Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study
Abstract
1. Introduction
2. Materials and Methods
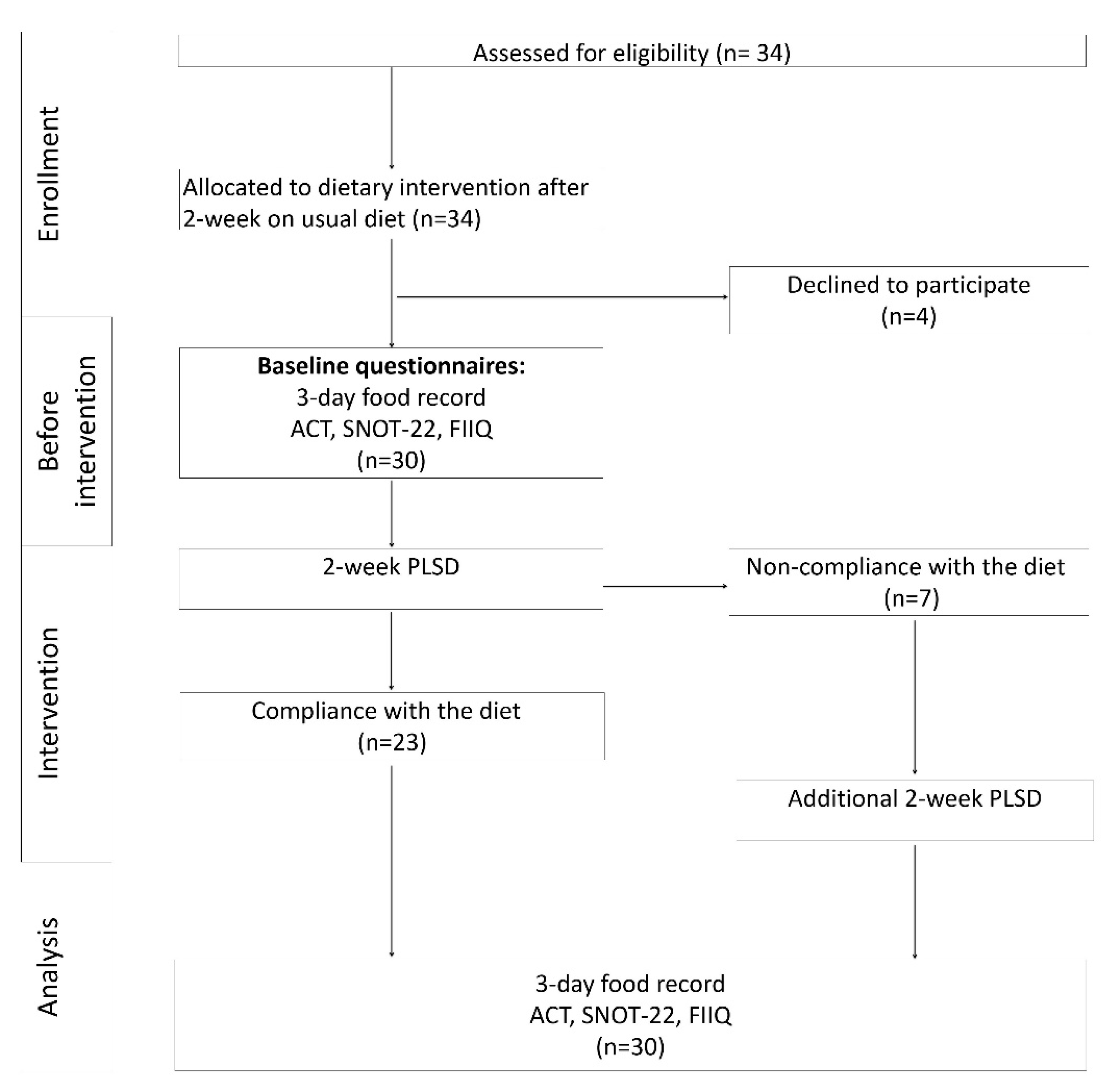
2.1. Study Design
2.2. Study Participants
- Age > 18 years old;
- A medical diagnosis of hypersensitivity to NSAIDs confirmed by an allergist;
- Persistent self-reported symptoms, typical of salicylates hypersensitivity (rhinitis and/or asthma, and/or urticaria), despite pharmacological treatment and NSAIDs avoidance;
- Intake of a stable dose of medication for at least 2 months prior to the commencement.
