Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment and Ethical Approval
2.2. Chemicals
2.3. Preparation for RG and Saponin Extract
2.4. Cell Culture
2.5. Biochemical Analysis
2.6. Histological Analysis of the Liver
2.7. Western Blot Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.9. Cytotoxicity
2.10. Statistical Analysis
3. Results
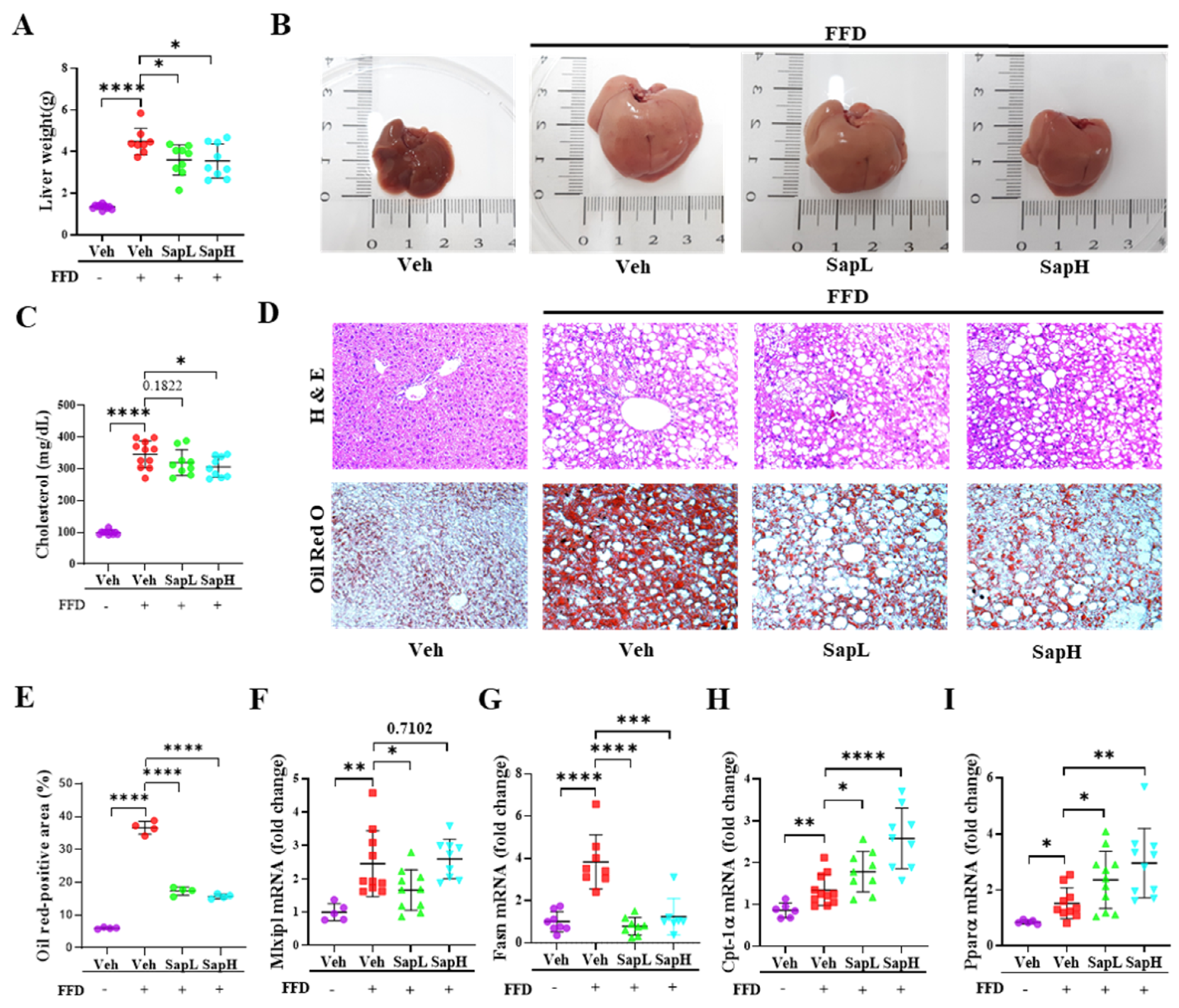
3.1. Saponin Extract Improved FFD-Induced Hepatic Steatosis
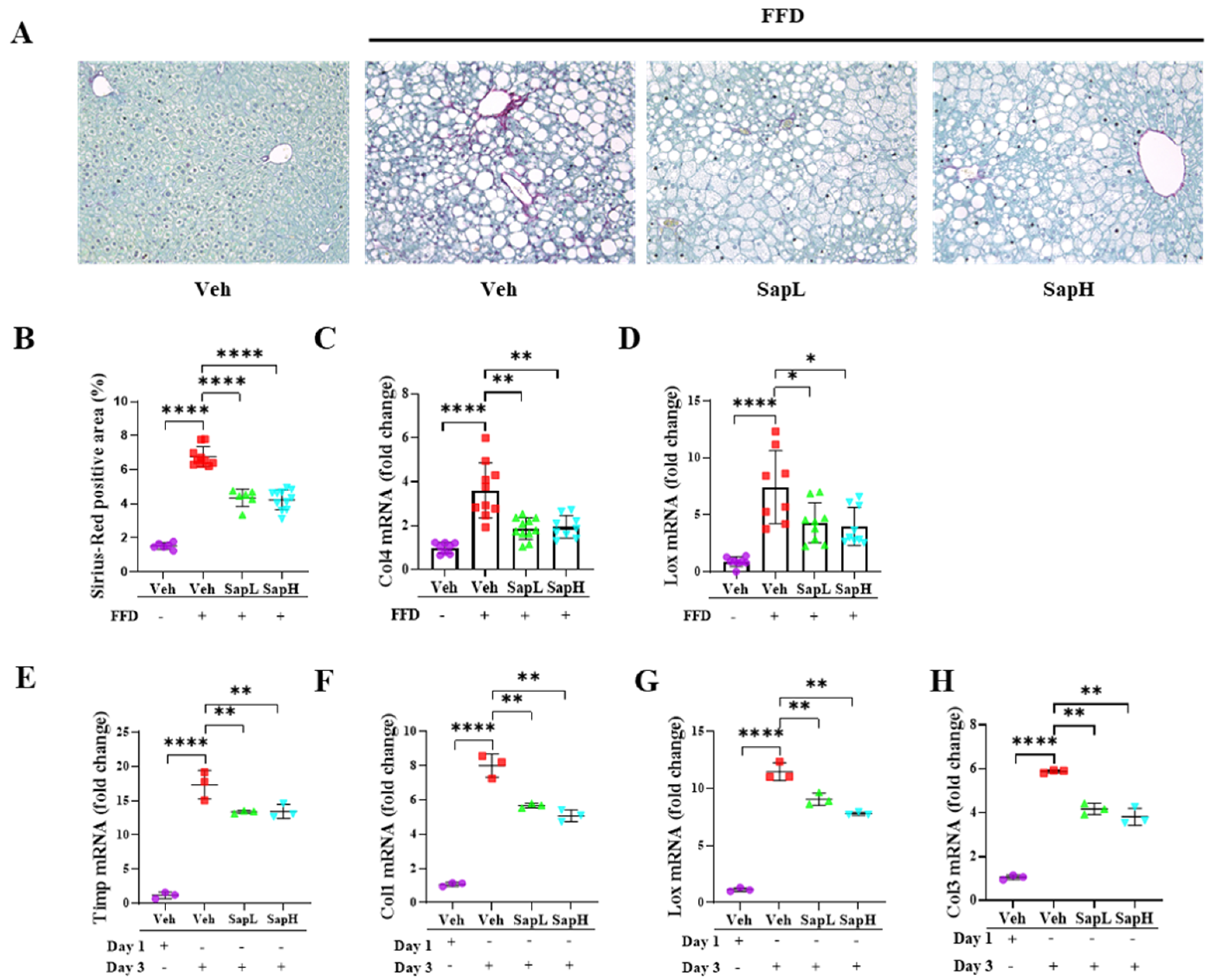
3.2. Saponin Extract Alleviated FFD-Induced Steatofibrosis
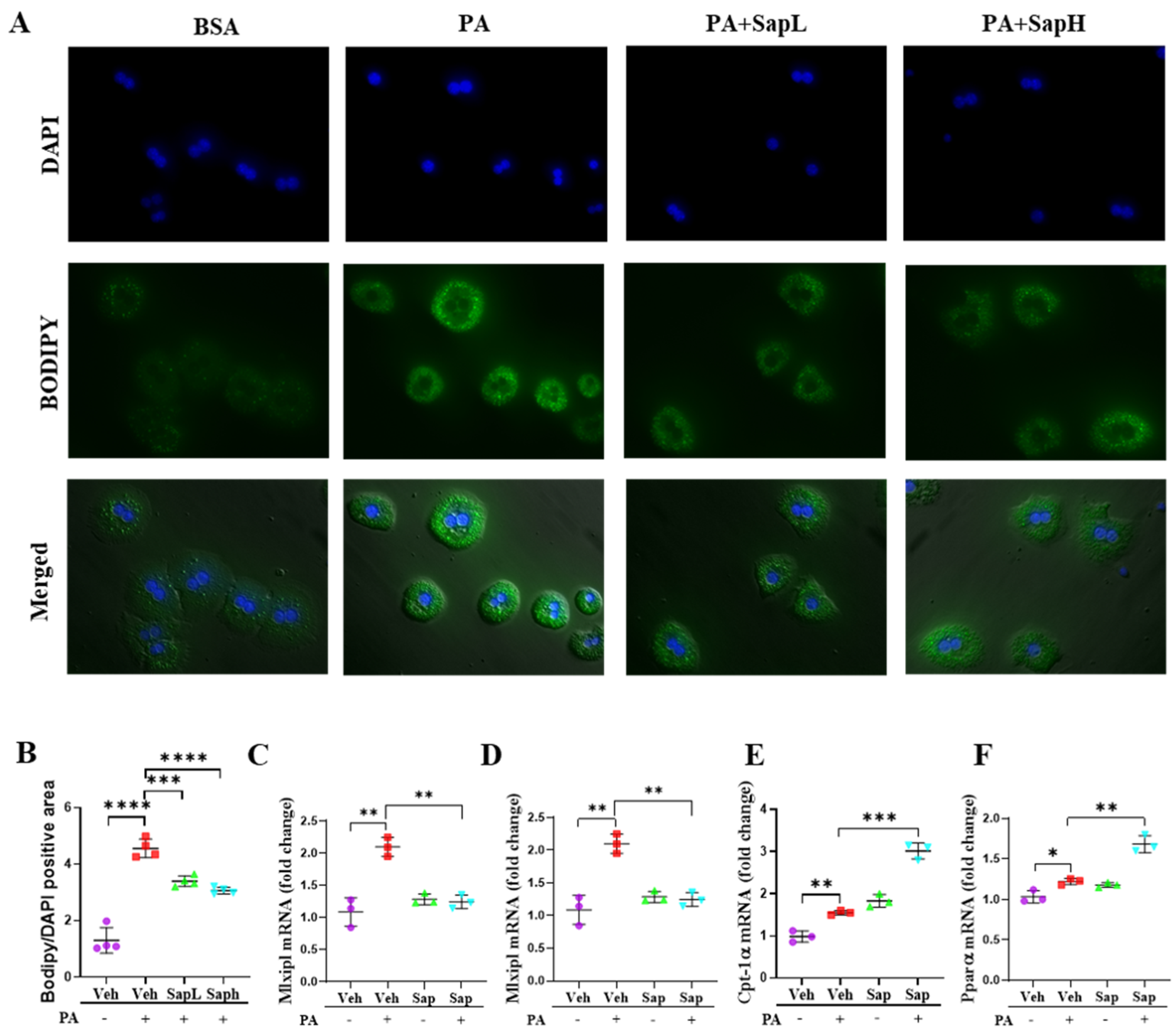
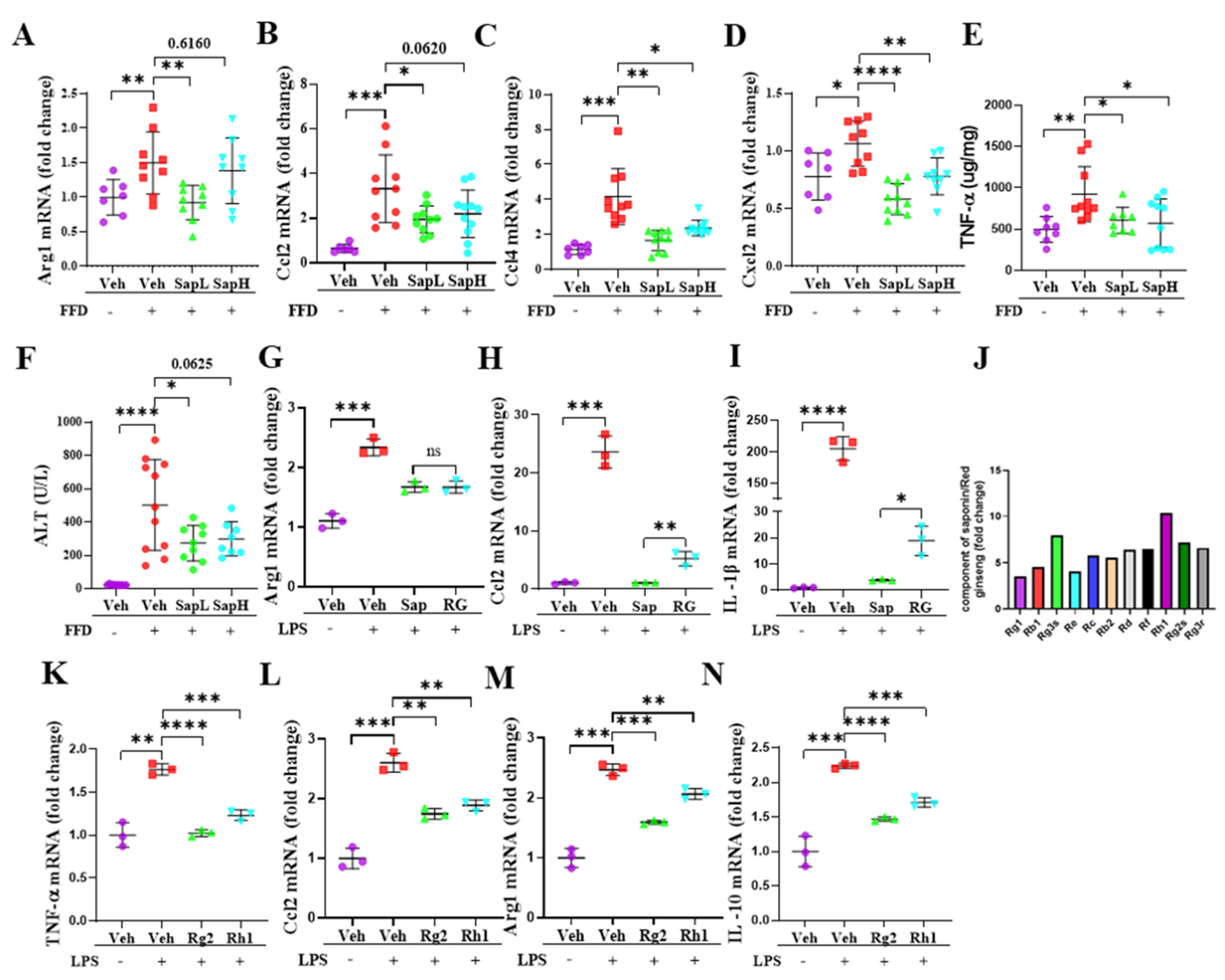
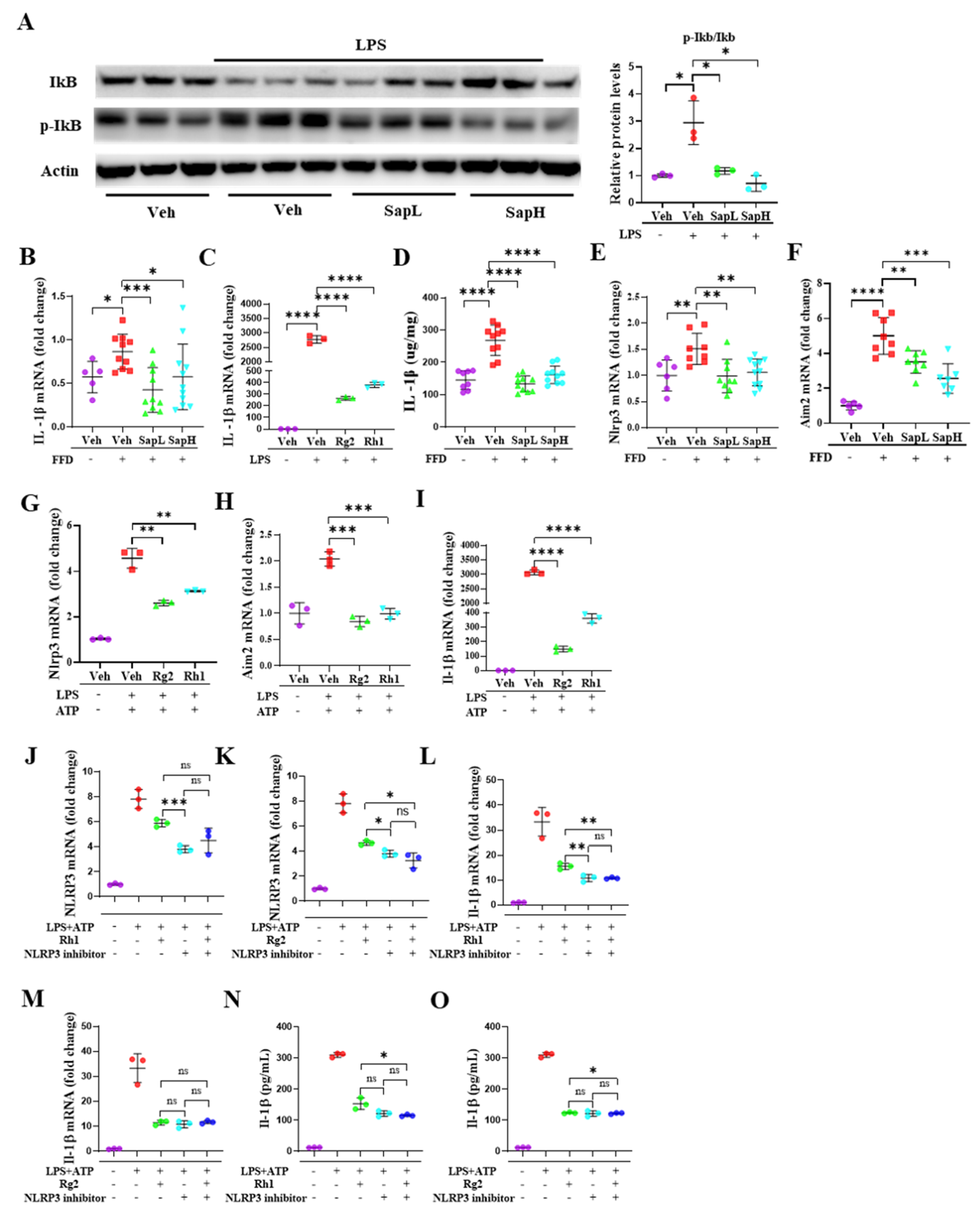
3.3. Saponin Extract Was Found to Contain High Contents of Ginsenosides Rh1 and Rg2, Which Exerted Anti-Inflammatory Effects
3.4. Rh1 and Rg2 Treatment Inhibited NLRP3 Inflammasome Activation
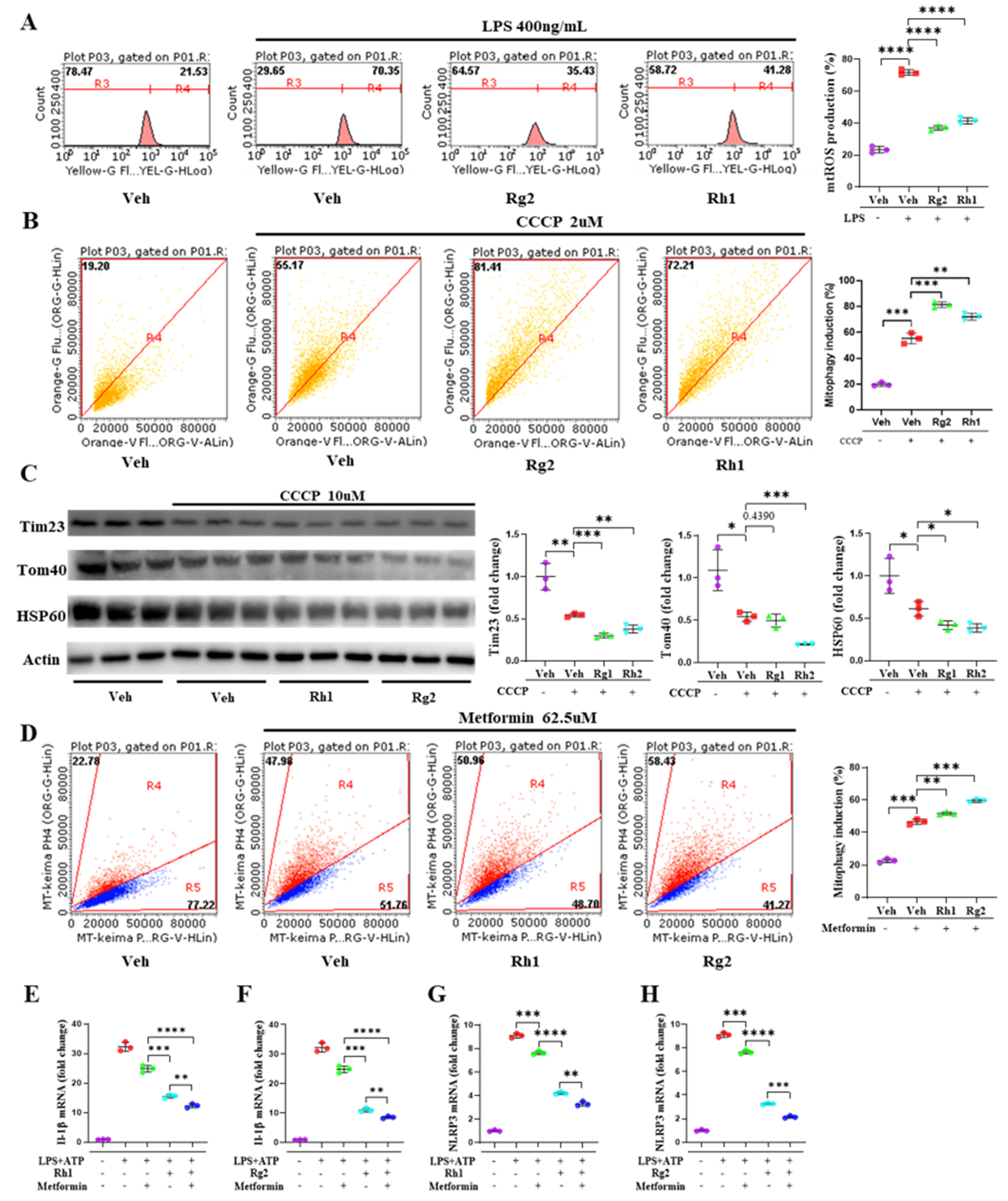
3.5. Ginsenosides Rh1 and Rg2 Alleviated NLRP3 Activation by Promoting Mitophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amarapurkar, D.N.; Hashimoto, E.; Lesmana, L.A.; Sollano, J.D.; Chen, P.J.; Goh, K.L.; Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the asia-pacific region and are there local differences? J. Gastroenterol. Hepatol. 2007, 22, 788–793. [Google Scholar] [CrossRef]
- Smits, M.M.; Ioannou, G.N.; Boyko, E.J.; Utzschneider, K.M. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: Results of a us national survey in three ethnic groups. J. Gastroenterol. Hepatol. 2013, 28, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of nafld and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M. Cirrhosis and liver failure in nonalcoholic fatty liver disease: Molehill or mountain? Hepatology 2008, 47, 1431–1433. [Google Scholar] [CrossRef]
