.; Lin, C.S.; Sorrentino, V.; He, M.X.; Wall, C.E.; Li, H.; Liddle, C.; Yu, R.T.; Atkins, A.R.; et al. PPARδ Promotes Running Endurance by Preserving Glucose. Cell Metab. 2017, 25, 1186–1193.e4. [Google Scholar] [CrossRef]
- Sharman, I.M. Nutrition and Athletic Performance. Nutr. Bull. 1973, 2, 36–43. [Google Scholar] [CrossRef]
- Ishihara, K.; Oyaizu, S.; Onuki, K.; Lim, K.; Fushiki, T. Chronic (-)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. J. Nutr. 2000, 130, 2990–2995. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Zou, F.; Xu, S.; Liu, P. Phosphorylation and function of DGAT1 in skeletal muscle cells. Biophys. Rep. 2015, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, X.; Cheol, S.C.; Shulman, G.I.; Klaus, K.; Nair, K.S.; Schwartz, G.J.; Zhang, Y.; Goldberg, I.J.; Yu, Y.H. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes 2009, 58, 2516–2524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, T.; Xu, D.; Zuo, B.; Lei, M.; Xiong, Y.; Chen, H.; Zhou, Y.; Wu, X. Ectopic overexpression of porcine DGAT1 increases intramuscular fat content in mouse skeletal muscle. Transgenic Res. 2013, 22, 187–194. [Google Scholar] [CrossRef]
- Yang, F.; Wei, Z.; Ding, X.; Liu, X.; Ge, X.; Song, G.; Li, G.; Guo, H. Upregulation of triglyceride synthesis in skeletal muscle overexpressing DGAT1. Lipids Health Dis. 2013, 12, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Chantranupong, L.; Scaria, S.M.; Saxton, R.A.; Gygi, M.P.; Shen, K.; Wyant, G.A.; Wang, T.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 2016, 165, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef]
- Watanabe, N.; Komiya, Y.; Sato, Y.; Watanabe, Y.; Suzuki, T.; Arihara, K. Oleic acid up-regulates myosin heavy chain (MyHC) 1 expression and increases mitochondrial mass and maximum respiration in C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2020, 525, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.C.; Roche, H.M. The potential role of olive oil-derived MUFA in insulin sensitivity. Mol. Nutr. Food Res. 2007, 51, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Marika, M.; Egeria, S.; Maria Annunziata, C.; Giuseppe, S.; Tiziano, V.; Raffaele, D.C.; Nadia, C. Effects of Olive Oil on Blood Pressure: Epidemiological, clinical, and mechanistic evidence. Nutrients 2020, 12, 1548. [Google Scholar]


| Ingredient (g/kg) | Control | Olive Oil |
|---|---|---|
| Casein | 200 | 200 |
| l-Cystine | 3 | 3 |
| Corn Starch | 397.486 | 397.486 |
| Maltodextrin 10 | 132 | 132 |
| Sucrose | 100 | 100 |
| Cellulose, BW200 | 50 | 50 |
| Soybean oil | 70 | - |
| Olive oil | - | 70 |
| Mineral Mix S10022G | 35 | 35 |
| Vitamin Mix V10037 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 |
| t-Butylhydroquinone | 0.028 | 0.028 |
| FD&C Yellow Dye, #5 | 0.05 | - |
| FD&C Red Dye, #40 | - | 0.05 |
| Fatty Acid | Soybean Oil | Olive Oil |
|---|---|---|
| C16:0 (Palmitic acid) | 10.4 | 11.5 |
| C16:1 (Palmitoleic acid) | - | 1.2 |
| C18:0 (Stearic acid) | 3.8 | 2.3 |
| C18:1 (Oleic acid) | 24.3 | 70.5 |
| C18:2 (Linoleic acid) | 53.5 | 13.0 |
| C18:3 (Linolenic acid) | 7.8 | 0.6 |
| C20:0 (Arachidic acid) | - | 0.4 |
| C20:1 (Eicosenoic acid) | - | 0.2 |
| Others | 0.2 | 0.3 |
| SFA | 14.2 | 14.2 |
| MUFA | 24.3 | 71.9 |
| PUFA | 61.3 | 13.6 |
| Gene | Primer Sequences (5′-3′) | Gene | Primer Sequences (5′-3′) |
|---|---|---|---|
| SREBP1 | F: GGTTTTGAACGACATCGAAGA | HSL | F: GCGCTGGAGGAGTGTTTTT |
| R: CGGGAAGTCACTGTCTTGGT | R: CGCTCTCCAGTTGAACCAAG | ||
