Efficacy of Functional Foods, Beverages, and Supplements Claiming to Alleviate Air Travel Symptoms: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Scoping Review
2.1.1. Search Strategy
2.1.2. Product Inclusion and Exclusion Criteria
2.1.3. Product Database Formation
2.1.4. Ingredient Synthesis of Products within the Database
2.2. Systematic Review
2.2.1. Search Strategy
2.2.2. Eligibility Criteria
- Population: healthy adults aged over 18 years without pre-existing health conditions that would impact the primary outcome of the intervention.
- Intervention: the administration of a food, beverage, or nutritional supplement to participants at any time before, during, or after a commercial air flight or simulation.
- Comparator: an appropriate control or comparison group receiving no intervention, a placebo, or standard management and underwent the same air flight or simulation as the intervention group.
- Outcomes: any qualitative or quantitative measurement of physical or cognitive symptoms associated with air travel.
- Conducted under military or space flight settings, as the conditions of speed and altitude are not comparable to commercial air travel.
- Examined a combination pharmacological (other than caffeine and melatonin) or non-pharmacological therapies, whereby the specific effect of the test food, beverage, or nutritional supplement could not be ascertained.
- Non-English texts.
- Full paper was not available.
2.2.3. Study Selection
2.2.4. Data Extraction
2.2.5. Data Synthesis and Analysis
2.2.6. Quality Assessment
3. Results
3.1. Scoping Review
3.2. Systematic Review
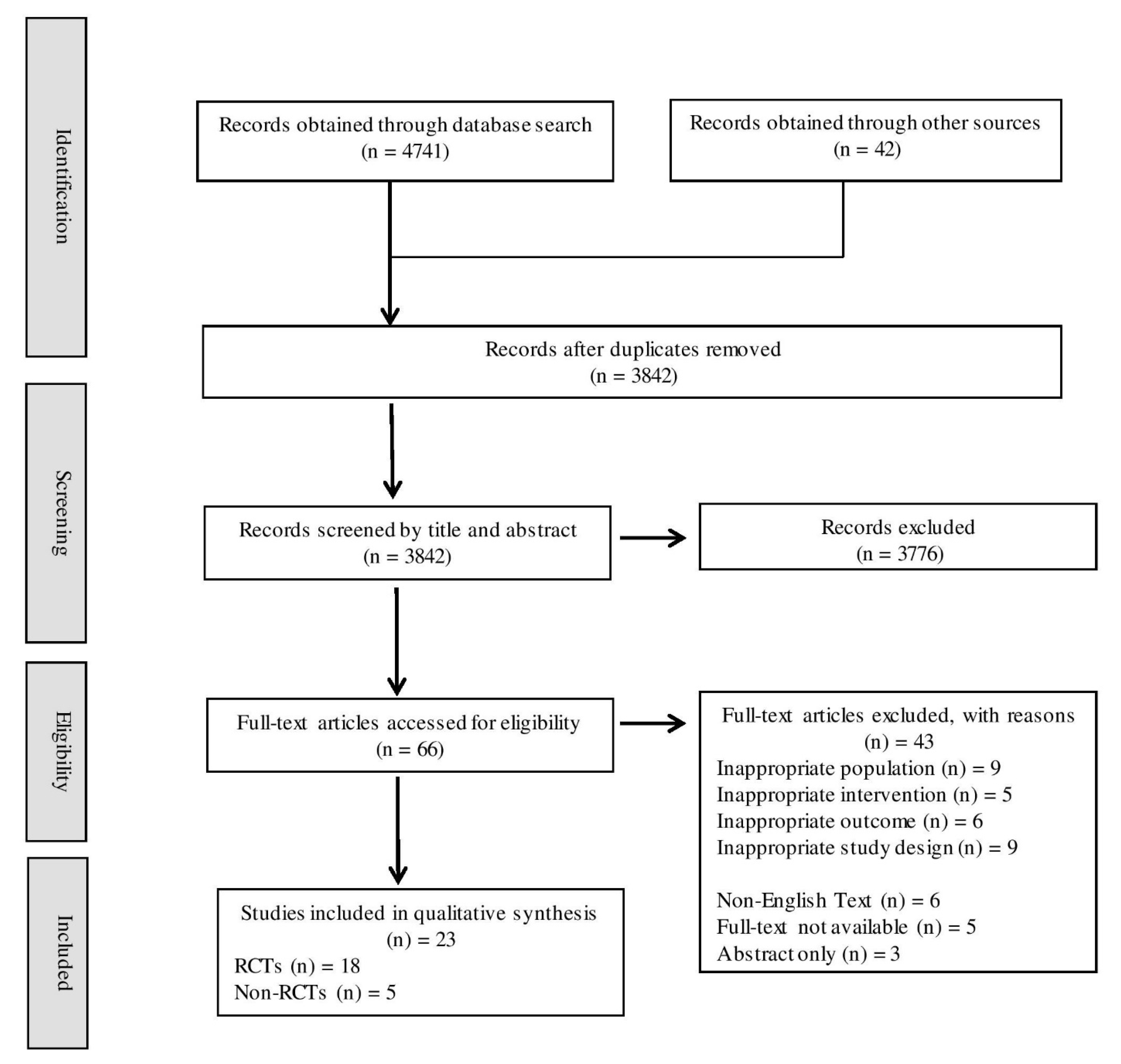
3.2.1. Study Selection
3.2.2. Characteristics of All Included Studies
| (a) | ||||||
|---|---|---|---|---|---|---|
| Author, Year, Study Design 1 | Agent 1 | Flight Conditions 1 | Trial Arms (n) 1 | Participant Characteristics 1 | Intervention Description 1 | Duration 1 |
| Cesarone et al., 2001, RCT [20] | Centella asiatica | Commercial air flight (economy class) Length: 3–14 h Direction: NR Country: NR | n = 66 I = 33 C = 33 Power: NR | Age: range 30–50 years Gender: 50% male No previous deep vein thrombosis or flight within the previous 7 days | Two days prior to flight until one day post flight, participants consumed: I = 60 mg Centellase tablet three times per day C = no drug or other treatment, no further information provided. Unclear if participants were blinded | Follow up period: <4 h post flight % followed up: 91% Excluded: dropped out (n) = 6, group NR Compliance: 97% |
| Tiralongo et al., 2016, RCT [21] | Elderberry | Commercial air flight (economy class) Length: 7+ h Direction: NR Country: Australia | n = 325 I = 163 C = 162 Power: n = 140 per intervention arm (α ≤ 0.05, β ≥ 0.80) | Age: mean (SD) 51 (16) years Gender: 44% male Good general health with no known plant allergy or existing respiratory disease; 54% received flu vaccination. | 10–2 days prior to flight consumed 2 capsules/day and 1 day prior to flight until 4–5 days after arrival at destination consumed 3 capsules/day that contained: I = 300 mg/capsule elderberry extract C = placebo, no further information Participants were blinded | Follow up period: 4–5 days post flight % followed up: 87% Excluded: Discontinued (n): I = 10, C = 9 No intervention (n): I = 5, C = 8 Lost to follow up (n): I = 4, C = 6 Compliance: 60% |
| Tiralongo et al., 2012, RCT [22] | Echinacea | Commercial air flight (economy class) Length: 15–25 h Direction: NR Country: Australia | n = 170 I = 85 C = 85 Power: n = 180 (α = 0.05, β = 0.80) | Age: mean (SD) 43 (14) Gender: 33% male Good general health with no known plant allergy, existing respiratory disease | 14–3 days before flight: 1 tablet twice/day. 2 days prior to flight until 7 days post arrival at destination: 2 tablets twice/day. 8 days post arrival until 3 days prior to return flight: 1 tablet twice/day. 2 days prior to return flight until 7 days post return: 2 tablets twice/day. 8–14 days post return 1 tablet twice/day. I = Echinacea (112.5 mg Echinacea purpurea and 150 mg Echinacea angustifolia) C = placebo Note: Sick dose 3 tables twice a day Participants were blinded | Follow up period: 4 weeks post return flight % followed up: 82% Excluded: Excluded due to various reasons (n): I = 3, C = 2 No intervention (n): I = 3, C = 2 Lost to follow up (n): I = 17, C = 10 Compliance: I = 93% and C = 95% |
| Arendt et al., 1988, cross over-RCT [23] | Melatonin | Commercial air flight Length: NR Direction: Both Country: UK, Australia or New Zealand | n = 61 I = 57 C = 56 Power: NR | Age: NR Gender: 72% male | 2 days prior to flight until day prior to arrival 1 tablet at 2 am destination time. Day of arrival until 4 days post arrival 1 tablet at local bedtime. Stay: >14 days Return protocol: repeated with the other intervention arm I = 5.0 mg melatonin C = placebo Participants were blinded | Follow up: 7 days post flight % followed up: 85% Excluded: (n): I = 5, C = 4, only completed single flight direction excluded from within subject comparison Compliance: NR |