- Dixon, L.J.; Flask, C.A.; Papouchado, B.G.; Feldstein, A.E.; Nagy, L.E. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS ONE 2013, 8, e56100. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic fatty liver disease and insulin resistance: New insights and potential new treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.D.; Lamkanfi, M.; Nunez, G. Intracellular nod-like receptors in host defense and disease. Immunity 2007, 27, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate immunity and inflammation in nafld/nash. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef]
- Wenfeng, Z.; Yakun, W.; Di, M.; Jianping, G.; Chuanxin, W.; Chun, H. Kupffer cells: Increasingly significant role in nonalcoholic fatty liver disease. Ann. Hepatol. 2014, 13, 489–495. [Google Scholar] [CrossRef]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhou, R.; Tschopp, J. The nlrp3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. The inflammasome: A remote control for metabolic syndrome. Cell Res. 2012, 22, 1095–1098. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Csak, T. Inflammasomes in liver diseases. J. Hepatol. 2012, 57, 642–654. [Google Scholar] [CrossRef]
- Ma, X.; McKeen, T.; Zhang, J.; Ding, W.X. Role and mechanisms of mitophagy in liver diseases. Cells 2020, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Czaja, M.J.; Ding, W.X.; Donohue, T.M., Jr.; Friedman, S.L.; Kim, J.S.; Komatsu, M.; Lemasters, J.J.; Lemoine, A.; Lin, J.D.; Ou, J.H.; et al. Functions of autophagy in normal and diseased liver. Autophagy 2013, 9, 1131–1158. [Google Scholar] [CrossRef]
- Yin, X.M.; Ding, W.X.; Gao, W. Autophagy in the liver. Hepatology 2008, 47, 1773–1785. [Google Scholar] [CrossRef]
- Ding, W.X. Role of autophagy in liver physiology and pathophysiology. World J. Biol. Chem. 2010, 1, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef]