| PPARγ | F: GAAAGACAACGGACAAATCACC | LPL | F: TTTGTGAAATGCCATGACAAG |
| R: GGGGGTGATATGTTTGAACTTG | R: CAGATGCTTTCTTCTCTTGTTTGT | ||
| PPARδ | F: CTCACCGAGTTCGCCAAG | ATGL | F: TGACCATCTGCCTTCCAGA |
| R: AGGGTCACCTGGTCATTGAG | R: TGTAGGTGGCGCAAGACA | ||
| PPARα | F: TTCCAAAGCAAGGTCTGAGG | CPT1β | F: GTCATGGCACTGGGTATGGT |
| R: GGATGGCACCAAGGACAGTA | R: GGGATGCGTGTAGTGTTGAA | ||
| ACSL1 | F: AAAGATGGCTGGTTACACACG | PDK4 | F: CGCTTAGTGAACACTCCTTCG |
| R: CGATAATCTTCAAGGTGCCATT | R: CTTCTGGGCTCTTCTCATGG | ||
| DGAT1 | F: GTCAAGGCCAAAGCTGTCTC | GCK | F: TCCCTGTAAGGCACGAAGAC |
| R: AACACAAAGTAGGAGCAAAGATGA | R: ACGATGTTGTTCCCTTCTGC | ||
| DGAT2 | F: GGCGCTACTTCCGAGACTAC | GLUT4 | F: GATTCTGCTGCCCTTCTGTC |
| R: TGGTCAGCAGGTTGTGTGTC | R: CGGTCAGGCGCTTTAGAC | ||
| CD36 | F: TGGAGCTGTTATTGGTGCAGT | MPC1 | F: TGAATAGCCGAGAGTCCCTAAA |
| R: GGTTCCTTCTTCAAGGACAACTT | R: TGATGAAGACAAATAAGGTTTAGCA | ||
| FASN | F: GCTGCTGTTGGAAGTCAGC | UCP3 | F: TACCCAACCTTGGCTAGACG |
| R: AGTGTTCGTTCCTCGGAGTG | R: GTCCGAGGAGAGAGCTTGC | ||
| ACACA | F: GCGTCGGGTAGATCCAGTT | PGC1α | F: TGAAAGGGCCAAACAGAGAG |
| R: CTCAGTGGGGCTTAGCTCTG | R: GTAAATCACACGGCGCTCTT | ||
| PLIN5 | F: GTCGGAGAAGCTGGTGGAC | MyHC1 | F: GAGCAGCAGGTGGATGATCT |
| R: TCAGCTGCCAGGACTGCTA | R: GCTTGGCTCGCTCTAGGTC | ||
| TBP | F: GGGGAGCTGTGATGTGAAGT | MyHC2A | F: AAAGCTCCAAGGACCCTCTT |
| R: CCAGGAAATAATTCTGGCTCAT | R: AGCTCATGACTGCTGAACTCAC |
| Control | Olive Oil | |||||
|---|---|---|---|---|---|---|
| Body weight gain and food intake | ||||||
| Final body weight (g) | 25.8 | ± | 0.18 | 25.1 | ± | 0.72 |
| Body weight gain (g) | 3.88 | ± | 0.36 | 3.50 | ± | 0.51 |
| Total food intake (g) | 149.1 | ± | 2.3 | 144.4 | ± | 0.3 |
| Tissue weight (mg) | ||||||
| Soleus muscle | 12.0 | ± | 0.4 | 11.2 | ± | 0.3 |
| EDL muscle | 12.1 | ± | 0.4 | 11.9 | ± | 0.3 |
| Gastrocnemius muscle | 276.6 | ± | 9.2 | 271.4 | ± | 6.4 |
| Epididymal fat | 479.7 | ± | 47.3 | 496.9 | ± | 44.2 |
| Perirenal fat | 193.9 | ± | 11.5 | 154.1 | ± | 20.1 |
| Inguinal fat | 381.7 | ± | 32.0 | 349.5 | ± | 34.2 |
| Brown adipose tissue | 175.4 | ± | 17.2 | 161.9 | ± | 13.0 |
| Liver | 1109.8 | ± | 23.9 | 1073.0 | ± | 45.1 |
| Serum biochemical component | ||||||
| Glucose (mg/100 mL) | 206.4 | ± | 17.4 | 212.3 | ± | 4.7 |
| TAG (mg/100 mL) | 102.1 | ± | 17.8 | 115.8 | ± | 19.6 |
| NEFA (mEq/L) | 0.87 | ± | 0.20 | 0.99 | ± | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komiya, Y.; Sugiyama, M.; Ochiai, M.; Osawa, N.; Adachi, Y.; Iseki, S.; Arihara, K. Dietary Olive Oil Intake Improves Running Endurance with Intramuscular Triacylglycerol Accumulation in Mice. Nutrients 2021, 13, 1164. https://doi.org/10.3390/nu13041164
Komiya Y, Sugiyama M, Ochiai M, Osawa N, Adachi Y, Iseki S, Arihara K. Dietary Olive Oil Intake Improves Running Endurance with Intramuscular Triacylglycerol Accumulation in Mice. Nutrients. 2021; 13(4):1164. https://doi.org/10.3390/nu13041164
Chicago/Turabian StyleKomiya, Yusuke, Makoto Sugiyama, Masaru Ochiai, Nanako Osawa, Yuto Adachi, Shugo Iseki, and Keizo Arihara. 2021. "Dietary Olive Oil Intake Improves Running Endurance with Intramuscular Triacylglycerol Accumulation in Mice" Nutrients 13, no. 4: 1164. https://doi.org/10.3390/nu13041164
APA StyleKomiya, Y., Sugiyama, M., Ochiai, M., Osawa, N., Adachi, Y., Iseki, S., & Arihara, K. (2021). Dietary Olive Oil Intake Improves Running Endurance with Intramuscular Triacylglycerol Accumulation in Mice. Nutrients, 13(4), 1164. https://doi.org/10.3390/nu13041164







