Dietary Fucose Affects Macrophage Polarization and Reproductive Performance in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing
2.2. Experimental Groups
2.3. Sample and Tissue Collection
2.4. Metagenomic Analysis
2.5. Flow Cytometry Analysis (FC)
2.6. Macrophage Phagocytosis Assay
2.7. Mitochondrial Respiration and Anaerobic Glycolysis
2.8. Data Analysis and Statistics
3. Results
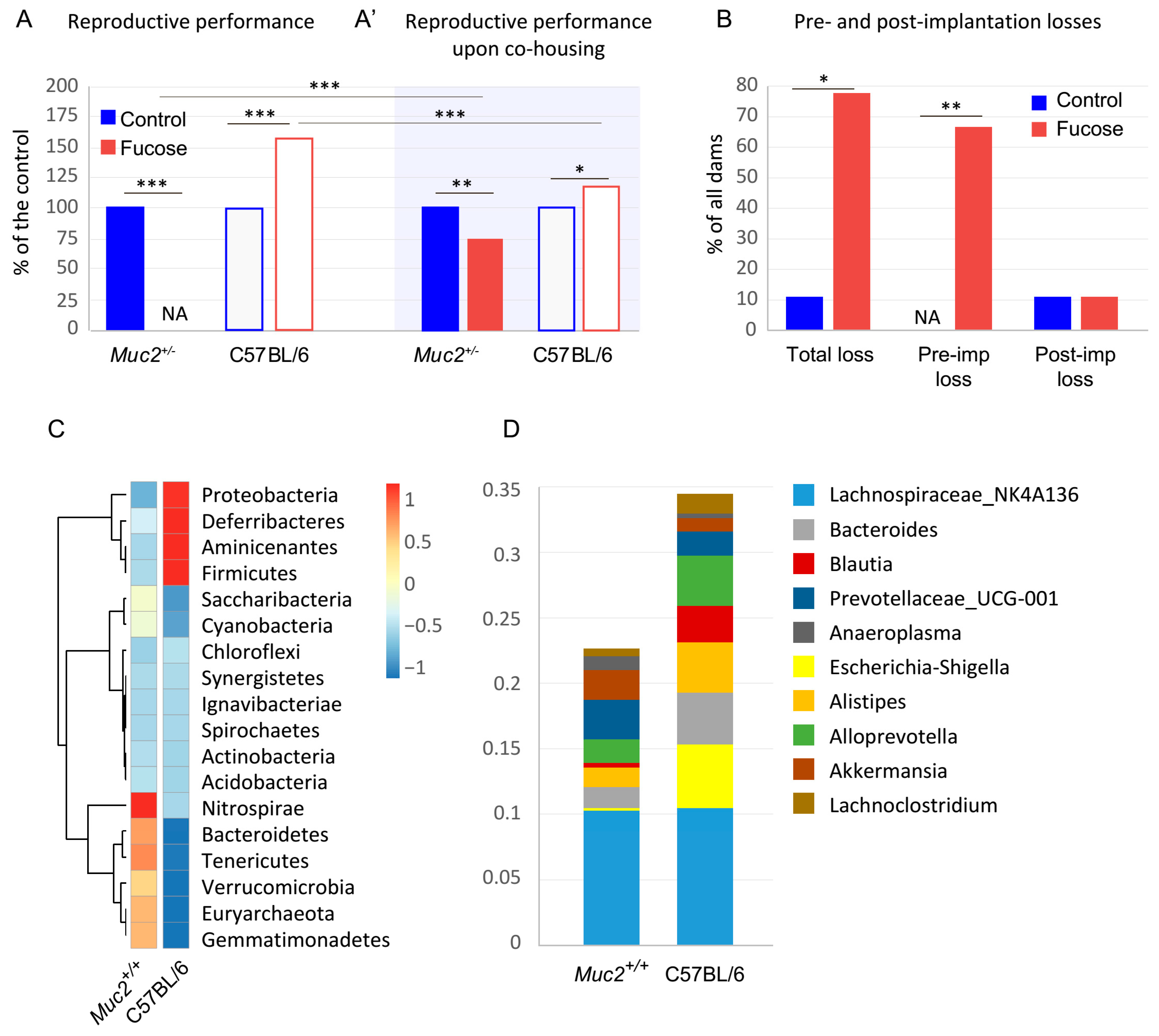
3.1. The Effect of Fucose on Reproductive Performance of Muc2+/− and C57BL/6 Females
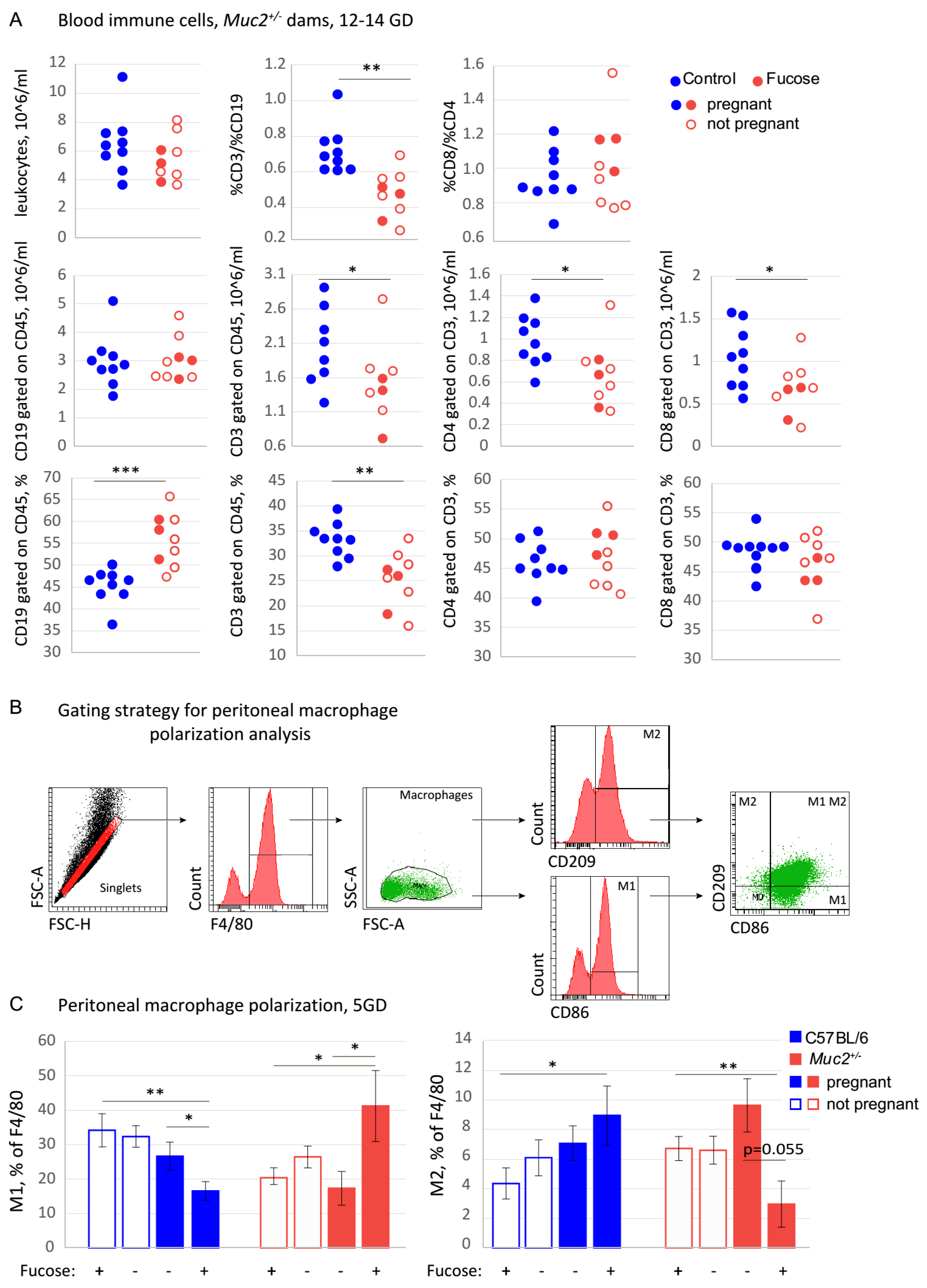
3.2. The Effect of Fucose on the Systemic Immune Response
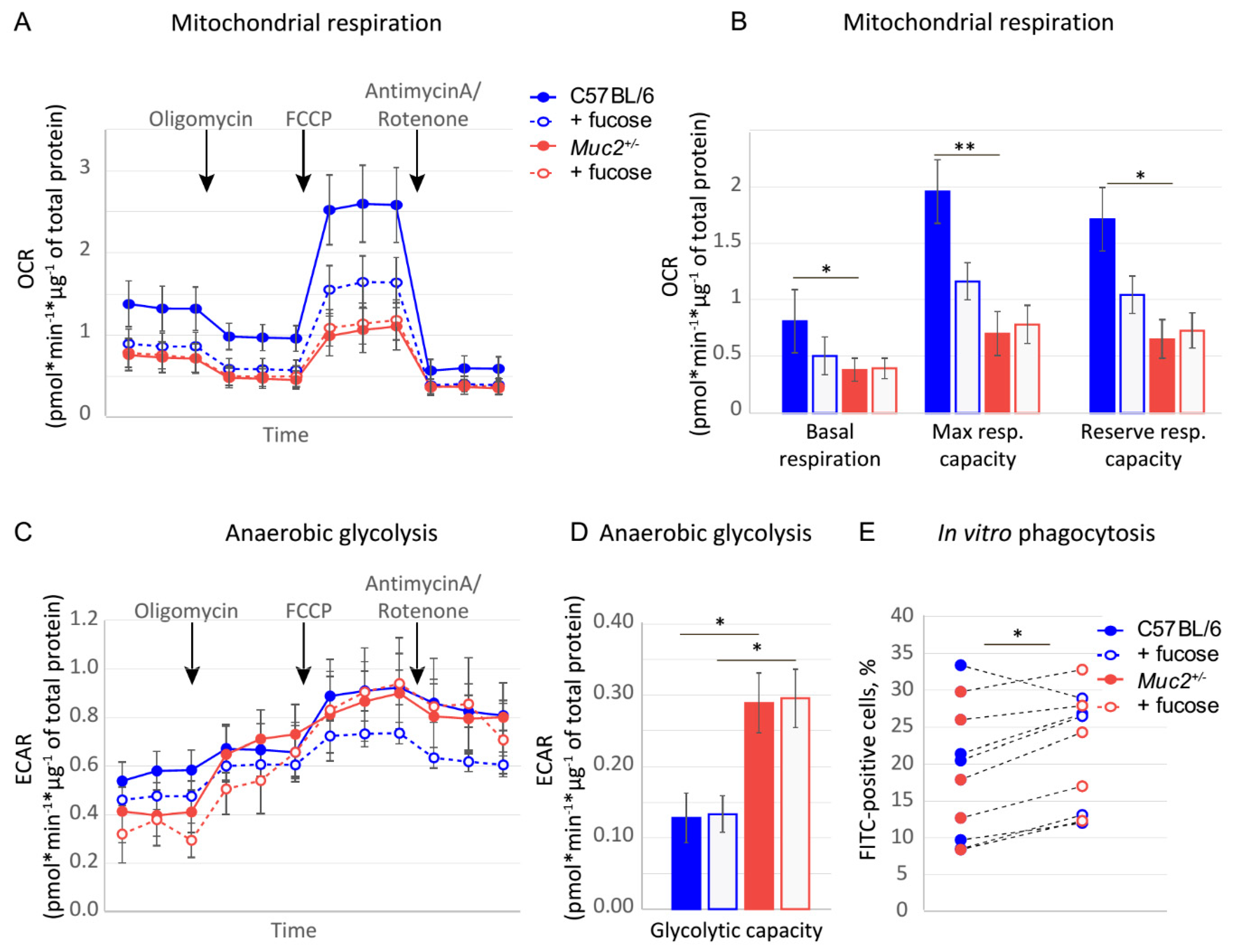
3.3. Fucose Regulates Macrophage Activity and Metabolism In Vitro
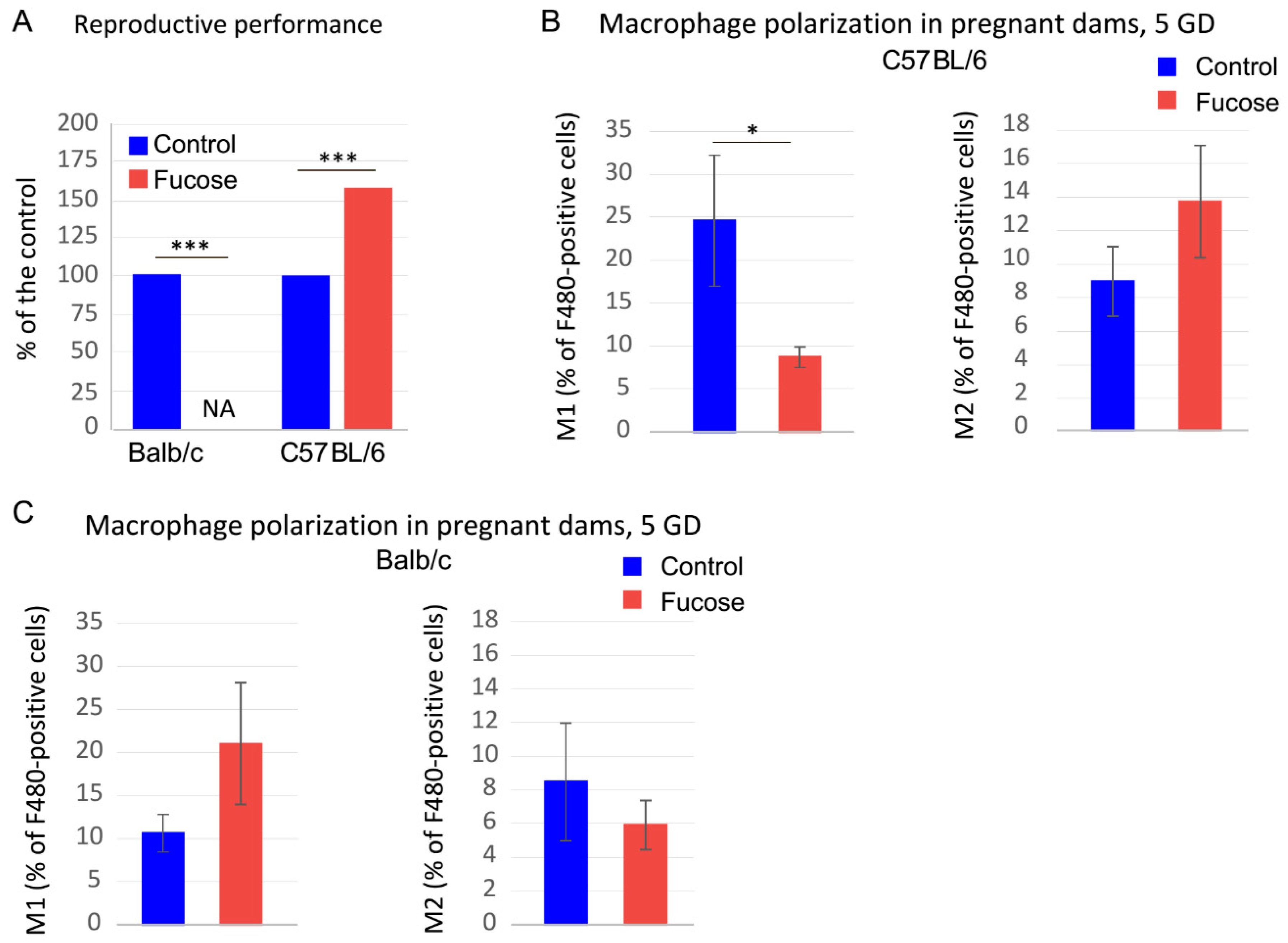
3.4. Fucose Affects Reproductive Performance and Macrophage Polarization in BALB/c Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. 2014, 16, 1004–1013. [Google Scholar] [CrossRef]
- Tomkovich, S.; Jobin, C. Microbiota and host immune responses: A love-hate relationship. Immunology 2016, 147, 1–10. [Google Scholar] [CrossRef]
- Nashida, J.; Nishi, N.; Takahashi, Y.; Hayashi, C.; Igarashi, M.; Takahashi, D.; Toshima, K. Systematic and Stereoselective Total Synthesis of Mannosylerythritol Lipids and Evaluation of Their Antibacterial Activity. J. Org. Chem. 2018, 83, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Kaliannan, K.; Strain, C.R.; Ross, R.P.; Stanton, C.; Kang, J.X. Maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Burrello, C.; Garavaglia, F.; Cribiu, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Ravel, J.; Regan, M. Clinical