1. Introduction
Bioadhesion and development of pathogenic biofilms on non-shedding solid surfaces are the key features for the initiation of caries, gingivitis, periodontitis as well as the increasing periimplantitis [
1,
2,
3,
4,
5]. Due to this fact, one approach in developing new prophylactic mouthwashes is the search for agents that allow targeted and sustainable modulation of initial bioadhesion. The first step of bioadsorption in the oral cavity is the formation of the pellicle. This layer is composed of proteins, peptides, glycoproteins but also metabolites and lipids from the oral fluids [
6,
7,
8]. Over the past decade, several in situ studies have demonstrated a noteworthy correlation between compositional and ultrastructural pellicle modifications and the variation of the pellicles’ protective properties at the tooth surface [
9,
10,
11,
12]. In this context, more and more food components and plant extracts have come into focus. Due to their biocompatibility, substances such as lipids and polyphenolic compounds might get integrated into or interfere with the natural adsorption processes at the tooth surface [
12,
13].
There are controversial data on the impact of oil mouthrinses on (bacterial) biofilm formation and erosion prevention under in vitro or in situ conditions [
10,
14,
15,
16,
17,
18]. Nevertheless, lipids are still of considerable interest due to their lipophilic character [
7]. They account for 20% of the pellicle’s dry weight, but the lack of suitable and reliable methods to obtain the biological material in situ and to detect the specific components have in the past been clear limitations in this field of research [
7,
19,
20]. Hydrophobic interactions are suggested to be a significant driving force for pellicle formation. The topical adsorption of lipophilic substances at the tooth surface after different lipid-containing mouthrinses was shown in a few in situ studies [
7,
10,
17,
19,
21]. However, it remains uncertain whether and how the applied macromolecules are integrated into the in situ pellicle and whether the pellicle’s composition, structural pattern and properties can be altered sustainably, for example, by mouthrinses with edible oils. Precise detection of the applied lipid components is a prerequisite for corresponding investigations.
Up to now, there are only a few studies on the lipid and fatty acid composition of the pellicle layer [
20,
21,
22,
23]. For that reason, we have already investigated the fatty acid content of the in situ pellicle with highly sensitive methods [
19,
24]. Based on gas chromatography coupled with electron impact ionization mass spectrometry, a pellicle-characteristic fatty acid profile with little interindividual variability was determined. Distinct differences were only detected regarding the total amount of adsorbed fatty acids, which also depended on the pellicles’ formation time [
19]. Furthermore, the ultrastructure of the in situ pellicle and the initial biofilm had been characterized in detail by transmission electron microscopy [
25,
26,
27]. Alterations were observed by TEM for a short period after mouthrinses with safflower oil [
10]. In comparison to the native control, 2 h in situ pellicles that had been formed after the application of the oil mouthrinse appeared to be less electron-dense with a rather loose protein accumulation. Following this, an accumulation of lipid droplets at the in situ pellicle’s surface has only recently been visualized by environmental scanning electron microscopy for up to 9.5 h after rinsing with safflower oil [
21].
Based on the knowledge from previous investigations, the present study aimed to clarify if rinses with a specific edible oil can modify the composition and ultrastructure of the pellicle layer sustainably. Linseed oil was selected for this purpose as it contains linolenic acid (18:3), which is not a physiological component of the saliva and accordingly served as a marker molecule. The obtained results will be relevant for the targeted development of new strategies in preventive dentistry that rely on the modulation of native bioadhesion processes. In this context, lipids could also serve as carriers that deliver protective substances to the tooth surface. In addition, valuable information may be gained about the dynamics of biomolecule adsorption at the tooth surface and the turnover of the in situ pellicle in general.
2. Materials and Methods
2.1. Subjects
As a preliminary investigation for this study, native saliva samples were collected from 12 subjects and the fatty acid profile was determined by GC-MS (see below). All volunteers had given their informed written consent about participation in the study. They underwent a clinical oral examination by an experienced dentist to ensure that they had no unrestored carious lesions, periodontal diseases, or an unphysiological salivary flow rate. Due to the high methodical effort, further in situ experiments regarding the effects of mouthrinses with linseed oil on saliva and the in situ pellicle were continued with five participants (aged 21–36). The study design was approved by the Ethics committee of the Medical Faculty, Technische Universität Dresden, Germany (vote: EK 475112016). Individual upper jaw splints were prepared for every participant to enable the exposure of bovine enamel specimens to the oral cavity for intraoral pellicle formation. Enamel slabs were obtained from the incisors of 2-year-old cattle, which are recognized as a suitable substitute for human enamel [
28]. For in situ exposure, all enamel slabs were prepared as described in numerous previous publications [
10,
11,
17,
24]. The slabs’ surfaces were wet-ground and polished according to a standardized protocol with up to 4000 grit abrasive paper, and the resulting smear layer was then removed by ultrasonication in 3% NaOCl for 3 min. Two washing cycles in distilled water for 5 min were followed by disinfection of the specimens in 70% ethanol, all activated by ultrasonication. Finally, the bovine enamel specimens were stored in distilled water at 4 °C for up to 24 h before intraoral exposure.