2.3. Dietary Intervention
- Salicylate-free products, safe for consumption, such as millet, barley, wheat, poultry, fish, eggs, milk, butter, lard, peeled pears or some varieties of apples;
- Containing salicylates, which should be limited to a certain amount per day.
2.4. Questionnaires
- The asthma control test (ACT) was used to assess asthma symptoms [34]. The test was conducted before the dietary intervention at the educational visit and on the last day of the PLSD use during the final visit. Higher scores indicated better asthma control;
- The sino-nasal outcome test (SNOT-22) was used to assess the symptoms of rhinosinusitis [35]. The test was conducted before the intervention, during the educational visit and on the last day of the PLSD use, during the final visit. Higher scores indicated greater severity of rhinosinusitis symptoms;
- The food-item itch questionnaire (FIIQ) was used to assess pruritus induced by urticaria [36]. The test was performed 3 times before the dietary intervention, and 3 times during the last 3 days of the intervention. The results obtained were presented as the mean of 3 repetitions. The results are presented as the mean of three replications. Higher scores indicated a higher severity of pruritus.
2.5. Assessment of Salicylates Intake
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swain, A.R.; Dutton, S.P.; Truswell, A.S. Salicylates in foods. J. Am. Diet. Assoc. 1985, 85, 950–960. [Google Scholar]
- Paterson, J.R.; Srivastava, R.; Baxter, G.J.; Graham, A.B.; Lawrence, J.R. Salicylic acid content of spices and its implications. J. Agric. Food Chem. 2006, 54, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Baxter, G.; Thies, F.; Kyle, J.; Duthie, G. A systematic review of salicylates in foods: Estimated daily intake of a Scottish population. Mol. Nutr. Food Res. 2011, 55, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Gibson, P.R.; Barrett, J.S.; Muir, J.G. Naturally occurring dietary salicylates: A closer look at common Australian foods. J. Food Compos. Anal. 2017, 57, 31–39. [Google Scholar] [CrossRef]
- Swain, A.R. The Role of Natural Salicylates in Food Intolerance. Ph.D. Thesis, University of Sydney, Sydney, Australia, 1988. [Google Scholar]
- Aresta, A.; Zambonin, C. Simultaneous determination of salicylic, 3-methyl salicylic, 4-methyl salicylic, acetylsalicylic and benzoic acids in fruit, vegetables and derived beverages by SPME-LC-UV/DAD. J. Pharm. Biomed. Anal. 2016, 20, 63–68. [Google Scholar] [CrossRef]
- Pierpoint, W.S. Salicylic acid and its derivatives in plants: Medicines, metabolites and messenger molecules. Adv. Bot. Res. 1994, 20, 164–193. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Venema, D.P.; Hollman, P.C.H.; Janssen, P.L.T.M.K.; Katan, M.B. Determination of acetylsalicylic acid and salicylic acid in foods, using HPLC with fluorescence detection. J. Agric. Food Chem. 1996, 44, 1762–1767. [Google Scholar] [CrossRef]