| Nickelsen et al., 1991, non-RCT [24] | Melatonin | Commercial air flight Length: 6–11 h Direction: Both Country: West Germany or North America | n = 36 I = 18 C = 18 Power: NR | Age: mean (SD) 26 (3) years Gender: 72% male | Following westbound flight: 1 capsule for 7 days at bedtime. Participants stayed at for >14 days. Following eastbound flight: 1 capsule for 5 days at bedtime I = 5.0 mg melatonin C = placebo Participants were blinded | Follow up: 7 and 5-days post west- or eastbound flights respectively: % followed up: NR Excluded: NR Compliance: NR |
| Petrie et al., 1989, Cross Over RCT [25] | Melatonin | Commercial air flight Length: 26 h Direction: Both Country: New Zealand or UK | n = 20 I = 20 C = 20 Power: NR | Age: range 26–68 Gender: 60% male | 3 days prior to flight and day of flight: capsule at 10:00–12:00 local time. 1–3 days post arrival: capsule at 22:00–24:00 destination time. Stay: 3 weeks. Return protocol: repeated with the other arm. I = 5.0 mg melatonin C = placebo Participants were blinded | Follow up: 10 days post flight % followed up: 100% Excluded (n) = 0 Compliance: NR |
| Petrie et al., 1993, RCT [26] | Melatonin | Commercial air flight Length: NR Direction: West Country: UK | n = 52 I1 = 14 * I2 = 15 * C = 15 * * included in day 6 analysis Power: NR | Age: mean (SD) 35 (8) years Gender: 50% male Air New Zealand Cabin Crew rostered on same 9-day duty. Return trip from New Zealand to UK. Study completed on westbound return journey | 2 days prior to flight: 2–3 a.m. NZST Day of flight: 12 p.m. NZST 1–5 days post arrival: 10–12 pm NZST I1 = 5.0 mg melatonin capsule I2 = 0.5 mg melatonin (+placebo on 2 days prior to flight) capsules C = placebo capsule Participants were blinded | Follow up: 6 days post flight % followed up: 85% Excluded: (n) = 8, final questionnaire not completed group NR Compliance: NR |
| Arendt et al., 1987, RCT [27] | Melatonin | Commercial air flight Length: NR, 8 time zones crossed Direction: East Country: America | n = 17 I = 8 C = 9 Power: NR | Age: mean (SEM) 49 (2) years Gender: 41% male Good general health Return trip from London to Los Angeles with 2 weeks stay. Study completed on eastbound return journey. | 2 days prior to flight and day of flight: 18.00 h local time 1–4 days post arrival: bedtime I = 5.0 mg melatonin capsule C = placebo capsule Participants were blinded | Follow up: 22 days post flight % followed up: 100% Excluded: no jetlag symptoms (n): C = 2 Compliance: NR |
| Claustrat et al., 1992, non-RCT [28] | Melatonin | Commercial air flight Length: NR Direction: East Country: North America | n = 37 I = 20 C = 20 * n = 3 cross over Power: NR | Age: NR Gender: 49% male Good general health Return trip from Lyon to North America with minimum 1 week stay. Study completed on eastbound return journey. | Day of flight: 22-n hours (where n is time lag between departure and destination) 1–3 days post flight: 10–11 pm local time I = 8.0 mg melatonin capsule C = placebo capsule Participants were blinded | Follow up: 7 days post flight % followed up: 72% Excluded: dropped out, (n): I = 5, C = 5, reasons NR but not due to side effects Compliance: NR |
| Edwards et al., 2000, RCT matched pairs [29] | Melatonin | Commercial air flight Length: 24 h Direction: East Country: UK | n = 34 I = 17 C = 17 Power: NR | Age: mean (SD) I = 41 (13) years, C = 41 (12) years Gender: 90% male (participants included) | Day of flight: 18:00–19:00 local time 1–4 days post arrival: 22:00–23:00 local time I = 5.0 mg melatonin capsule C = placebo capsule Participants were blinded | Follow up: 6 days post flight % followed up: 82% Excluded: Illness (n): I = 3 Incomplete dataset (n): C = 3 Compliance: NR |
| Spitzer et al., 1999, RCT [30] | Melatonin | Commercial air flight Length: 6 h Direction: East Country: America | n = 257 * I1 = 64 * I2 = 70 * I3 = 63 * C = 60 * * completersPower: NR | Age: mean (SD) 44 (7) years * Gender: 79% male * Attendees of a pharmaceutical-company-sponsored educational program. * completers Return trip from Norway to New York with 5 days stay. Study conducted on return trip | Day of flight until 5 days post arrival, participants consumed capsules: I1 = 5.0 mg melatonin at bedtime I2 = 0.5 mg melatonin at bedtime I3 = 0.5 mg melatonin 11 h after wake C = placebo Cointerventions: sleep mask on airplane, alcohol avoidance, no sleep medication Participants were blinded | Follow up: 6 days post flight % followed up: 76% Excluded: Noncompleters (n) = 82, reason and group NR Compliance: NR |
| Suhner et al., 2001, RCT [31] | Melatonin | Commercial air flight Length: mean (SD) 12 (4) hours, 6–9 time zones crossed Direction: East Country: America | n = 160 I1 = 40 I2 = 40 I3 = 40 C = 40 Power: NR | Age: mean (SD) 41 (NR) years * Gender: 51% male * Return trip from Switzerland to America with minimum 1 week stay. Study completed on eastbound return journey. * completers | Day of flight: 1700–2100 departure time 1–4 days post arrival at local bedtime I1 = 5.0 mg melatonin + placebo capsules I2 = 10.0 mg zolpidem + placebo capsules I3 = 5.0 mg melatonin + 10.0 mg zolpidem capsules C = placebo + placebo capsules Participants were blinded | Follow up: 4 days post flight (with 4-day baseline measurement 2 weeks post flight) % followed up: 86% Excluded: Noncompliant (n) = 9, group NRAdverse effects (n) = 14, group NR Compliance: NR |