Relevance of Gastrointestinal Microbiota during Pregnancy: A Primer for Nurses. Biol. Res. Nurs. 2018, 20, 84–102. [Google Scholar] [CrossRef]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; de Wiele, T.V.; Schuller, S.; Juge, N.; et al. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol. Rev. 2019, 43, 457–489. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Moreno, I.; Codoner, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazan, J.; Alonso, R.; Alama, P.; Remohi, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Otsuki, K.; Imai, N. Effects of lactoferrin in 6 patients with refractory bacterial vaginosis. Biochem. Cell Biol. 2017, 95, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Bergstrom, K.S.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Linden, S.K.; Florin, T.H.; McGuckin, M.A. Mucin dynamics in intestinal bacterial infection. PLoS ONE 2008, 3, e3952. [Google Scholar] [CrossRef] [PubMed]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.; Rice, P.; Ha, T.; Li, C.; Kelley, J.; Ensley, H.; Lopez-Perez, J.; Kalbfleisch, J.; Lowman, D.; Margl, P.; et al. A (1-->3)-beta-D-linked heptasaccharide is the unit ligand for glucan pattern recognition receptors on human monocytes. Microbes Infect. 2001, 3, 789–797. [Google Scholar] [CrossRef]
- Nonaka, Y.; Izumo, T.; Izumi, F.; Maekawa, T.; Shibata, H.; Nakano, A.; Kishi, A.; Akatani, K.; Kiso, Y. Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production. Int. Arch. Allergy Immunol. 2008, 145, 249–257. [Google Scholar] [CrossRef]