- Scotter, M.J.; Roberts, D.P.T.; Wilson, L.A.; Howart, F.A.C.; Davis, J.; Mansell, N. Free salicylic acid and acetyl salicylic acid content of foods using gas chromatography-mass spectrometry. Food Chem. 2007, 105, 273–279. [Google Scholar] [CrossRef]
- Kęszycka, P.K.; Szkop, M.; Gajewska, D. Overall content of salicylic acid and salicylates in food available on the European market. J. Agric. Food Chem. 2017, 65, 11085–11091. [Google Scholar] [CrossRef]
- Chiang, H.L.; Venter, C.; Syue, P.C.; Ku, K.L.; Wu, C.H. Which Fruits and Vegetables Should Be Excluded from a Low-Salicylate Diet? An Analysis of Salicylic Acid in Foodstuffs in Taiwan. Int. Arch. Allergy Immunol. 2018, 176, 198–204. [Google Scholar] [CrossRef]
- Blacklock, C.J.; Lawrence, J.R.; Wiles, D.; Malcolm, E.A.; Gibson, I.H.; Kelly, C.J.; Paterson, J.R. Salicylic acid in the serum of subjects not taking aspirin. Comparison of salicylic acid concentrations in the serum of vegetarians, non-vegetarians, and patients taking low dose aspirin. J. Clin. Pathol. 2001, 54, 553–555. [Google Scholar] [CrossRef]
- Morgan, G. Could vitamin S (salicylate) protect against chilhood cancer? Med. Hypotheses 2005, 64, 661–664. [Google Scholar] [CrossRef]
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Spadafranca, A.; Bertoli, S.; Fiorillo, G.; Testolin, G.; Battezzati, A. Circulating salicylic acid is related to fruit and vegetable consumption in healthy subjects. Br. J. Nutr. 2007, 98, 802–806. [Google Scholar] [CrossRef]
- Siniorakis, E.; Arvanitakis, S.; Zarreas, E.; Saridakis, M.; Balanis, A.; Tzevelekos, P.; Bokos, G.; Limberi, S. Mediterranean diet: Natural salicylates and other secrets of the pyramid. Int. J. Cardiol. 2013, 166, 538–539. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Peter, R.; Baxter, G.J.; Robson, J.; Graham, A.B.; Paterson, J.R. Urinary excretion of salicyluric and salicylic acids by non-vegetarians, vegetarians, and patients taking low dose aspirin. J. Clin. Pathol. 2003, 56, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Agache, I.; Bavbek, S.; Bakirtas, A.; Blanca, M.; Bochenek, G.; Bonini, M.; Heffler, E.; Klimek, L.; Laidlaw, T.M.; et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy 2019, 74, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Warin, R.P. The effect of aspirin in chronic urticaria. Br. J. Dermatol. 1960, 72, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Calnan, C.D. Urticarial Reactions. Br. Med. J. 1964, 2, 649–655. [Google Scholar] [CrossRef]
- Samter, M.; Beers, R.F., Jr. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann. Intern. Med. 1968, 68, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Szczeklik, A.; Nizankowska, E.; Duplaga, M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur. Respir. J. 2000, 16, 432–436. [Google Scholar] [CrossRef]