- Allaire, M.; Rautou, P.E.; Codogno, P.; Lotersztajn, S. Autophagy in liver diseases: Time for translation? J. Hepatol. 2019, 70, 985–998. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in nlrp3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the nalp3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Palacin, M.; Zorzano, A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009, 89, 799–845. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Won, J.H.; Hwang, I.; Hong, S.; Lee, H.K.; Yu, J.W. Defective mitochondrial fission augments nlrp3 inflammasome activation. Sci. Rep. 2015, 5, 15489. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, R.; Zhou, G.; Zhou, X.; Kou, Z.; Sui, F.; Li, C.; Tang, L.; Wang, Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (burk.) f.H. Chen: A review. J. Ethnopharmacol. 2016, 188, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Hu, Y.; Wu, W.Y.; Ye, M.; Guo, D.A. Saponins in the genus panax l. (araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef]
- Nah, S.Y.; McCleskey, E.W. Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as mu-type opioids. J. Ethnopharmacol. 1994, 42, 45–51. [Google Scholar] [CrossRef]
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, C.; Zhang, S.; Li, J.; Xiao, Q.; Huang, W. Ginsenoside rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biol. Pharm. Bull. 2018, 41, 1638–1644. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, T.; Yang, L.; Wang, H.Y. Ginsenoside rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of sirt1 and activation of ampk. Int. J. Mol. Sci. 2017, 18, 1063. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, H.J.; Kim, J.W.; Hong, S.H.; Kim, J.A.; Lee, Y.B.