2.2. Adopted Mouth Rinse and Rinsing Procedure
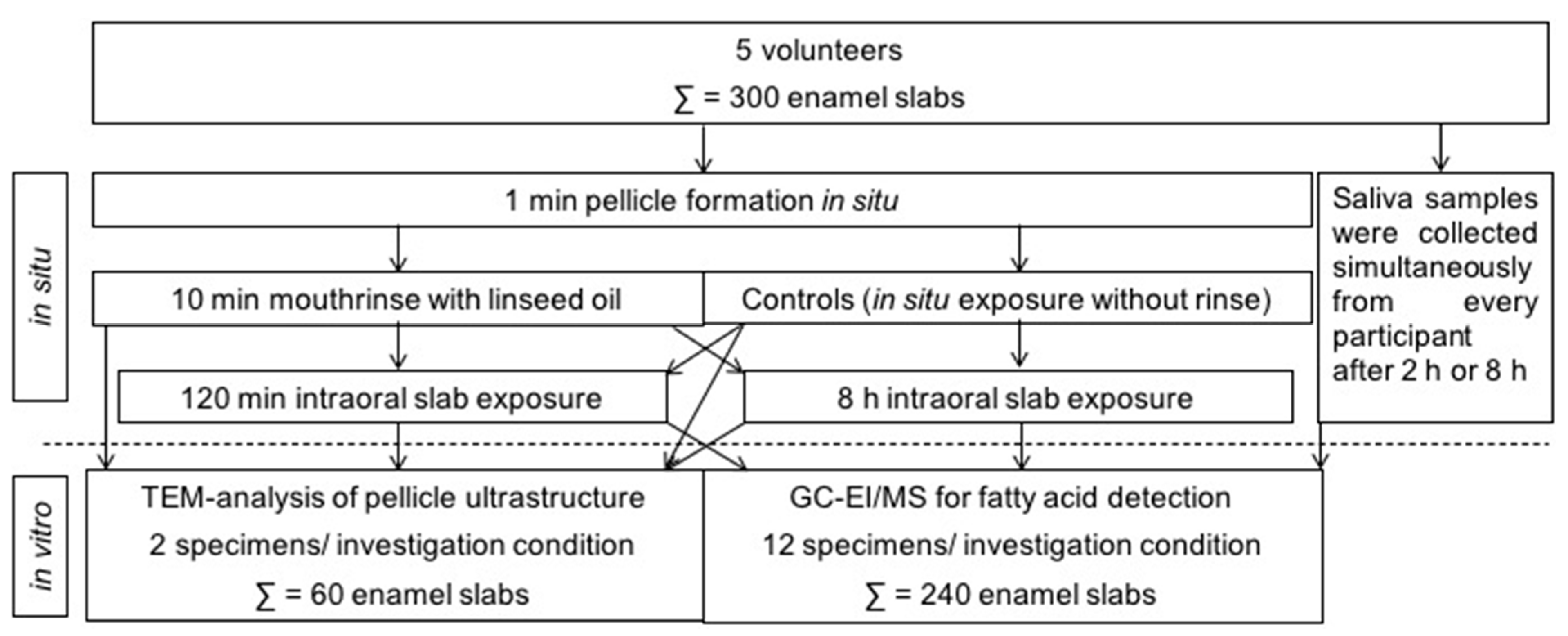
Different in situ experiments were carried out on separate days allowing a wash-out period of at least 48 h in which the subjects were asked to perform their regular oral hygiene. Two hours before an intraoral test period, the volunteers had to brush their teeth without toothpaste. In the following, no food or drinks other than water were to be consumed until the collection of in situ pellicle/biofilm samples. All participants had to carry their individual splints intraorally for either 120 min or 8 h overnight, with the enamel slabs attached in little cavities in the buccal and palatal region of the premolars and the first molar. Twelve enamel slabs per splint were exposed at a time to obtain sufficient in situ pellicle material necessary for the lipid analyses. TEM was performed on two pellicle samples per time of investigation and volunteer. The in situ rinsing protocol and exposure times were planned on the basis of earlier studies in this field [
10,
12,
15,
29], see
Figure 1 for an overview of the experimental setup. After 1 min of pellicle formation in situ, mouthrinses were performed for 10 min with 10 mL of linseed oil. This specific edible oil was chosen as it contains linolenic acid (18:3), which under physiological conditions cannot be detected in the fatty acid profile of human saliva or salivary in situ pellicles. The fatty acid composition of the linseed oil used in this study is shown in
Table 1.