- Bergstrom, K.S.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, Y.; Li, Z.; Fang, W.; Yang, Y.; Li, X.; Li, Z.; Xiong, B.; Chen, Z.; Wang, J.; et al. MicroRNA profiling in Muc2 knockout mice of colitis-associated cancer model reveals epigenetic alterations during chronic colitis malignant transformation. PLoS ONE 2014, 9, e99132. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Li, J.; Bao, Y.; Guo, Y.; Yang, W. The Dynamic Changes of Gut Microbiota in Muc2 Deficient Mice. Int. J. Mol. Sci. 2018, 19, 2809. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol. Pharm. Bull. 2012, 35, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.; Liu, X.; Zhao, Y.; Gao, N.; Wu, Q.; Song, K.; Cui, Y.; Li, Y.; McDaniel, J.M.; McGee, S.; et al. Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice. Gastroenterology 2016, 151, 152–164 e111. [Google Scholar] [CrossRef] [PubMed]
- Morampudi, V.; Dalwadi, U.; Bhinder, G.; Sham, H.P.; Gill, S.K.; Chan, J.; Bergstrom, K.S.; Huang, T.; Ma, C.; Jacobson, K.; et al. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016, 9, 1218–1233. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; van der Sluis, M.; Bouma, J.; Korteland-van Male, A.M.; Lu, P.; Van Seuningen, I.; Boehm, G.; van Goudoever, J.B.; Renes, I.B. Colitis development during the suckling-weaning transition in mucin Muc2-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G667–G678. [Google Scholar] [CrossRef]