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Ginsenoside mc1 improves liver steatosis and insulin resistance by attenuating er stress. J. Ethnopharmacol. 2020, 259, 112927. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Park, J.S.; Shon, C.H.; Lee, C.Y.; Ryu, J.M.; Son, D.J.; Hwang, B.Y.; Yoo, H.S.; Cho, Y.C.; Lee, J.; et al. Fermented korean red ginseng extract enriched in rd and rg3 protects against non-alcoholic fatty liver disease through regulation of mtorc1. Nutrients 2019, 11, 2963. [Google Scholar] [CrossRef]
- Kim, J.C.; Jeon, J.Y.; Yang, W.S.; Kim, C.H.; Eom, D.W. Combined amelioration of ginsenoside (rg1, rb1, and rg3)-enriched korean red ginseng and probiotic lactobacillus on non-alcoholic fatty liver disease. Curr. Pharm. Biotechnol. 2019, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, J.J.; Eun, S.H.; Kim, D.H. Anti-inflammatory effects of ginsenoside rg1 and its metabolites ginsenoside rh1 and 20(s)-protopanaxatriol in mice with tnbs-induced colitis. Eur. J. Pharmacol. 2015, 762, 333–343. [Google Scholar] [CrossRef]
- Huynh, D.T.N.; Baek, N.; Sim, S.; Myung, C.S.; Heo, K.S. Minor ginsenoside rg2 and rh1 attenuates lps-induced acute liver and kidney damages via downregulating activation of tlr4-stat1 and inflammatory cytokine production in macrophages. Int. J. Mol. Sci. 2020, 21, 6656. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the nfkappab system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. Nf-kappab signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yoon, J.H.; Ryu, J.H. Mitophagy: A balance regulator of nlrp3 inflammasome activation. BMB Rep. 2016, 49, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Sun, N.; Malide, D.; Liu, J.; Rovira, I.I.; Combs, C.A.; Finkel, T. A fluorescence-based imaging method to measure in vitro and in vivo mitophagy using mt-keima. Nat. Protoc. 