Table 1.
Fatty acid composition of major fatty acids (in %, in relation to total fatty acids) in linseed oil with linolenic acid (18:3) being the predominant fatty acid. Comparison of the composition between oil used as a mouthrinse in this study (composition was determined by the GC-MS method described in this paper) and data provided in literature [
30,
31].
Table 1.
Fatty acid composition of major fatty acids (in %, in relation to total fatty acids) in linseed oil with linolenic acid (18:3) being the predominant fatty acid. Comparison of the composition between oil used as a mouthrinse in this study (composition was determined by the GC-MS method described in this paper) and data provided in literature [
30,
31].
| Fatty Acid | Linseed Oil
(Used in this Study) | Linseed Oil
(Literature Values) |
|---|
| 16:0 | 4–9% | 4–6% |
| 18:0 | 2–9% | 2–3% |
| 18:1 | 12–14% | 10–22% |
| 18:2 | 12–14% | 12–18% |
| 18:3 | 58–66% | 56–71% |
Finally, all enamel slabs were removed from the splints and subjected to in vitro investigation methods. Simultaneously, unstimulated saliva samples were obtained from every participant. These samples were centrifuged at 6000× g for 10 min and sterile filtered (0.2 µm) before analysis. The same experimental runs were made without the use of oil. This allowed the collection of enamel slabs, as well as saliva, which had not been exposed to the oil and served as control.
2.3. GC-MS Analysis of the Fatty Acid Composition
The analysis of fatty acids in saliva and pellicle desorbates was performed as described in detail previously [
24].
2.4. Sample Preparation
Tridecanoic and nonadecanoic acid were used as internal standards and were added to the desorbed pellicle samples before all sample preparation steps. The pellicle lipids were initially separated from the matrix by liquid/liquid extraction. For this step, an adapted Folch extraction procedure was used, in which the lipids were extracted from the desorbed pellicle sample with a mixture of methanol and chloroform. Rapid transesterification (1 h; 100 °C) of all fatty acids (FAs) containing lipids into fatty acid methyl esters (FAME) was carried out in methanol using concentrated HCl (35%, w/w) as an acidic catalyst. The FAMEs were then extracted by adding hexane and deionized water. The hexane phase was isolated, evaporated under a gentle stream of nitrogen, and the residue was redissolved in 0.1 mL of hexane. For GC-MS analyses, 1 µL of this solution was injected.
2.5. GC-MS (Instrumental Conditions)
GC/EI-MS was performed with an ISQ™ 7000 Single Quadrupole GC-MS system (Thermo Scientific, Dreieich, Germany). The injector was operated in splitless mode at 260 °C. For the separation of the target compounds, a TRACE™ TR FAME fused silica capillary column (30 m × 0.25 mm × 0.25 µm; Thermo Scientific, Dreieich, Germany) and helium as carrier gas was used with a constant flow of 1.5 mL/min. The oven temperature was programmed at 50 °C for 5 min, followed by an increase of 6.5 °C/min until the final temperature of 260 °C was reached and then held for 8 min. The electron energy was 70 eV, and the ion source temperature was set to 270 °C. A mass range of m/z 60–400 was recorded in full scan mode. Additionally, selected ion monitoring (SIM), recording fragment ions including m/z 74, m/z 79, m/z 81, and m/z 87 was performed throughout the run.