- Lu, P.; Burger-van Paassen, N.; van der Sluis, M.; Witte-Bouma, J.; Kerckaert, J.-P.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. Colonic gene expression patterns of mucin muc2 knockout mice reveal various phases in colitis development1. Inflammatory Bowel Diseases 2011, 17, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Mandal, M.; Goldsmith, L.; Kashani, B.; Ponzio, N. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 2015, 171. [Google Scholar] [CrossRef]
- Robertson, S.; Petroff, M.; Hunt, J. Immunology of Pregnancy; Academic Press: London, UK, 2015; Volume 2, pp. 1835–1874. [Google Scholar]
- Zhang, Y.; Li, X.; Luo, Z.; Ma, L.; Zhu, S.; Wang, Z.; Wen, J.; Cheng, S.; Gu, W.; Lian, Q.; et al. ECM1 is an essential factor for the determination of M1 macrophage polarization in IBD in response to LPS stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 3083–3092. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Xu, C.; Wang, Y.; Wang, Z.; Chen, M.; Jiang, Z.; Pan, J.; Yang, C.; Li, X.; et al. Cross-talk between the gut microbiota and monocyte-like macrophages mediates an inflammatory response to promote colitis-associated tumourigenesis. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.-R.; Bain, C.C.; Fenton, T.M.; Kelly, A.; Brown, S.L.; Ivens, A.C.; Travis, M.A.; Cook, P.C.; MacDonald, A.S. Dynamics of Colon Monocyte and Macrophage Activation During Colitis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, E.C.; Plevy, S.E. The Role of Macrophages and Dendritic Cells in the Initiation of Inflammation in IBD. Inflamm. Bowel Dis. 2013, 20, 166–175. [Google Scholar] [CrossRef]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Anti-inflammatory potential of probiotic Lactobacillus spp. on carrageenan induced paw edema in Wistar rats. Int. J. Biol. Macromol. 2015, 81, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef]