| Suhner et al., 1998, RCT [32] | Melatonin | Commercial air flight Length: NR, 6–8 time zones crossed Direction: East Country: Switzerland or America | n = 320 I1 = 80 I2 = 80 I3 = 80 C = 80 Power: NR | Age: mean (SD) 36 (NR) years Gender: 54% male Good general health | 1–4 days post arrival at local bedtime I1 = 0.5 mg fast release melatonin I2 = 5.0 mg fast release melatonin I3 = 2.0 mg controlled release melatonin C = placebo Participants were blinded | Follow up: 4 days post flight % followed up: 73% Excluded: Noncompliant (n) = 75, group NR Withdrew (medical reasons) (n) = 2, group NR Travel illness (n) = 9, group NR Compliance: 77% |
| Cesarone et al., 2003, RCT [33] | Pinokinase | Commercial air flight Length: 7–8 h Direction: Both Country: UK or America | n = 224 I = 110 C = 114 Power: NR | Age: mean (SD) I = 48 (12) years, C = 50 (13) years. * Gender: 51% male * High risk of deep vein thrombosis but no recent thrombosis (<6 months) * completers | 2 capsules with 250 mL water 2 h prior to flight, repeated 6 h later. I = 150 mg Pinokinase (per capsule, 300 mg total dosage) C = Placebo * SM: exercise and regular water drinking Unclear if participants were blinded | Follow up: acute % followed up: 83% Excluded: Drop out (n) = 18 poor compliance or flight connections, group NR Unclear reason (n) = 20, group NR Compliance: NR |
| Belcaro et al., 2018, non-RCT [34] | Pycnogenol | Commercial air flight (economy class) Length: 8+ h Direction: NR Country: NR | n = 295 I = 90 C1 = 99 C2 = 106 Power: NR | Age: NR Gender: 52% male Participants of varying risk of edema and DVT but no recent thrombosis (<6 months) | 3 days prior to flight until 3 days post flight: I = 50 mg Pycnogenol capsule three times per day (150 mg total dosage) C1 = SM C2 = SM + compression stockings * SM: exercise and regular water drinking Participants were not blinded | Follow up: acute % followed up: 100% Excluded (n) = 0 Compliance: NR |
| Belcaro et al., 2008, non-RCT [35] | Pycnogenol | Study 1: Commercial air flight (economy/business) Length: 10–14 h Direction: West Country: NR | n = 68 I = 38 C = 30 Power: NR | Age: mean (SD) I = 48 (12) years, C = 45 (7) years * Gender: 57% male * Subgroup: mild hypertension treated with anti-hypertensive medication * completers | 2 days prior to flight until 4 days post arrival: I = 50 mg Pycnogenol capsules 3 times per day (150 mg total dosage) C = NR Unclear if participants were blinded | Follow up: 48 h % followed up: 88% Excluded: Non-medical issues or loss of contact (n) = 8, group NR Compliance: NR |
| Study 2: Commercial air flight (economy/business) Length: 7–9 h Direction: NR Country: NR | n = 65 I = 34 C = 31 Power: NR | Age: average 54 (6) years Gender: 52% male Subgroup: mild hypertension treated with anti-hypertensive medication | Follow up: 28 h % followed up: 92% Excluded: Non-medical issues or loss of contact (n) = 5, group NR Compliance: NR | |||
| Belcaro et al., 2004, RCT [36] | Pycnogenol | Commercial air flight Length: 7–12 h Direction: NR Country: NR | n = 244 I = 110 C = 114 Power: NR | Age: NR Gender: NR Moderate-high risk of DVT but no recent thrombosis (<6 months) | 2 capsules with 250 mL water 2–3 h prior to flight, repeated 6 h later. 1 capsule the following day. I = 100 mg Pycnogenol (per capsule, 200 mg total dosage) C = Placebo Unclear if participants were blinded | Follow up: <2 h % followed up: 81% Excluded: lost at end of flight (n) = 13, group NR non-medical reasons (n) = 33, group NR Compliance: NR |
| Cesarone et al., 2005, non-RCT [37] | Pycnogenol | Commercial air flight Length: 7–12 h Direction: NR Country: NR | n = 211 I = 106 C = 105 Power: NR | Age: average (SD) 45 (8) years Gender: NR No recent thrombosis (<6 months) | 2 capsules with 250 mL water 2–3 h prior to flight, repeated 6 h later. 1 capsule the following day. I = 100 mg Pycnogenol (per capsule) C = Placebo Unclear if participants were blinded | Follow up: acute % followed up: 80% Excluded: reasons NR (n): I = 25, C = 17 Compliance: NR |
| (b) | ||||||
| Author, Year, Study Design 1 | Agent 1 | Flight Conditions 1 | Trial Arms (n) 1 | Participant Characteristics 1 | Intervention Description 1 | Duration 1 |
| Caska et al., 2007, RCT [38] | Caffeine | Computer simulation Length: 10 min Direction: NR Country: Australia | n = 30 I1 = 10 I2 = 10 C = 10 Power: NR | Age: mean (SD) 23 (4) years Gender: NR Held current Class 1 Aviation Medical Certificate and abstained from caffeine for 6 h. | Consumed a lemon-based solution after baseline measurement that contained: I1 = 1.0 mg/kg of body weight caffeine I2 = 3.0 mg/kg of body weight caffeine C = 0.0 mg/kg caffeine Participants were blinded | Follow up period: acute % followed up: 100% Excluded: (n) = 0 Compliance: 100% |
| Dagan et al., 2006, Cross over RCT [39] | Caffeine | Computer simulation Length: 15 min Direction: NR Country: NR | n = 24 I1 = 24 I2 = 24 C = 24 Power: NR | Age: range 25–31 years Gender: 100% male No prior experience operating flight simulator | Consumed 1 pill at 23.00 h that contained: I1 = 200 mg modafinil I2 = 200 mg caffeine C = 200 mg starch Washout period: 2 weeks Participants were blinded | Follow up period: acute % followed up: 100% Excluded: (n) = 0 Compliance: 100% |