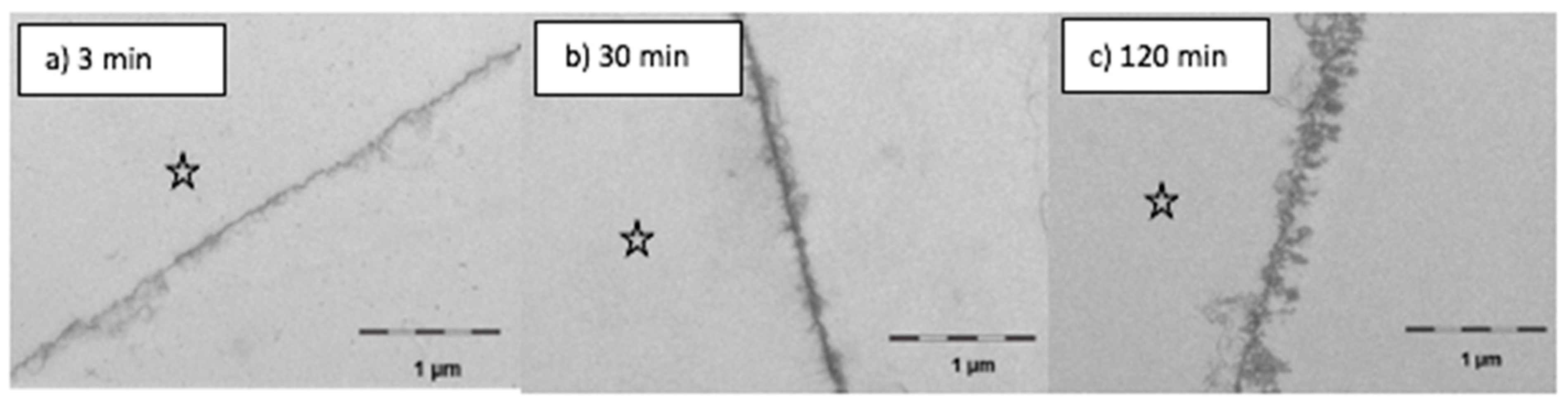
In order to visualize the impact of mouthrinses with linseed oil on the ultrastructure of the in situ pellicle over time, transmission electron microscopy was performed on pellicle samples directly after the rinse, after 2 h or after 8 h of oral exposure, respectively. The findings were compared with control images of a characteristic native 3 min, 30 min, 120 min and an 8 h in situ pellicle. After completion of the oral exposure times, the enamel specimens were carefully rinsed with distilled water and fixed in 2.5% glutaraldehyde and 0.1 M cacodylate buffer for 1 h at 4 °C. Five washing cycles were performed in phosphate buffer before 1% osmium tetroxide was applied for 1 h to visualize the organic structures. The samples were then dehydrated in an ascending alcohol series and embedded in Araldite CY212 (Agar Scientific Ltd., Stansted, United Kingdom). All enamel residues were decalcified with 0.1 M HCl, and the remaining pellicle samples were re-embedded in Araldite. Ultrathin sections of the embedded pellicle samples were cut in series with a diamond knife in an ultramicrotome (Ultracut E, Reichert, Bensheim, Germany). The samples were mounted on pioloform coated copper grids (Plano, Wetzlar, Germany), and uranyl acetate and lead citrate were applied to enhance contrasts. TEM-analysis was performed at magnification from 20,000 to 100,000-fold using a TEM TECNAI 12 Biotwin (FEI, Eindhoven, The Netherlands). Representative images were taken during the investigation of the entire pellicle samples.
4. Discussion
In recent years, few in situ studies have been published that made a scientifically sound contribution to the incomplete knowledge about the involvement of lipids in initial bioadhesion processes at the tooth surface [
10,
15,
19,
21,
32]. Clearly, as had already been suggested earlier, lipids and lipophilic molecules must be regarded as essential components of the in situ pellicle at various formation times [
19,
20,
33]. At the same time, it appears that a modification of the lipid components provided in the oral cavity may have a detectable effect on the pellicle’s composition, structure, and functional properties [
10,
17,
21]. However, only modern and highly sensitive analytical methods will give greater certainty about the actual integration of lipid components into the pellicle layer and their alteration due to remodeling processes.