2017, 12, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Si, P.P.; Zhen, J.L.; Cai, Y.L.; Wang, W.J.; Wang, W.P. Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress. Neurosci. Lett. 2016, 618, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sapper, T.N.; Mah, E.; Rudraiah, S.; Schill, K.E.; Chitchumroonchokchai, C.; Moller, M.V.; McDonald, J.D.; Rohrer, P.R.; Manautou, J.E.; et al. Green tea extract provides extensive nrf2-independent protection against lipid accumulation and nfkappab pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 2016, 60, 858–870. [Google Scholar] [CrossRef]
- Liou, C.J.; Huang, Y.L.; Huang, W.C.; Yeh, K.W.; Huang, T.Y.; Lin, C.F. Water extract of helminthostachys zeylanica attenuates lps-induced acute lung injury in mice by modulating nf-kappab and mapk pathways. J. Ethnopharmacol. 2017, 199, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Liu, H.; Yuan, Y.; Xie, K.; Xu, P.; Liu, X.; Wen, J. Genetic factors associated with risk of metabolic syndrome and hepatocellular carcinoma. Oncotarget 2017, 8, 35403–35411. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the nf-kappab inflammation pathway through activating ampkalpha-sirt1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84. [Google Scholar] [CrossRef]
- George, K.S.; Roberts, C.B.; Beasley, S.; Fox, M.; Rashied-Henry, K.; Brooklyn Partnership to Drive Down, D. Our health is in our hands: A social marketing campaign to combat obesity and diabetes. Am. J. Health Promot. AJHP 2016, 30, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, S.; Yang, C.; Du, R.; Zhao, J.; Li, J.; Xu, Y.; Qin, Y.; Gao, Y.; Huang, W. Ginsenoside rg1 ameliorates palmitic acid-induced hepatic steatosis and inflammation in hepg2 cells via the ampk/nf-kappab pathway. Int. J. Endocrinol. 2019, 2019, 7514802. [Google Scholar] [CrossRef]