| Lindseth et al., 2013, Cross Over RCT [40] | Fluid | Computer simulation Length: 20 min Direction: NR Country: America | n = 40 I = 40 C = 40 Power: n = 35 (α = 0.05, β = 0.80) | Age: mean (SD) 20 (2) years Gender: predominately male, no further information Third term in collegiate aviation program | 2-week fluid diet, no alcoholic beverages and caffeine limited to <90 mg/day. I = high fluid (>80 ounces) C = low fluid (<40 ounces) Washout period: 2 weeks Unclear if participants were blinded | Follow up period: acute % followed up: 100% Excluded: (n) = 0 Compliance: NR |
| Hinninghofen, et al., 2006, Cross over RCT [4] | Fiber | Altitude Simulation Length: 8 h Direction: NR Location: NR | n = 16 I = NR C = NR Power: NR | Age: mean (SD) 26 (6) years Gender: 100% male Good general health and no history of gastrointestinal dysfunction | Overnight fasted subjects consumed test meal within 10 min: I = high fiber (20 g) C = low fiber (2 g) Washout period: separate days Participants were blinded for altitude but not for fiber content of test meal | Follow up period: acute % followed up: 100% Excluded: (n) = 0 Compliance: 100% |
| Lindseth et al., 2011, Cross over RCT [41] | Macro-nutrients | Computer simulation Length: 20 min Direction: NR Country: America | n = 45 I1 = 45 I2 = 45 I3 = 45 C = 45 Power: n = 35 (α = 0.05, β = 0.80) | Age: mean (SD) 21 (2) years Gender: NR Participants held current federal aviation administration medical certificates and was in their third semester of commercial plot aviation course | 4-day diet consisting of: I1 = high carbohydrate (56% carbohydrate, 22% fat, 22% protein) I2 = high protein (56% protein, 22% carbohydrate, 22% fat) I3 = high fat (56% fat, 22% carbohydrate, 22% protein) C = control diet (50% carbohydrate, 35% fat, and 15% protein) Washout period: 2 weeks Participants were blinded | Follow up: acute % followed up: 100% Excluded: (n) = 2 reason and group NR Compliance: NR |
3.2.3. Characteristics of Studies Conducted within Flight Settings
3.2.4. Characteristics of Studies Conducted in Simulated Flight Settings
3.2.5. Key Outcomes of Studies Conducted within Flight Settings
3.2.6. Key Outcomes of Studies Conducted within Simulated Flight Settings
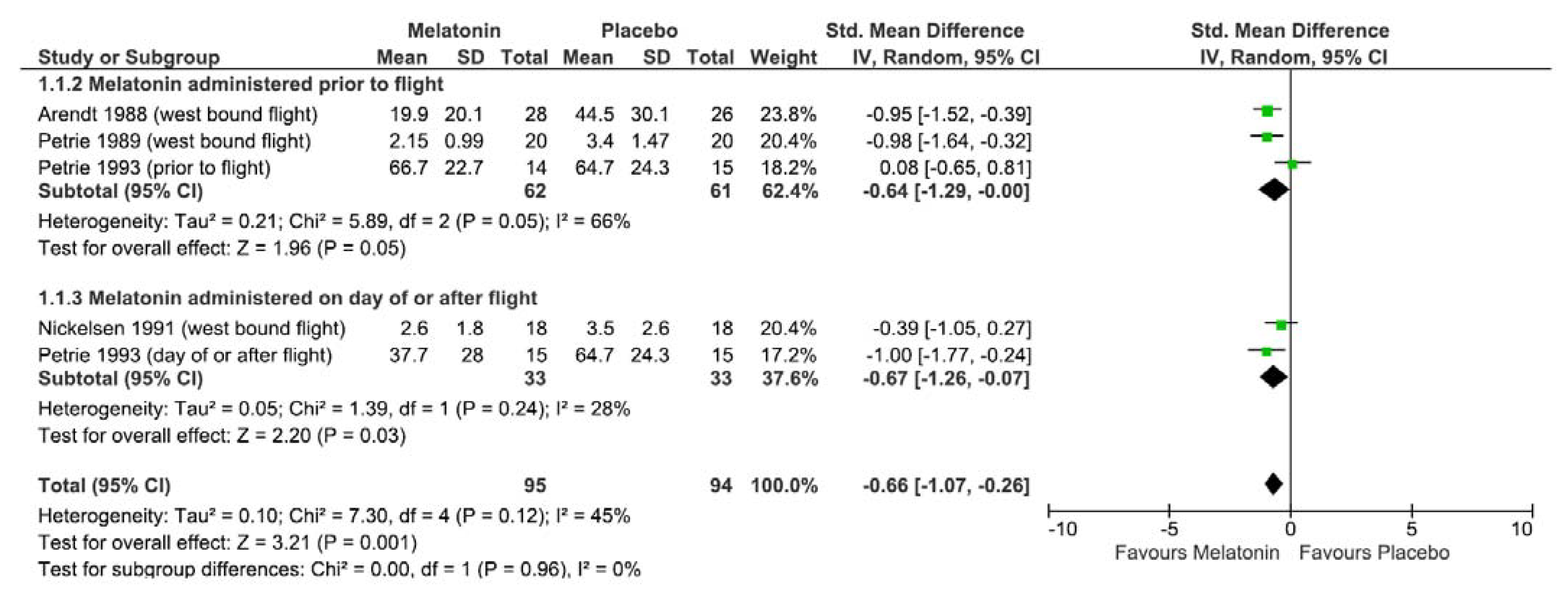
3.2.7. Impact of Melatonin on Self-Reported Jetlag Following Westbound Travel
3.2.8. Impact of Melatonin on Self-Reported Jetlag Following Eastbound Travel
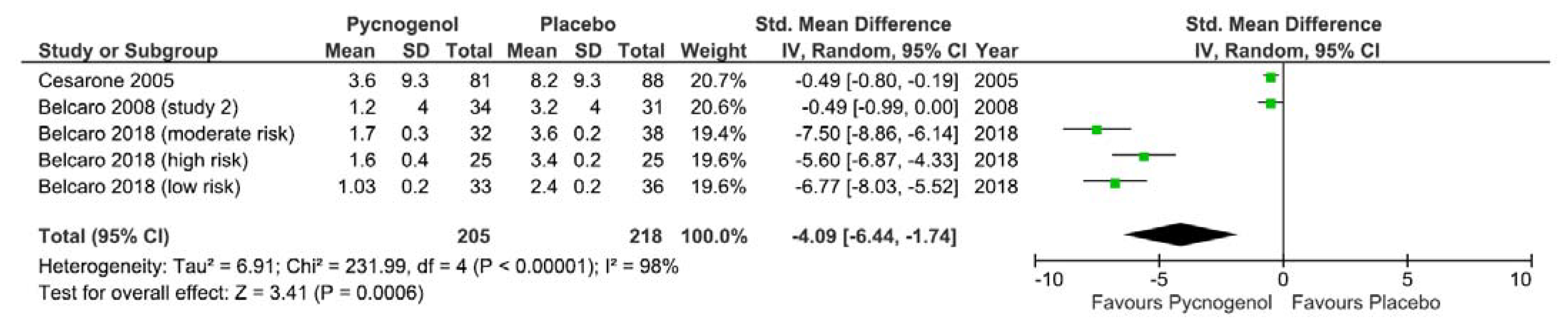
3.2.9. Impact of Pycnogenol on Edema
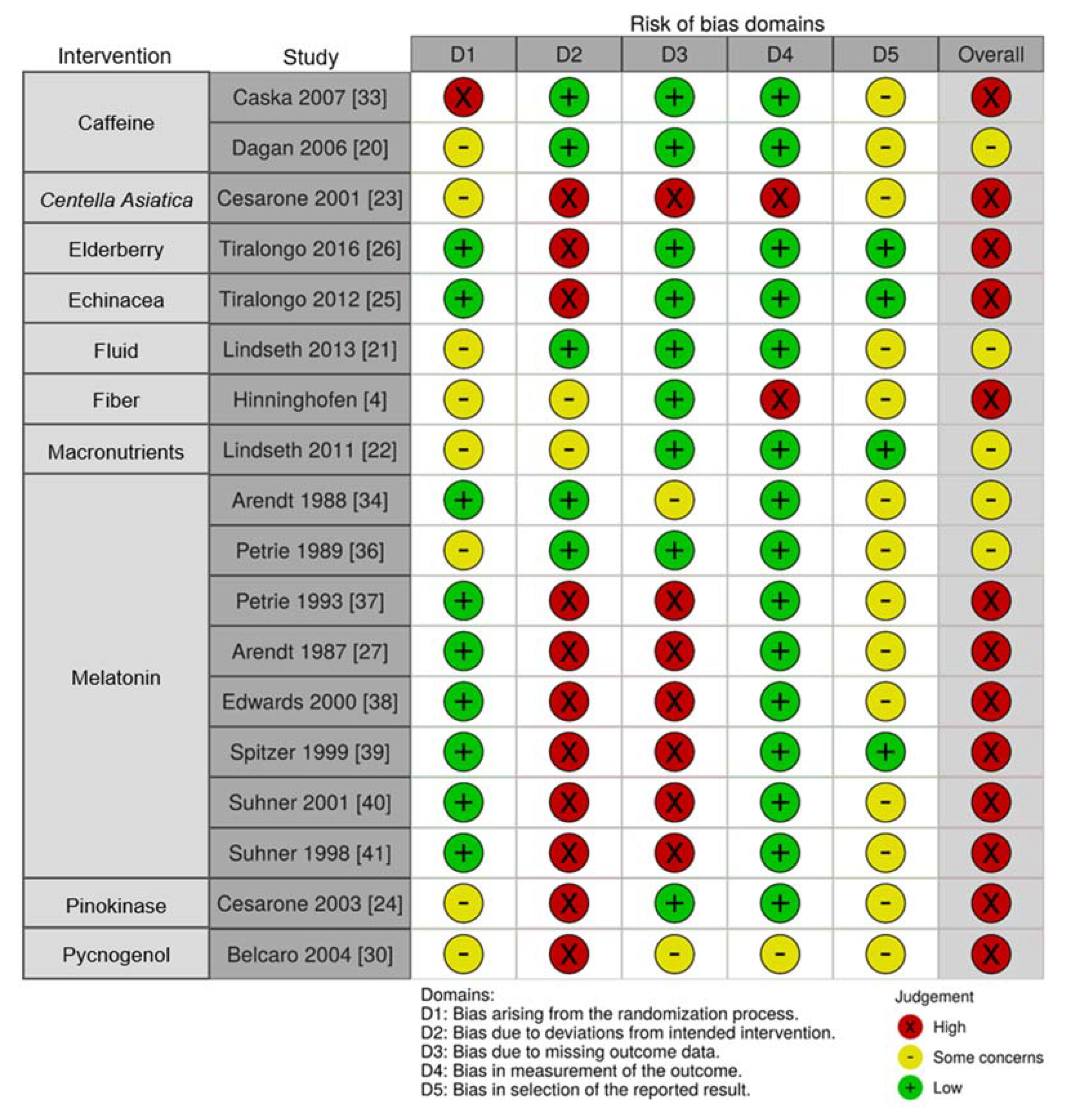
3.2.10. Risk of Bias of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annual Review 2019. Available online: https://www.iata.org/contentassets/c81222d96c9a4e0bb4ff6ced0126f0bb/iata-annual-review-2019.pdf (accessed on 14 October 2020).
- Herxheimer, A.; Petrie, K.J. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst. Rev. 2002, Cd001520. [Google Scholar] [CrossRef]
- Waterhouse, J.; Reilly, T.; Atkinson, G.; Edwards, B. Jet lag: Trends and coping strategies. Lancet 2007, 369, 1117–1129. [Google Scholar] [CrossRef]
- Hinninghofen, H.; Musial, F.; Kowalski, A.; Enck, P. Gastric emptying effects of dietary fiber during 8 hours at two simulated cabin altitudes. Aviat. Space Env. Med. 2006, 77, 121–123. [Google Scholar]
- Hinninghofen, H.; Enck, P. Passenger well-being in airplanes. Auton. Neurosci. 2006, 129, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, M.; Fries, D.; Innerhofer, P.; Schobersberger, B.; Klingler, A.; Partsch, H.; Fischbach, U.; Gunga, H.-C.; Koralewski, E.; Kirsch, K.; et al. Formation of Edema and Fluid Shifts During a Long-haul Flight. J. Travel Med. 2006, 10, 334–339. [Google Scholar] [CrossRef]
- Mangili, A.; Gendreau, M.A. Transmission of infectious diseases during commercial air travel. Lancet 2005, 365, 989–996. [Google Scholar] [CrossRef]
- Silverman, D.; Gendreau, M. Medical issues associated with commercial flights. Lancet 2005, 13, 2067–2077. [Google Scholar] [CrossRef]
- Bin, Y.S.; Postnova, S.; Cistulli, P.A. What works for jetlag? A systematic review of non-pharmacological interventions. Sleep Med. Rev. 2019, 43, 47–59. [Google Scholar] [CrossRef] [PubMed]
- DeHart, R.L. Health issues of air travel. Ann. Rev. Public Health 2003, 24, 133–151. [Google Scholar] [CrossRef]
- Bin, Y.S.; Ledger, S.; Nour, M.; Postnova, S.; Stamatakis, E.; Cistulli, P.A.; de Chazal, P.; Allman-Farinelli, M.; Caillaud, C.; Bauman, A.; et al. How do travelers manage jetlag and travel fatigue? A survey of passengers on long-haul flights. Chronobiol. Int. 2020, 1621–1628. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Chan, V.; Allman-Farinelli, M. Efficacy of Functional Foods, Beverages, and Supplements Claiming to Alleviate Air Travel Symptoms: Protocol for a Systematic Review. JMIR Res. Protoc. 2020, 9, e16155. [Google Scholar] [CrossRef] [PubMed]
- Search Filters: Randomised Controlled Trials Scottish Intercollegiate Guidelines Network. Available online: https://www.sign.ac.uk/assets/search-filters-randomised-controlled-trials.docx (accessed on 12 October 2020).
- Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0 (Updated July 2019). Available online: www.training.cochrane.org/handbook (accessed on 12 October 2020).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Incandela, L.; De Sanctis, M.T.; Belcaro, G.; Geroulakos, G.; Griffin, M.; Lennox, A.; Di Renzo, A.D.; Cacchio, M.; Bucci, M. Flight microangiopathy in medium- to long-distance flights: Prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology 2001, 52 (Suppl. 2), S33–S37. [Google Scholar] [CrossRef]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry Supplementation Reduces Cold Duration and Symptoms in Air-Travellers: A Randomized, Double-Blind Placebo-Controlled Clinical Trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Tiralongo, E.; Lea, R.A.; Wee, S.S.; Hanna, M.M.; Griffiths, L.R. Randomised, double blind, placebo-controlled trial of echinacea supplementation in air travellers. Evid. Based Complement. Altern. Med. 2012, 2012, 417267. [Google Scholar] [CrossRef][Green Version]
- Arendt, J.; Aldhous, M. Further evaluation of the treatment of jet-lag by melatonin: A double-blind crossover study. Ann. Rev. Chronopharmacol. 1988, 5, 53–55. [Google Scholar]
- Nickelsen, T.; Lang, A.; Bergau, L. The effect of 6-, 9-and 11-hour time shifts on circadian rhythms: Adaptation of sleep parameters and hormonal patterns following the intake of melatonin or placebo. Adv. Pineal Res. 1991, 5, 303–306. [Google Scholar]
- Petrie, K.; Conaglen, J.V.; Thompson, L.; Chamberlain, K. Effect of melatonin on jet lag after long haul flights. Br. Med. J. 1989, 298, 705–707. [Google Scholar] [CrossRef]
- Petrie, K.; Dawson, A.G.; Thompson, L.; Brook, R. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol. Psychiatry 1993, 33, 526–530. [Google Scholar] [CrossRef]
- Arendt, J.; Aldhous, M.; English, J.; Marks, V.; Arendt, J.; Marks, M.; Folkard, S. Some effects of jet-lag and their alleviation by melatonin. Ergonomics 1987, 30, 1379–1393. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; David, M.; Sassolas, G.; Chazot, G. Melatonin and jet lag: Confirmatory result using a simplified protocol. Biol. Psychiatry 1992, 32, 705–711. [Google Scholar] [CrossRef]
- Edwards, B.J.; Atkinson, G.; Waterhouse, J.; Reilly, T.; Godfrey, R.; Budgett, R. Use of melatonin in recovery from jet-lag following an eastward flight across 10 time-zones. Ergonomics 2000, 43, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Terman, M.; Williams, J.B.; Terman, J.S.; Malt, U.F.; Singer, F.; Lewy, A.J. Jet lag: Clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am. J. Psychiatry 1999, 156, 1392–1396. [Google Scholar]
- Suhner, A.; Schlagenhauf, P.; Hofer, I.; Johnson, R.; Tschopp, A.; Steffen, R. Effectiveness and tolerability of melatonin and zolpidem for the alleviation of jet lag. Aviat. Space Environ. Med. 2001, 72, 638–646. [Google Scholar]
- Suhner, A.; Schlagenhauf, P.; Johnson, R.; Tschopp, A.; Steffen, R. Comparative study to determine the optimal melatonin dosage form for the alleviation of jet lag. Chronobiol. Int. 1998, 15, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Nicolaides, A.N.; Ricci, A.; Geroulakos, G.; Ippolito, E.; Brandolini, R.; Vinciguerra, G.; Dugall, M.; Griffin, M.; et al. Prevention of venous thrombosis in long-haul flights with Flite Tabs: The LONFLIT-FLITE randomized, controlled trial. Angiology 2003, 54, 531–539. [Google Scholar] [CrossRef]
- Belcaro, G.; Cornelli, U.; Dugall, M.; Hosoi, M.; Cotellese, R.; Feragalli, B. Long-haul flights, edema, and thrombotic events: Prevention with stockings and Pycnogenol supplementation (LONFLIT Registry Study). Minerva Cardiol. Angiol. 2018, 66, 152–159. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Steigerwalt, R.J.; Di Renzo, A.; Grossi, M.G.; Ricci, A.; Stuard, S.; Ledda, A.; Dugall, M.; Cornelli, U.; et al. Jet-lag: Prevention with Pycnogenol. Preliminary report: Evaluation in healthy individuals and in hypertensive patients. Minerva Cardiol. Angiol. 2008, 56, 3–9. [Google Scholar]
- Belcaro, G.; Cesarone, M.R.; Rohdewald, P.; Ricci, A.; Ippolito, E.; Dugall, M.; Griffin, M.; Ruffini, I.; Acerbi, G.; Vinciguerra, M.G.; et al. Prevention of venous thrombosis and thrombophlebitis in long-haul flights with pycnogenol. Clin. Appl. Thromb. Hemost. 2004, 10, 373–377. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Rohdewald, P.; Pellegrini, L.; Ippolito, E.; Scoccianti, M.; Ricci, A.; Dugall, M.; Cacchio, M.; Ruffini, I.; et al. Prevention of edema in long flights with Pycnogenol. Clin. Appl. Thromb. Hemost. 2005, 11, 289–294. [Google Scholar] [CrossRef]
- Caska, T.J.; Molesworth, B.R. The effects of low dose caffeine on pilot performance. Int. J. Appl. Aviat. Stud. 2007, 7, 244–255. [Google Scholar]
- Dagan, Y.; Doljansky, J.T. Cognitive performance during sustained wakefulness: A low dose of caffeine is equally effective as modafinil in alleviating the nocturnal decline. Chronobiol. Int. 2006, 23, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Lindseth, P.D.; Lindseth, G.N.; Petros, T.V.; Jensen, W.C.; Caspers, J. Effects of hydration on cognitive function of pilots. Mil. Med. 2013, 178, 792–798. [Google Scholar] [CrossRef]
- Lindseth, G.N.; Lindseth, P.D.; Jensen, W.C.; Petros, T.V.; Helland, B.D.; Fossum, D.L. Dietary Effects on Cognition and Pilots’ Flight Performance. Int. J. Aviat. Psychol. 2011, 21, 269–282. [Google Scholar] [CrossRef]
- Waterhouse, J.; Edwards, B.; Nevill, A.; Atkinson, G.; Reilly, T.; Davies, P.; Godfrey, R. Do subjective symptoms predict our perception of jet-lag? Ergonomics 2000, 43, 1514–1527. [Google Scholar] [CrossRef]