With this aim in mind, the performed application of gas chromatography coupled with electron impact ionization mass spectrometry (GC–EI/MS) as well as electron microscopic techniques (TEM) were suitable methods to detect and quantify (specific) lipid components in the in situ pellicle samples. At the time the study was conducted, micellar molecule aggregations had already been visualized in pellicle samples shortly after mouthrinses with edible oils and lipids containing food components [
10,
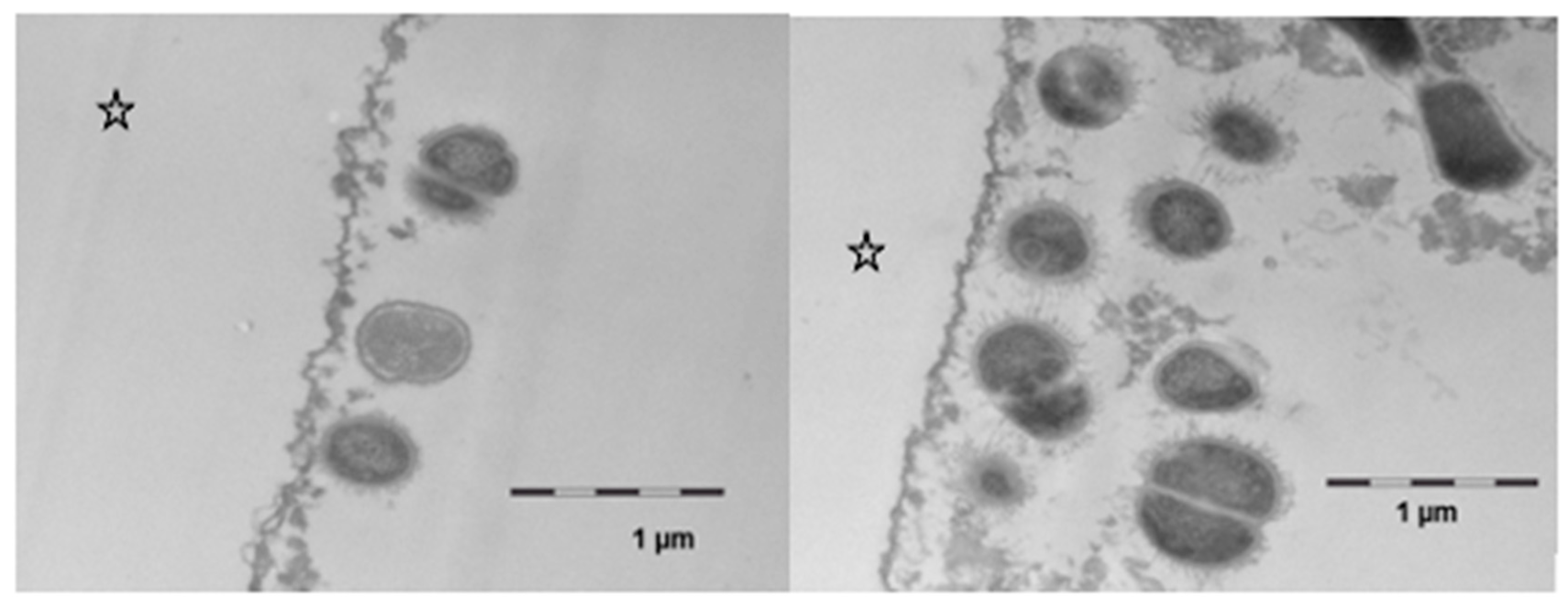
11]. Similar observations were made in the present investigation. In comparison to the native control, the ultrastructure of the in situ pellicle after mouthrinses with linseed oil showed a slightly more heterogeneous pattern during the first 120 min of pellicle formation (
Figure 6,
Figure 7 and
Figure 8). It must be assumed that lipid components were adsorbed to the pellicle layer and that the resulting molecular interactions had a notable effect on the adsorption and distribution of proteins at the tooth surface. Most biological lipids (phospholipids, sphingolipids, glycolipids), as well as free fatty acids, are amphiphilic molecules with hydrophilic and lipophilic properties [
20]. On the one hand, hydrophobic repellent forces of the long hydrocarbon chains of fatty acids could be the reason for the slightly loosened ultrastructure of the second pellicle layer. On the other hand, in aqueous solution, fatty acids tend to form micellar aggregations (
Figure 7b). It is even conceivable that these acted as carrier systems that delivered non-polar proteins to the tooth surface [
22]. Two hours after the mouthrinse with linseed oil, a subsurface pellicle was occasionally detected, and parts of the basal pellicle appeared slightly more electron dense. The affinity of lipids to bind amphiphilic molecules or proteins, respectively, and to therefore alter the ultrastructure of the in situ pellicle had been described earlier in case of mouthrinses with bovine milk [
17]. However, considering the dynamics of dental biofilm formation and the salivary clearance, it remained uncertain if these pellicle modifications had any sustainable effect for more than 2 h. Furthermore, details on the type of lipids or fatty acids accumulated in the pellicle were preferable to possibly understand their impact on pellicle and initial bacterial biofilm formation.