- Song, B.; Sun, Y.; Chu, Y.; Wang, J.; Zheng, H.; Liu, L.; Cai, W.; Zhang, H. Ginsenoside rb1 alleviated high-fat-diet-induced hepatocytic apoptosis via peroxisome proliferator-activated receptor gamma. BioMed Res. Int. 2020, 2020, 2315230. [Google Scholar] [CrossRef] [PubMed]
- Reccia, I.; Kumar, J.; Akladios, C.; Virdis, F.; Pai, M.; Habib, N.; Spalding, D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metab. Clin. Exp. 2017, 72, 94–108. [Google Scholar] [CrossRef]
- Ma, X.; Liu, S.; Zhang, J.; Dong, M.; Wang, Y.; Wang, M.; Xin, Y. Proportion of nafld patients with normal alt value in overall nafld patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N. The role of cholesterol in the pathogenesis of nash. Trends Endocrinol. Metab. TEM 2016, 27, 84–95. [Google Scholar] [CrossRef]
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From nafld to nash to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef]
- Csak, T.; Ganz, M.; Pespisa, J.; Kodys, K.; Dolganiuc, A.; Szabo, G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011, 54, 133–144. [Google Scholar] [CrossRef]
- Rambold, A.S.; Lippincott-Schwartz, J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 2011, 10, 4032–4038. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, L.; Lao, Y.; Liao, W.; Liao, M.; Luo, X.; Wu, J.; Xie, W.; Zhang, Y.; Xu, N. Microrna-181a suppresses parkin-mediated mitophagy and sensitizes neuroblastoma cells to mitochondrial uncoupler-induced apoptosis. Oncotarget 2016, 7, 42274–42287. [Google Scholar] [CrossRef]