- Ledger, S.; Bin, Y.S.; Nour, M.; Cistulli, P.; Bauman, A.; Allman-Farinelli, M.; Naismith, S.L.; Stamamtakis, E.; Caillaud, C.; De Chazal, P.; et al. Internal consistency and convergent and divergent validity of the Liverpool jetlag questionnaire. Chronobiol. Int. 2020, 37, 218–226. [Google Scholar] [CrossRef]
- Complementary Medicines Overview. Available online: https://www.tga.gov.au/complementary-medicines-overview (accessed on 14 October 2020).
- Food and Medicine Regulation. Available online: https://www.tga.gov.au/community-qa/food-and-medicine-regulation (accessed on 14 October 2020).
- Bero, L.A. Why the Cochrane Risk of Bias Tool Should Include Funding Source as a Standard Item. Cochrane Database Syst. Rev. 2013, 12, ED000075. [Google Scholar]


| Health Claim Category 1 | n 2 | Percentage of Products |
|---|---|---|
| Fatigue | 42 | 37.8 |
| Immunity | 41 | 36.9 |
| Jetlag | 36 | 32.4 |
| Sleep | 36 | 32.4 |
| Hydration Status | 31 | 27.9 |
| Anxiety | 29 | 26.1 |
| Cardiovascular | 24 | 21.6 |
| Cognitive Ability | 16 | 14.4 |
| Gastrointestinal Symptoms | 16 | 14.4 |
| Radiation/Oxidative Stress | 15 | 13.5 |
| Nausea | 11 | 9.9 |
| Inflammation | 5 | 4.5 |
| Ingredient | Product (n) 1 | Percentage of Products Containing |
|---|---|---|
| Vitamins | 44 | 39.6 |
| A | 0 | 0.0 |
| B (not further defined) | 16 | 14.4 |
| B1 | 8 | 7.2 |
| B2 | 7 | 6.3 |
| B3 | 7 | 6.3 |
| B5 | 5 | 4.5 |
| B6 | 14 | 12.6 |
| B7 | 3 | 2.7 |
| B9 | 3 | 2.7 |
| B12 | 8 | 7.2 |
| C | 22 | 19.8 |
| D | 4 | 3.6 |
| E | 4 | 3.6 |
| Minerals | 41 | 36.9 |
| Electrolytes (not further defined) | 13 | 11.7 |
| Sodium | 6 | 5.4 |
| Potassium | 7 | 6.3 |
| Calcium | 3 | 2.7 |
| Magnesium | 19 | 17.1 |
| Chloride | 4 | 3.6 |
| Bicarbonate | 1 | 0.9 |
| Zinc | 20 | 18.0 |
| Other | 18 | 16.2 |
| Macronutrients | 35 | 31.5 |
| Glucose/Sugar/Carbohydrate | 5 | 4.5 |
| Amino Acids/Protein | 26 | 23.4 |
| Dietary Fiber | 2 | 1.8 |
| Pharmacological | 16 | 14.4 |
| Caffeine | 3 | 2.7 |
| Melatonin | 14 | 12.6 |
| Herbal/Supplement | 74 | 66.7 |
| Pycnogenol | 7 | 6.3 |
| Other | 72 | 64.9 |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Author, Year, Citation | Agent | Key Outcome and Measurement Method(s) 1 | Key Results 1 | Adverse Effects 1 | Funding and Conflicts of Interest 1 | Overall Risk of Bias 2 |
| Cesarone et al., 2001 [20] | Centella asiatica | Edema: subjective analogue scale line before and after flight Rate of ankle swelling method: NR | Edema: supplementation was associated with reduced edema after 9 h of flight (I = 2.6, C = 3.6, p < 0.05) when compared to control Rate of Ankle Swelling: supplementation reduced rate of swelling after 3 h of flight (I = 1.2, C = 1.7, p < 0.05) when compared to control | None | Funding: NR | High |
| Tiralongo et al., 2016 [21] | Elderberry | Cold diagnosis and length: Jackson score (daily) | Cold diagnosis: NS difference between number of participants diagnosed with colds (I = 12, C = 17, p = 0.2). Placebo group had longer collective cold episode in days (I = 57, C = 117, p = 0.05) and higher symptom score (I = 247, C = 583, p = 0.02) than intervention. | n = 5–cold like symptoms, fatigue and kidney pain | Industry provided capsules, partial involvement in study design and results publication | High |
| Tiralongo et al., 2012 [22] | Echinacea | Quality of Life: Wisconsin Upper Respiratory Symptom Survey (WURSS-44) at 14 days prior to travel, <1 week and 4 weeks after return flight Respiratory disorder symptom score (RDS+): A WURSS-44 score of 17+ at same time points | Quality of Life: Placebo group had a higher median WURSS-44 score than Echinacea group (I = 13, C = 26, p = 0.05) at within 1 week return time point. NS for baseline (14 days prior) and follow-up (4 weeks post) RDS+: Percentage of participants reporting respiratory illness (WURSS-44 > 17) was lower in Echinacea group than placebo (I = 43%, C = 57%, p = 0.05) at 1 week return time point and 4 week time point (I = 25%, C = 39%, p = 0.03). Baseline NS. | n = 3: vomiting, headache, heart burn, diarrhea n = 2, tingling, burning of tongue and mouth | Industry funding leveraged from an AusIndustry grant through Australian Government | High |
| Arendt et al., 1988 [23] | Melatonin | Jetlag: self-reported using 10 cm visual analogue scale on 6–7 days post flight. | Jetlag (eastbound): Melatonin improved self-reported jetlag ratings compared to placebo (mean (SD): I = 21.4 (19.4 *), C = 39.2 (30.7 *), p = 0.01015) Jetlag (westbound): Melatonin improved self-reported jetlag ratings compared to placebo (mean (SD): 19.9 (20.1 *), C = 44.5 (30.1 *), p = 0.00136 * SD back calculated as per Cochrane handbook [16] | n = 6 Headache n = 5 Nausea n = 4 worsened symptoms | Funding: NR | Some Concerns |
| Nickelsen et al., 1991 [24] | Melatonin | Jetlag: self-reported using visual analogue scale daily and overall retrospective rating. | Jetlag (eastbound): NS in overall self-reported jetlag between melatonin and placebo group (mean (SD): I = 5.2 (2.5), C = 6.6 (2.1), p = 0.071) Jetlag (westbound): NS in overall self-reported jetlag between melatonin and placebo group (mean (SD): I = 2.6 (1.8), C = 3.5 (2.6), p = 0.214) | NR | Funding: NR | Serious |
| Petrie et al., 1989 [25] | Melatonin | Jetlag: self-reported using visual analogue scale on arrival and 16:00 days 1–5, 7 and 10 | Jetlag (both east- and westbound): Melatonin group reported less jetlag than placebo on day 10 (mean (SD): I = 2.15 (0.99), C = 3.40 (1.47), p < 0.01) | NR | Funding: NR | Some concerns |