- Cleophas, M.C.P.; Ratter, J.M.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.S.; Netea, M.G.; Joosten, L.A.B. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Stunault, M.I.; Bories, G.; Guinamard, R.R.; Ivanov, S. Metabolism Plays a Key Role during Macrophage Activation. Mediat. Inflamm. 2018, 2018, 2426138. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; O’Neill, L.A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 2016, 46, 13–21. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. Inflammatory signaling in macrophages: Transitions from acute to tolerant and alternative activation states. Eur. J. Immunol. 2011, 41, 2477–2481. [Google Scholar] [CrossRef]
- Vishnyakova, P.; Elchaninov, A.; Fatkhudinov, T.; Sukhikh, G. Role of the Monocyte-Macrophage System in Normal Pregnancy and Preeclampsia. Int. J. Mol. Sci. 2019, 20, 3695. [Google Scholar] [CrossRef]
- Hunt, J.S.; Robertson, S.A. Uterine macrophages and environmental programming for pregnancy success. J. Reprod. Immunol. 1996, 32, 1–25. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages Infiltrate the Human and Rat Decidua During Term and Preterm Labor: Evidence That Decidual Inflammation Precedes Labor1. Biol. Reprod. 2012, 86. [Google Scholar] [CrossRef]
- Larsson, J.M.H.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.V.; Eklund, L.; Sjövall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef]