- Li, J.; Qi, W.; Chen, G.; Feng, D.; Liu, J.; Ma, B.; Zhou, C.; Mu, C.; Zhang, W.; Chen, Q.; et al. Mitochondrial outer-membrane e3 ligase mul1 ubiquitinates ulk1 and regulates selenite-induced mitophagy. Autophagy 2015, 11, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Storek, K.M.; Monack, D.M. Bacterial recognition pathways that lead to inflammasome activation. Immunol. Rev. 2015, 265, 112–129. [Google Scholar] [CrossRef]
- Song, D.H.; Lee, J.O. Sensing of microbial molecular patterns by toll-like receptors. Immunol. Rev. 2012, 250, 216–229. [Google Scholar] [CrossRef]
- Zhao, C.; Gillette, D.D.; Li, X.; Zhang, Z.; Wen, H. Nuclear factor e2-related factor-2 (nrf2) is required for nlrp3 and aim2 inflammasome activation. J. Biol. Chem. 2014, 289, 17020–17029. [Google Scholar] [CrossRef]
| Component | Western Diet (g) | Sugar Solution (g/L) |
|---|---|---|
| Methionine, DL | 3 | 0 |
| Lodex 10 | 100 | 0 |
| Solka floc, FCC200 | 50 | 0 |
| Corn oil | 10 | 0 |
| Calcium phosphate, dibasic | 4 | 0 |
| V10001C | 1 | 0 |
| Cholesterol, NF | 1.5 | 0 |
| Casein, lactic, 30 mesh | 195 | 0 |
| Sucrose, finely granulated | 350 | 0 |
| Starch, corn | 50 | 0 |
| Butter, anhydrous | 200 | 0 |
| S10001A | 17.5 | 0 |
| Choline bitartrate | 2 | 0 |
| Ethoxyquin | 0.04 | 0 |
| Glucose | 0 | 18.9 |
| Sucrose | 0 | 23.1 |
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| GAPDH | ACGGCAAATTCAACGGCACAG | AGACTCCACGACATACTCAGCAC |
| MLXIPL | CTGGGGACCTAAACAGGAGC | GAAGCCACCCTATAGCTCCC |
| FASN | AGGTGCTAGAGGCCCTGCTA | AGGTGCTAGAGGCCCTGCTA |
| CPT-1α | CGGTTCAAGAATGGCATCATC | TCACACCCACCACCACGAT |
| PPARα | GCTACCACTACGCAGTTCACG | GCTCCGATCACAACTTGTCGT |
| SREBP-1c | GGAGCCATGGATTGCACATT | AGGAAGGCTTCCAGAGAGGA |
| COL4 | AGGAGAGAAGGGTGAACAAGG | CCAGGAGTGCCAGGTAAGCC |
| TIMP1 | TCTGGCATCTGGCATCCTCTTG | TCTGGCATCTGGCATCCTCTTG |
| LOX | GGTTACTTCCAGTACCGTCTCC | GCAGCGCATCTCAGGTTGT |
| COL1 | TAGGCCATTGTGTATGCAGC | ACATGTTCAGCTTTGTGGAC |