- Szczeklik, A.; Stevenson, D.D. Aspirin-induced asthma: Advances in pathogenesis, diagnosis, and management. J. Allergy Clin. Immunol. 2003, 111, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Makowska, J.S.; Burney, P.; Jarvis, D.; Keil, T.; Tomassen, P.; Bislimovska, J.; Brozek, G.; Bachert, C.; Baelum, J.; Bindslev-Jensen, C.; et al. Respiratory hypersensitivity reactions to NSAIDs in Europe: The global allergy and asthma network (GA2 LEN) survey. Allergy 2016, 71, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Rajan, J.P.; Wineinger, N.E.; Stevenson, D.D.; White, A.A. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J. Allergy Clin. Immunol. 2015, 135, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Grattan, C.E. Aspirin sensitivity and urticaria. Clin. Exp. Dermatol. 2003, 28, 123–127. [Google Scholar] [CrossRef]
- Cavkaytar, O.; ArikYilmaz, E.; Buyuktiryaki, B.; Sekerel, B.E.; Sackesen, C.; Soyer, O.U. Challenge-proven aspirin hypersensitivity in children with chronic spontaneous urticaria. Allergy 2015, 70, 153–160. [Google Scholar] [CrossRef]
- Sommer, D.D.; Hoffbauer, S.; Au, M.; Sowerby, L.J.; Gupta, M.K.; Nayan, S. Treatment of aspirin exacerbated respiratory disease with a low salicylate diet: A pilot crossover study. Otolaryngol. Head Neck Surg. 2015, 152, 42–47. [Google Scholar] [CrossRef]
- Sommer, D.D.; Rotenberg, B.W.; Sowerby, L.J.; Lee, J.M.; Janjua, A.; Witterick, I.J.; Monteiro, E.; Gupta, M.K.; Au, M.; Nayan, S. A novel treatment adjunct for aspirin exacerbated respiratory disease: The low-salicylate diet: A multicenter randomized control crossover trial. Int. Forum Allergy Rhinol. 2016, 6, 385–391. [Google Scholar] [CrossRef]
- Szkop, M.; Szkop, U.; Kęszycka, P.; Gajewska, D. A Simple and Robust Protocol for fast RP-HPLC Determination of Salicylates in Foods. Food Anal. Methods 2017, 10, 618–625. [Google Scholar] [CrossRef]
- Gajewska, D.; Kęszycka, P.K.; Szkop, M. Dietary salicylates in herbs and spices. Food Funct. 2019, 10, 7037. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the Asthma Control Test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Mędrek, K.; Szepietowski, J. Czteropunktowy kwestionariusz oceny świądu—Walidacja kwestionariusza. Dermatol. Rev. Prz. Dermatol. 2012, 99, 600–604. [Google Scholar]
- Kowalski, M.L.; Asero, R.; Bavbek, S.; Blanca, M.; Blanca-Lopez, N.; Bochenek, G.; Brockow, K.; Campo, P.; Celik, G.; Cernadas, J.; et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy 2013, 68, 1219–1232. [Google Scholar] [CrossRef]
- Settipane, R.A.; Constantine, H.P.; Settipane, G.A. Aspirin intolerance and recurrent urticaria in normal adults and children. Epidemiology and review. Allergy 1980, 35, 149–154. [Google Scholar] [CrossRef]
- Asero, R.; Bavbek, S.; Blanca, M.; Blanca-Lopez, N.; Cortellini, G.; Nizankowska-Mogilnicka, E.; Quaratino, D.; Romano, A.; Sanchez-Borges, M.; Torres-Jaen, M.J. Clinical management of patients with a history of urticaria/angioedema induced by multiple NSAIDs: An expert panel review. Int. Arch. Allergy Immunol. 2013, 160, 126–133. [Google Scholar] [CrossRef]