| Petrie et al., 1993 [26] | Melatonin | Jetlag: self-reported using visual analogue scale daily at 16:00 h for 6 days and day 6 retrospective rating | Jetlag (westbound): Early melatonin group had higher retrospective rating of jetlag on day 6 than late melatonin (mean (SD): I1 5.0 mg = 66.7 (22.7), I2 0.5 mg = 37.7 (28.0), p < 0.05) but similar to placebo group (mean (SD): C = 64.7 (24.3), p > 0.05) | n = 5 for early melatonin: sleeping difficulties, drowsiness, headaches and depression | Funding: NR | High |
| Arendt et al., 1987 [27] | Melatonin | Jetlag: self-reported using 10 cm visual analogue scale on day 7 after arrival | Jetlag (eastbound): melatonin group reported less jetlag than placebo group (mean (SD): I = 11.3 (9.3) *, C = 55.2 (38.2) *, p < 0.01 * values from previous systematic review on melatonin [2] | NR | Funding: Horner Ltd./Nabisco Airline and hotel supplied flights and accommodation | High |
| Claustrat et al., 1992 [28] | Melatonin | Treatment efficiency of melatonin on jetlag: self-reported on day 8 after arrival (10 cm visual analogue scale). | Treatment efficiency (eastbound): melatonin had a greater treatment efficiency score (median: I = 73, C = 48, p < 0.05) than placebo group. * values from previous systematic review on melatonin: mean (SD): I = 34.5 (30.9), C = 52.8 (36.2) [2] | n = 2 hypnotic effects n = 1 tachycardia n = 2 heavy head | Funding: DRET grant | Serious |
| Edwards et al., 2000 [29] | Melatonin | Jetlag: self-reported using visual analog scale (range 1–10) and Liverpool Jetlag Questionnaire (07:00 ± 08:00 h, 12:00 ± 13:00 h, 16:00 ± 17:00 h and 19:00 ± 20:00 h over 6 days) | Jetlag (eastbound): NS in subjective ratings of jetlag between melatonin and placebo groups over 6 days (p = 0.741) and day 6 time point (p = 0.833) | n = 6 headache, n = 4 dizziness, n = 6 “rocking” (n = 5 melatonin p = 0.036) | Funding: NR | High |
| Spitzer et al., 1999 [30] | Melatonin | Jetlag: Columbia Jetlag Scale daily over 7 days | Jetlag (eastbound): NS in ratings of jetlag between melatonin and placebo groups (p = 0.62) | n = 1 difficulty swallowing and breathing | Funding: New York State Office of Mental Health. Recruitment: pharmaceutical sponsored education program. | High |
| Suhner et al., 2001 [31] | Melatonin | Jetlag: Scale (range: 1–3) every evening and 100 mm visual analog scale on day 4 Treatment effectiveness: 100 mm visual analog scale on day 4 | Jetlag (eastbound): NS in subjective ratings between melatonin and placebo groups (p > 0.05) Treatment effectiveness (eastbound): Melatonin more effective than placebo (mean (SEM): I1 = 41.1 (4.9), C = 25.1 (4.4) p < 0.05). * values interpreted from figure. This study was excluded from meta-analysis | n = 17 including: diarrhea, fever, nausea, headache | Funding: NR | High |
| Suhner et al., 1998 [32] | Melatonin | Jetlag: symptoms questionnaire every evening on a 3-point scale | Jetlag (eastbound): NS in ratings of jetlag between melatonin and placebo group (p > 0.05) | Some–authors attributed to jetlag | Funding: NR | High |
| Cesarone et al., 2003 [33] | Pinokinase | Edema: score based on parametric data (edema tester, variations in ankle circumference, volume measurements) and subjective assessment of swelling and discomfort on an analogue scale line (range: 0–10) DVT: ultrasound scan of venous system | Edema: lower edema score after flight in Pinokinase group than control group (mean (SD): I = 7.54 (0.8), C = 9.8 (0.5), p < 0.05) DVT: reduced incidence of DVT in Pinokinase group than control group (n: I = 0, C = 5, p < 0.025) | NR | Funding: not sponsored by company producing materials quoted | High |
| Belcaro et al., 2018 [34] | Pycnogenol | Edema: score based on parametric data (edema tester, variations in ankle circumference, volume measurements) and subjective assessment of swelling and discomfort on an analogue scale line DVT: ultrasound scan of venous system >24 h before flight and >30 h return flight | Edema (low risk group): Pycnogenol group had lower edema than standard management (C1) and compression stockings (C2): mean (SD): I = 1.03 (0.2), C1 = 2.4 (0.2), C2 = 2.1 (0.3), p < 0.05 Edema (moderate risk group): Pycnogenol group had lower edema than standard management (C1) and stockings (C2): mean (SD): I = 1.7 (0.3), C1 = 3.6 (0.2), C2 = 3.4 (0.2), p < 0.05 Edema (high risk group): Pycnogenol group had lower edema than standard management and compression stockings: mean (SD): I = 1.6 (0.4), C1 = 3.4 (0.2), C2 = 3.4 (0.2), p < 0.05 DVT (low risk): Incidence: I = 0, C1 = 0, C2 = 0, nil p-value DVT (moderate risk): Incidence: I = 0, C1 = 1, C2 = 0, nil p-value DVT (high risk): Incidence: I = 0, C1 = 1, C2 = 0, nil p-value | NR | Funding: not sponsored by company producing materials quoted | Serious |
| Belcaro et al., 2008 [35] | Pycnogenol | Study 1: Jetlag: self-reported using visual analog scale (range 1–10) < 48 h post flight | Jetlag: duration (hours) of signs/symptoms of jetlag were reduced in Pycnogenol group when compared to controls (mean (SD): I = 12.2 (7), C = 39.3 (0.8), p < 0.05). | NR | Funding: Italian Society for Vascular Investigations (ISVI), Ministry of Scientific Research (MURST) and Department of Biomedical Sciences, G’D’Annunzio University | Serious |