- Pickard, J.M.; Chervonsky, A.V. Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 2015, 194, 5588–5593. [Google Scholar] [CrossRef]
- Haines-Menges, B.L.; Whitaker, W.B.; Lubin, J.B.; Boyd, E.F. Host Sialic Acids: A Delicacy for the Pathogen with Discerning Taste. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Newburg, D.S.; He, Y. Neonatal Gut Microbiota and Human Milk Glycans Cooperate to Attenuate Infection and Inflammation. Clin. Obstet. Gynecol. 2015, 58, 814–826. [Google Scholar] [CrossRef]
- Dias, A.M.; Pereira, M.S.; Padrao, N.A.; Alves, I.; Marcos-Pinto, R.; Lago, P.; Pinho, S.S. Glycans as critical regulators of gut immunity in homeostasis and disease. Cell. Immunol. 2018, 333, 9–18. [Google Scholar] [CrossRef] [PubMed]
- McKeen, S.; Young, W.; Fraser, K.; Roy, N.C.; McNabb, W.C. Glycan Utilisation and Function in the Microbiome of Weaning Infants. Microorganisms 2019, 7, 190. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- FELASA Working Group on Revision of Guidelines for Health Monitoring of Rodents and Rabbits; Mahler Convenor, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Smith, A.L.; Jones, T.A.; Gridley, D.S. Modification of immunologic and hematologic variables by method of CO2 euthanasia. Comp. Med. 2000, 50, 595–602. [Google Scholar]
- Stollings, L.M.; Jia, L.J.; Tang, P.; Dou, H.; Lu, B.; Xu, Y. Immune Modulation by Volatile Anesthetics. Anesthesiology 2016, 125, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Cobo, E.R.; Kissoon-Singh, V.; Moreau, F.; Chadee, K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of beta-defensin 2. Mucosal Immunol. 2015, 8, 1360–1372. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Takahashi, H.; Kuwabara, Y.; Takeshita, T. Innate immune cells in reproduction. J. Obstet. Gynaecol. Res. 2018, 44, 2025–2036. [Google Scholar] [CrossRef]