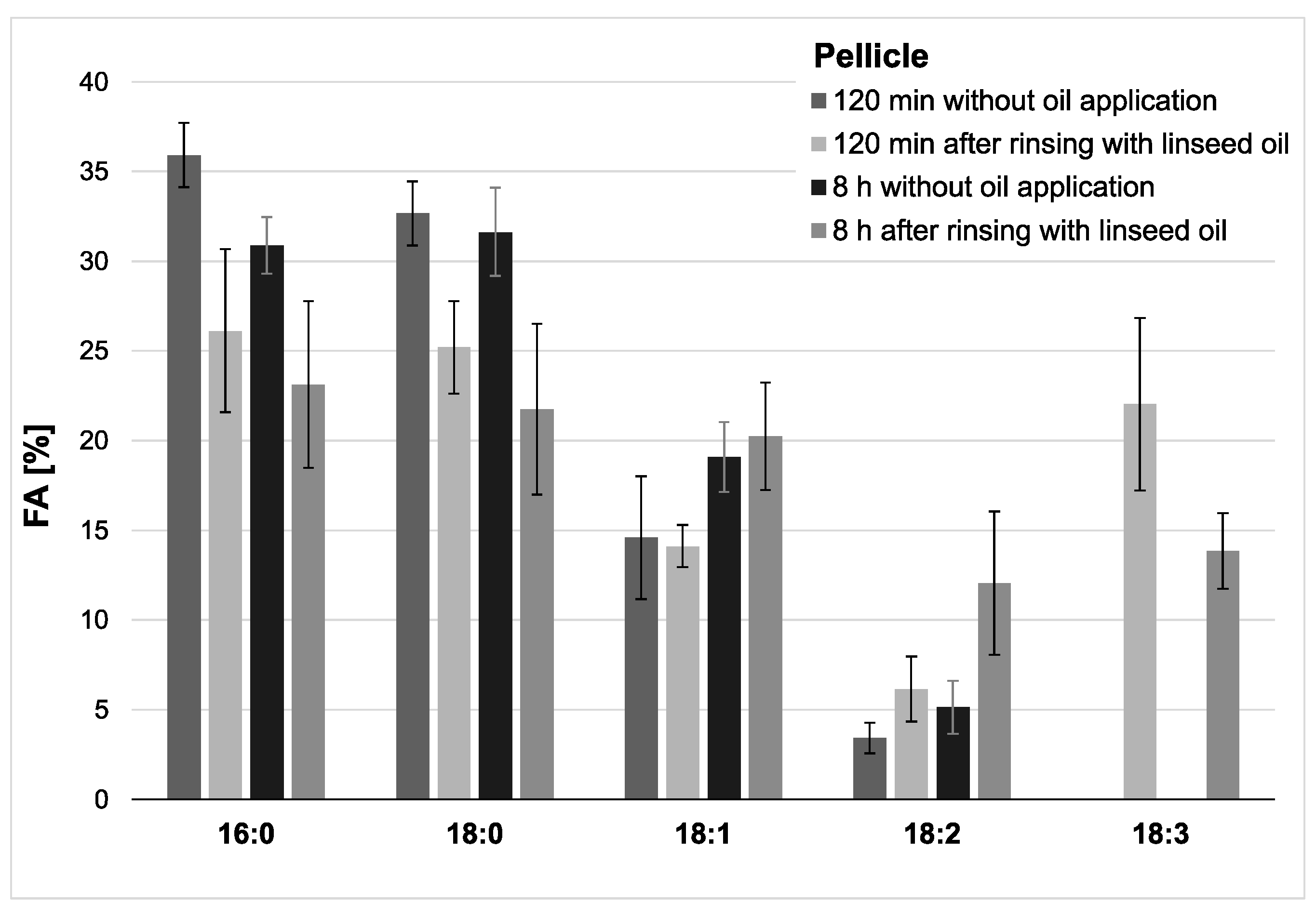
Characteristic distributions of major fatty acids were detected in both physiological saliva and pellicle samples by GC–EI/MS. A comparatively higher proportion of saturated fatty acids in the pellicle demonstrates the selective adsorption of salivary components on the tooth surface. During maturation of the in situ pellicle overnight, the fatty acid profile appeared to be relatively consistent.
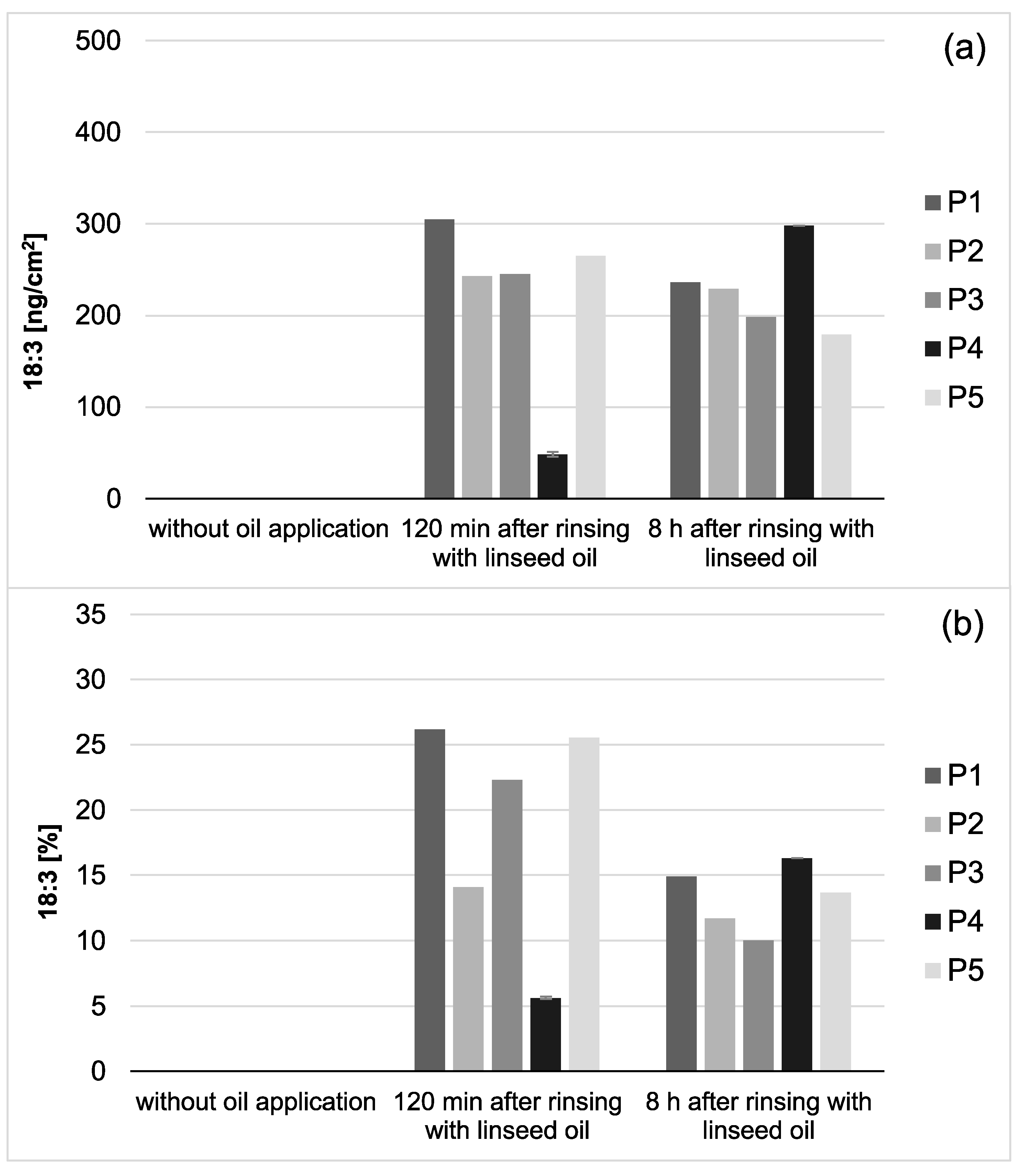
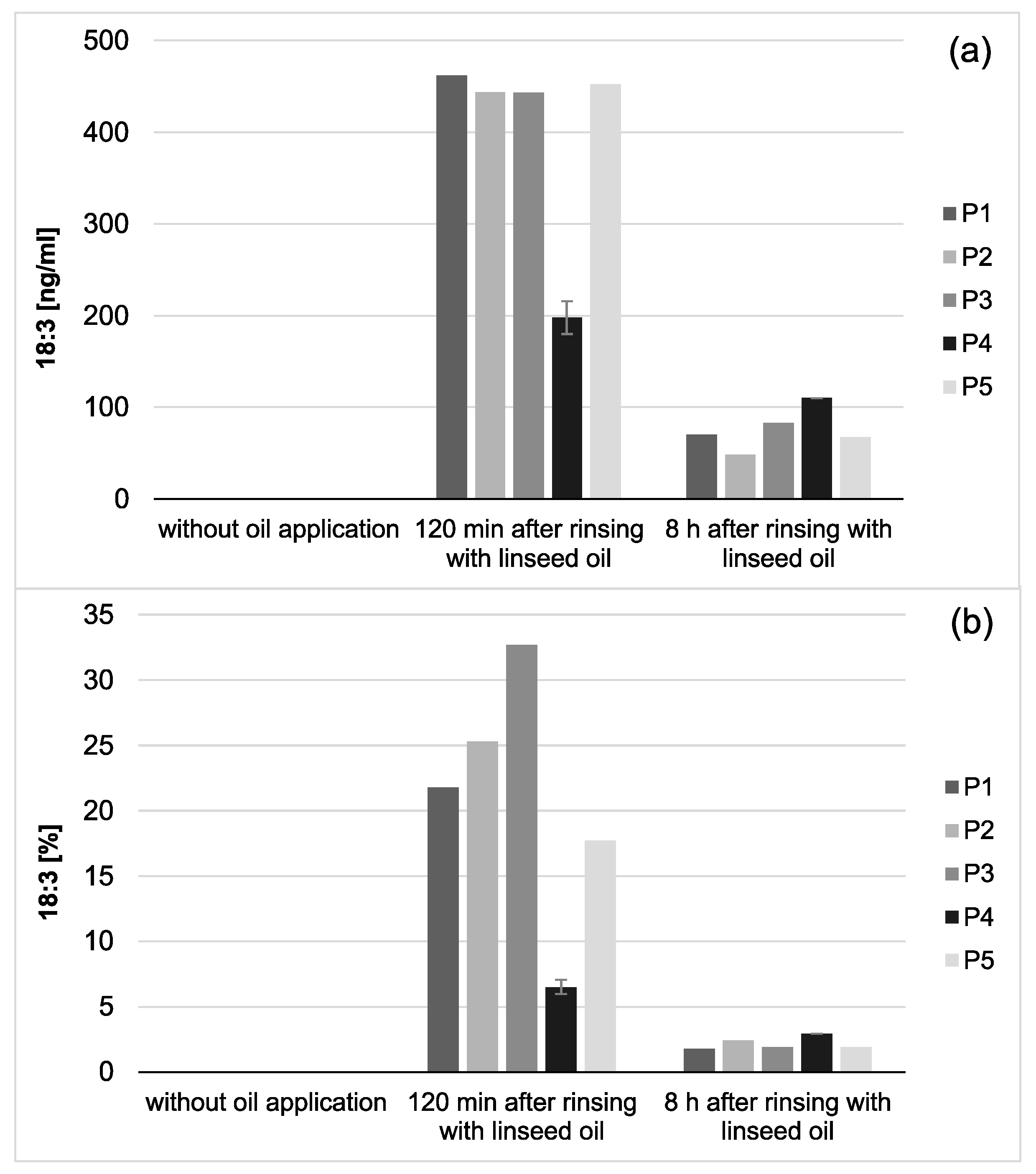
Choosing linseed oil for the mouthrinse allowed the definite detection of oil-associated lipid accumulations in all investigated saliva and pellicle samples by GC–EI/MS. Linolenic acid (18:3) is not included in the physiological fatty acid profile of the oral fluids, and its detection in the pellicle could reliably be correlated with its absorption from the oil mouthrinse. Therefore, this specific fatty acid served as a suitable biological marker for the tracking of bioadhesion processes and their durability at the tooth surface. Based hereon, it was shown that the mouthrinse initially induced a shift of the major fatty acid composition to a higher percentage of unsaturated fatty acids and a remarkable accumulation of linolenic acid in both the saliva and pellicle samples (2 h). As already indicated above, the higher percentage of unsaturated fatty acids and the