| Study 2: Edema: CT scan of brain < 28 h post flight and evaluated using cerebral CT edema scale (range: 0–5) | Edema: lower edema score in Pycnogenol group than control group (mean (SD): I = 1.2 (4.0), C = 3.2 (4.0), p < 0.05) * SD back calculated as per Cochrane handbook [16] | |||||
| Belcaro et al., 2004, RCT [36] | Pycnogenol | DVT/SVT: ultrasound scan < 90 min before flight and <2 h post flight | DVT: Pycnogenol group had a lower incidence than control group (I = 0, C = 1, nil p-value) SVT: Pycnogenol group had a lower incidence than control group (I = 0, C = 4, p < 0.05) | NR | Funding: not sponsored by company producing materials quoted | High |
| Cesarone et al., 2005 [37] | Pycnogenol | Edema: edema score (0–12) composed of: analogue line by measuring observer, edema perceived by participant, edema perceived by observer and associated edema signs or symptoms | Edema: The increase in edema score of Pycnogenol group was less than controls following flight (mean (SD) I = 3.6 (9.3), C = 8.2 (9.3), p < 0.05) * SD back calculated as per Cochrane handbook [16] | NR | Funding: NR Materials supplied by Pycnogenol company without conditions | Serious |
| (b) | ||||||
| Author, Year, Citation | Agent | Key Outcome and Measurement Method(s) 1 | Key Results 1 | Adverse Effects 1 | Funding and Conflicts of Interest 1 | Overall Risk of Bias 2 |
| Caska et al., 2007 [38] | Caffeine | Flight performance: horizontal and vertical deviations from prescribed flight path at baseline and 30 min post intervention | Flight performance: NS difference between groups in both mean horizontal (p = 0.60) and vertical deviations (p = 0.77) | NR | Funding: NR | High |
| Dagan et al., 2006 [39] | Caffeine | Flight performance: deviations from prescribed altitude and velocity at 23:00, 01:00, 03:00, 05:00, 07:00, 09:00, and 11:00 h. | Flight performance: Caffeine decreased deviations from altitude from baseline (mean difference = −191.1, p = 0.0093) at 03:00 and velocity from baseline (mean difference: −11.2, p = 0.0115) and control (mean difference: −8.8, p = 0.0444) at 05:00 compared within participants | NR | Funding: NR | Some concerns |
| Lindseth et al., 2013 [40] | Fluid | Flight performance: deviations from prescribed airspeed control, heading control, and altitude control. | Flight performance: NS within subject scores between fluid diet (mean (SD): I high fluid = 231,600.5 (315,627.7), C low fluid = 278,986.8 (194,077.3), p = 0.97) compared within participants. Subgroup analysis: flight performance of individuals that were dehydrated (1–3% participant weight loss) and on a low fluid diet was poorer than those without dehydration (mean (SD): 1–3% body weight loss = 449,005.2 (43,909.0), no weight loss 193,234.9 (72,055.9), p = 0.002) | NR | Funding: US Army Biomedical Research Command and National Institutes of Health | Some concerns |
| Hinninghofen, et al., 2006 [4] | Fiber | Gastric emptying: 13CO2 breath samples-% difference from baseline per minute and cumulatively over 4 h Symptom: Score (range: 1–5) of abdominal pain, distension, bloating, belching, heart burn, and general wellbeing | Gastric emptying: delayed at 2500 m altitude on a high fiber when compared to low fiber (mean (SD): I high fiber 146.31 (58.41) min, C low fiber 193.91 (54.34) min, p = 0.039) Symptoms: high reports of distention (mean (SD): I high fiber 1.33 (0.3), C low fiber 1.07 (0.15),0 p = 0.022) and bloating (mean: I high fiber 1.82 (0.47), C low fiber 1.34 (0.35), p = 0.016) at 2500 m altitude on a high fiber when compared to low fiber | High dietary fiber at 2500 altitude may increase gastrointestinal symptoms | Funding: NR | High |
| Lindseth et al., 2011 [41] | Macro-nutrients | Flight performance: deviations from prescribed airspeed control, heading control, and altitude control | Flight performance: I1 high carbohydrate, I3 high fat and C diets made fewer errors than I2 high protein diet group (mean (SD): I1 = 206.1 (97.6), I2 250.9 (109.8), I3 = 198.2 (100.3), C = 217.5 (135.9), p = 0.05) compared within participants. | NR | Funding: U.S. Army Biomedical Research Award and the National Institutes of Health | Some concerns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, V.; Wang, L.; Allman-Farinelli, M. Efficacy of Functional Foods, Beverages, and Supplements Claiming to Alleviate Air Travel Symptoms: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 961. https://doi.org/10.3390/nu13030961
Chan V, Wang L, Allman-Farinelli M. Efficacy of Functional Foods, Beverages, and Supplements Claiming to Alleviate Air Travel Symptoms: Systematic Review and Meta-Analysis. Nutrients. 2021; 13(3):961. https://doi.org/10.3390/nu13030961
Chicago/Turabian StyleChan, Virginia, Leanne Wang, and Margaret Allman-Farinelli. 2021. "Efficacy of Functional Foods, Beverages, and Supplements Claiming to Alleviate Air Travel Symptoms: Systematic Review and Meta-Analysis" Nutrients 13, no. 3: 961. https://doi.org/10.3390/nu13030961
APA StyleChan, V., Wang, L., & Allman-Farinelli, M. (2021). Efficacy of Functional Foods, Beverages, and Supplements Claiming to Alleviate Air Travel Symptoms: Systematic Review and Meta-Analysis. Nutrients, 13(3), 961. https://doi.org/10.3390/nu13030961