- Sweet, J.M.; Stevenson, D.D.; Simon, R.A.; Mathison, D.A. Long-term effects of aspirin desensitization--treatment for aspirin-sensitive rhinosinusitis-asthma. J. Allergy Clin. Immunol. 1990, 85, 59–65. [Google Scholar] [CrossRef]
- Philpott, C.M.; Smith, R.; Davies-Husband, C.R.; Erskine, S.; Clark, A.; Welch, A.; Hopkins, C.; Carrie, S.; Ray, J.; Sunkaraneni, V.; et al. Exploring the association between ingestion of foods with higher potential salicylate content and symptom exacerbation in chronic rhinosinusitis. Data from the National Chronic Rhinosinusitis Epidemiology Study. Rhinology 2019, 57, 303–312. [Google Scholar] [CrossRef]
- Levy, J.M.; Rudmik, L.; Peters, A.T.; Wise, S.K.; Rotenberg, B.W.; Smith, T.L. Contemporary management of chronic rhinosinusitis with nasal polyposis in aspirin-exacerbated respiratory disease: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2016, 6, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Dölle-Bierke, S.; Plank-Habibi, S.; Schäfer, C.; Ahrens, B.; Ballmer-Weber, B.; Beyer, K.; Blümchen, K.; Huttegger, I.; Jappe, U.; Kleine-Tebbe, J.; et al. Dietary implications in acetylsalicylic acid intolerance. Allergo J. Int. 2020, 29, 93–96. [Google Scholar] [CrossRef]
- Janssen, P.L.T.M.K.; Hollman, P.C.; Venema, D.P.; van Staveren, W.A.; Katan, M.B. Salicylates in Foods. Nutr. Rev. 1996, 54, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Williams, M.; Reeves, L.; Meyer, R.; Venter, C. Sensitivity to food additives, vaso-active amines and salicylates: A review of the evidence. Clin. Transl. Allergy 2015, 5, 34. [Google Scholar] [CrossRef]
- Esmaeilzedeh, H.; Esmaeilzadeh, E.; Faramarzi, M.; Nabavi, M.; Farhadi, M. Salicylate Food Intolerance and Aspirin Hypersensitivity in Nasal Polyposis. Iran. J. Immunol. 2017, 14, 81–88. [Google Scholar] [PubMed]
- Baxter, G.J.; Graham, A.B.; Lawrence, J.R.; Wiles, D.; Paterson, J.R. Salicylic acid in soups prepared from organically and non-organically grown vegetables. Eur. J. Nutr. 2001, 40, 289–292. [Google Scholar] [CrossRef]
- Paterson, J.R.; Baxter, G.; Dreyer, J.S.; Halket, J.M.; Flynn, R.; Lawrence, J.R. Salicylic acid sans aspirin in animals and man: Persistence in fasting and biosynthesis from benzoic acid. J. Agric. Food Chem. 2008, 56, 11648–11652. [Google Scholar] [CrossRef] [PubMed]
| Mean | SD | Min–Max | Median | |
|---|---|---|---|---|
| Age, years | 40.33 | 16.29 | 18.00–83.00 | 37.50 |
| Weight, kg | 63.36 | 10.17 | 47.00–83.70 | 62.55 |
| BMI, kg/m2 | 23.28 | 4.25 | 17.92–32.70 | 22.02 |
| Disease duration, years | 3.32 | 3.04 | 0.25–10.00 | 2.00 |
| Number of patients | Percent (%) | |||
| Smokers | 2 | 6.67 | ||
| Food allergies | 10 | 33.33 | ||
| Inhalation allergies | 4 | 13.33 | ||
| Contact allergies | 2 | 6.67 | ||
| No allergies | 14 | 46.67 | ||
| Family history of aspirin sensitivity | 7 | 23.33 | ||
| Symptoms | Number of patients | Percent (%) | ||
| Urticaria (only) | 2 | 6.67 | ||
| Rhinosinusitis (only) | 1 | 3.33 | ||
| Urticaria and asthma | 1 | 3.33 | ||
| Urticaria and rhinosinusitis | 7 | 23.33 | ||