high reactivity of their double bonds might have influenced the surface wettability and the molecular interactions during pellicle formation. As expected, after 8 h, the mouthrinse alterations of saliva’s major fatty acid profile were almost eliminated due to the salivary clearance. The content of linolenic acid decreased by approximately 85% from 2 to 8 h after the rinse, yet linolenic acid was still traceable (
Figure 3). Interestingly, pellicle maturation during these hours only depended to a certain extent on the availability of lipid components provided by the saliva. Oil-induced modifications of the pellicle’s fatty acid profile appeared to be notably more, continuing with a considerably lower turnover after 8 h of initial biofilm formation (~35% less linolenic acid from 2 to 8 h after the oil-rinse,
Figure 2). Simultaneously, augmentation of pellicles’ thickness and bacterial adhesion were shown in the electron microscopic analyses. However, the ultrastructural pattern did not differ notably from the native controls.
Concerning a potential modification of functional pellicle properties in terms of erosion prevention or bacterial biofilm formation, so far, there is no undoubted evidence that mouthrinses with edible oils have a convincing health-promoting effect [
10,
14,
15,
16,
20]. Nevertheless, the present TEM-images still indicate that components of the linseed oil initially affected the morphology of oral bacteria (
Figure 7). Damage to the cell wall integrity of bacteria can be a sign of reduced viability. Taking in vitro studies into account, it can be assumed that certain free fatty acids, possibly also linolenic acid, had an antibacterial effect on salivary bacteria [
34,
35]. Fatty acids might have affected the integrity of bacteria’s cell membranes and interfered in metabolic processes which could explain the deformed morphology of some bacteria shortly after the mouthrinse. However, these effects were only seen in the first 2 h after the mouthrinse. It is also conceivable that bacteria’s adhesion on the pellicle was influenced by the distribution pattern of lipid components in the in situ pellicle. Looking at the TEM-images after 8 h of biofilm formation, bacterial adhesion appeared to have proceeded unhampered, if not had even been enhanced by lipid components, respectively. No clear difference could be seen between the controls and the samples that had been exposed to the linseed oil mouthrinse regarding the morphology of the adhering bacteria. This is in line with previous in situ investigations that did not measure a significant effect of edible oil mouthrinses on the adherence or live/dead ratio of bacteria at the tooth surface after 8 h of biofilm formation [
15]. Further investigations in this field will be necessary.