| ARG1 | CTCCAAGCCAAAGTCCTTAGAG | CTCCAAGCCAAAGTCCTTAGAG |
| CCL2 | ATTGGGATCATCTTGCTGGT | CCTGCTGTTCACAGTTGCC |
| CCL4 | CTCTGCGTGTCTGCCCTCTC | TGGTCTCATAGTAATCCATCAC |
| CXCL2 | GCCAAGGGTTGACTTCAAGAACA | AGGCTCCTCCTTTCCAGGTCA |
| TNF- α | AGGGTCTGGGCCATAGAACT | CCACCACGCTCTTCTGTCTA |
| IL-1β | CTCGCAGCAGCACATCAACAAG | CCACGGGAAAGACACAGGTAGC |
| IL-10 | GCTGGACAACATACTGCTAACCG | GCTGGACAACATACTGCTAACCG |
| NLRP3 | ACTGAAGCACCTGCTCTGCAAC | AACCAATGCGAGATCCTGACAAC |
| AIM2 | GGTTGATGTTGAATCTAACCACGAA | GGTTGATGTTGAATCTAACCACGAA |
| Sample | Ginsenosides (mg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Rb1 | Rg3s | Re | Rc | Rb2 | Rd | Rf | Rh1 | Rg2s | Rg3r | |
| RG | 0.64 | 4.15 | 2.43 | 0.69 | 1.61 | 1.41 | 0.65 | 0.92 | 0.81 | 1.1 | 0.91 |
| Saponin extract | 2.27 | 18.92 | 19.25 | 2.79 | 9.37 | 7.84 | 4.12 | 5.95 | 8.38 | 7.82 | 5.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Park, J.-S.; Ma, Y.; Ma, H.; Lee, Y.-J.; Lee, G.-R.; Yoo, H.-S.; Hong, J.-T.; Roh, Y.-S. Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation. Nutrients 2021, 13, 856. https://doi.org/10.3390/nu13030856
Wang F, Park J-S, Ma Y, Ma H, Lee Y-J, Lee G-R, Yoo H-S, Hong J-T, Roh Y-S. Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation. Nutrients. 2021; 13(3):856. https://doi.org/10.3390/nu13030856
Chicago/Turabian StyleWang, Feng, Jeong-Su Park, Yuanqiang Ma, Hwan Ma, Yeo-Jin Lee, Gyu-Rim Lee, Hwan-Soo Yoo, Jin-Tae Hong, and Yoon-Seok Roh. 2021. "Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation" Nutrients 13, no. 3: 856. https://doi.org/10.3390/nu13030856
APA StyleWang, F., Park, J.-S., Ma, Y., Ma, H., Lee, Y.-J., Lee, G.-R., Yoo, H.-S., Hong, J.-T., & Roh, Y.-S. (2021). Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation. Nutrients, 13(3), 856. https://doi.org/10.3390/nu13030856