| Asthma and rhinosinusitis | 6 | 20.00 | ||
| Urticaria, asthma and rhinosinusitis | 13 | 43.33 | ||
| Group of Drugs | Number of Patients (n = 30) | Percent (%) |
|---|---|---|
| Antihistamines | 25 | 83.33 |
| Glucocorticosteroids | 21 | 70.00 |
| Leukotriene receptor antagonists | 5 | 16.67 |
| Hydroxyzine | 2 | 6.67 |
| Levothyroxine | 6 | 20.00 |
| Proton pump inhibitors | 3 | 10.00 |
| Beta-blockers | 2 | 6.67 |
| Statins | 1 | 3.33 |
| Anticoagulants 1 | 2 | 6.67 |
| Salicylate Intake (n = 30) | ||||
|---|---|---|---|---|
| Baseline | PLSD | Statistical Significance a | Δ PLSD vs. Baseline | |
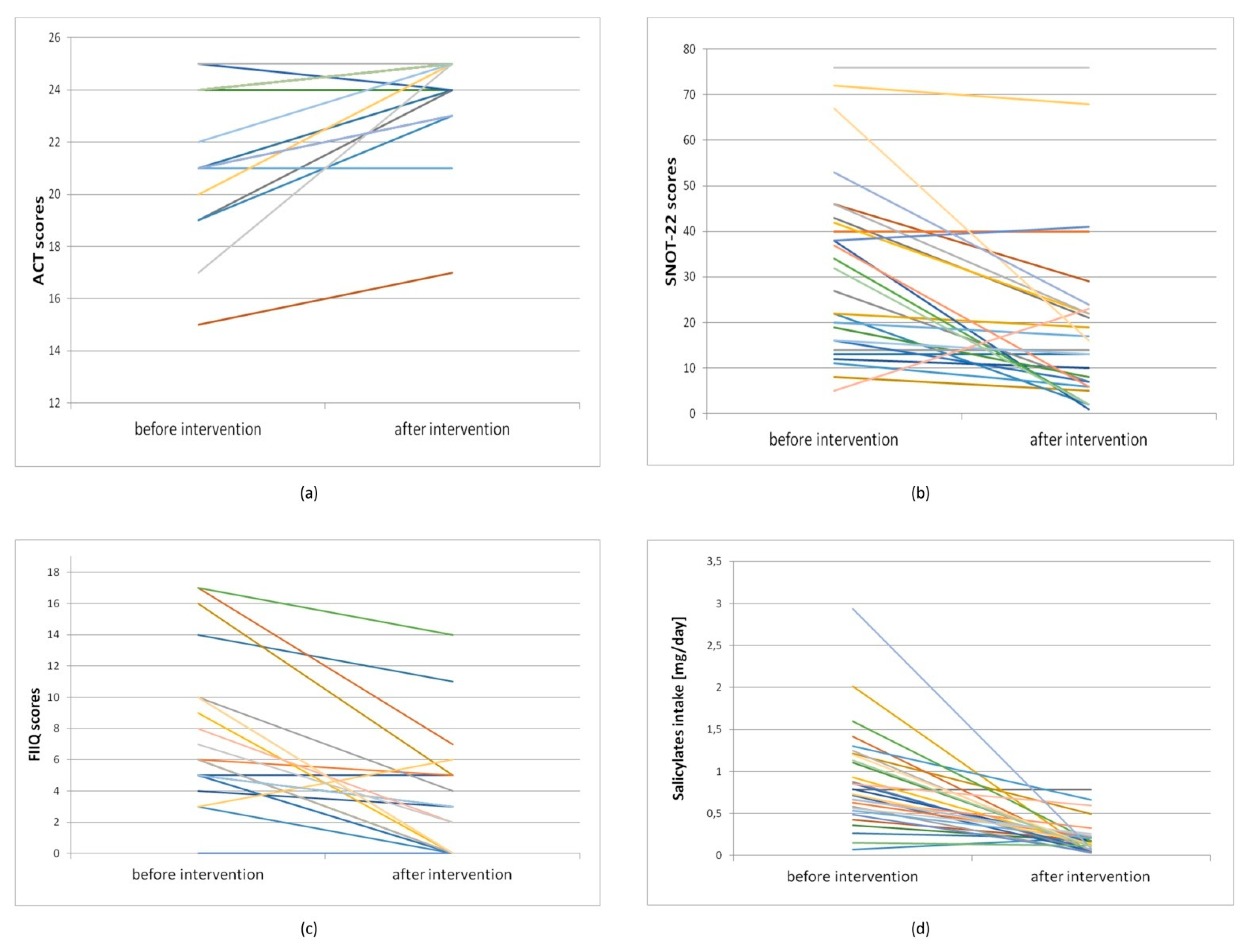
| Mean, mg/day | 0.90 | 0.20 | −0.70 | |
| Median, mg/day | 0.79 | 0.15 | p < 0.001 | −0.60 |
| Min, mg/day | 0.07 | 0.03 | −2.86 | |
| Max, mg/day | 2.94 | 0.79 | 0.14 | |
| SD | 0.58 | 0.19 | 0.61 | |
| Participants | Initial Test before Intervention | Retest after Intervention | p1 | ∆ after vs. before Intervention | |
|---|---|---|---|---|---|
| ACT score | |||||
| All N = 30 | Mean | 23.00 | 24.27 | 1.27 | |
| Median | 24.00 | 25.00 | 0.001332 | 0.00 | |
| Min | 15.00 | 17.00 | −1 | ||
| Max | 25.00 | 25.00 | 8 | ||
| SD | 2.75 | 1.66 | 2.02 | ||
| With asthma N = 20 | Mean | 22.00 | 23.90 | 1.90 | |
| Median | 23.00 | 25.00 | 0.001332 | 1.00 | |
| Min | 15.00 | 17.00 | −1.00 | ||
| Max | 25.00 | 25.00 | 8.00 | ||
| SD | 2.90 | 1.94 | 2.22 | ||
| SNOT-22 score | |||||
| All N = 30 | Mean | 28.97 | 17.13 | −12.07 | |
| Median | 24.50 | 13.00 | 0.000175 | −4.50 | |
| Min | 0 | 0 | −51.00 | ||
| Max | 76.00 | 76.00 | 11.00 | ||
| SD | 20.89 | 18.61 | 14.73 | ||
| With rhinosinusitis N = 27 | Mean | 32.19 | 19.04 | −13.41 | |
| Median | 32.00 | 14.00 | 0.000175 | −9.00 | |
| Min | 5.00 | 1.00 | −51 | ||
| Max | 76.00 | 76.00 | 11 | ||
| SD | 19.47 | 18.67 | 14.94 | ||
| FIIQ score | |||||
| All N = 30 | Mean | 5.90 | 2.43 | −3.57 | |
| Median | 5.00 | 0.00 | 0.000152 | −3.00 | |
| Min | 0 | 0 | −11 | ||
| Max | 17 | 14 | 3 | ||
| SD | 5.11 | 3.51 | 3.75 | ||
| With urticaria N = 23 | Mean | 7.70 | 3.04 | −4.65 | |
| Median | 6.00 | 2.00 | 0.000152 | −5.00 | |
| Min | 0 | 0 | −11 | ||
| Max | 17.00 | 14.00 | 3 | ||
| SD | 4.60 | 3.78 | 3.72 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kęszycka, P.K.; Lange, E.; Gajewska, D. Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study. Nutrients 2021, 13, 991. https://doi.org/10.3390/nu13030991
Kęszycka PK, Lange E, Gajewska D. Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study. Nutrients. 2021; 13(3):991. https://doi.org/10.3390/nu13030991
Chicago/Turabian StyleKęszycka, Paulina K., Ewa Lange, and Danuta Gajewska. 2021. "Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study" Nutrients 13, no. 3: 991. https://doi.org/10.3390/nu13030991
APA StyleKęszycka, P. K., Lange, E., & Gajewska, D. (2021). Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study. Nutrients, 13(3), 991. https://doi.org/10.3390/nu13030991