- Aplin, J.D.; Jones, C.J. Fucose, placental evolution and the glycocode. Glycobiology 2012, 22, 470–478. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Y.; Zhou, Q.; Wang, C.; Chen, L.; Di, W.; Zhang, Y. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 2020, 134, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Sadiku, P.; Walmsley, S.R. Hypoxia and the regulation of myeloid cell metabolic imprinting: Consequences for the inflammatory response. EMBO Rep. 2019, 20. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hsu, H.C.; Ding, Y.; Li, H.; Wu, Q.; Yang, P.; Luo, B.; Rowse, A.L.; Spalding, D.M.; Bridges, S.L., Jr.; et al. Inhibition of fucosylation reshapes inflammatory macrophages and suppresses type II collagen-induced arthritis. Arthritis Rheumatol. 2014, 66, 2368–2379. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Han, C.; Lin, R.; Qian, W.; Hou, X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int. Immunopharmacol. 2019, 73, 379–388. [Google Scholar] [CrossRef]
- Borisova, M.A.; Snytnikova, O.A.; Litvinova, E.A.; Achasova, K.M.; Babochkina, T.I.; Pindyurin, A.V.; Tsentalovich, Y.P.; Kozhevnikova, E.N. Fucose Ameliorates Tryptophan Metabolism and Behavioral Abnormalities in a Mouse Model of Chronic Colitis. Nutrients 2020, 12, 445. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litvinova, E.A.; Bets, V.D.; Feofanova, N.A.; Gvozdeva, O.V.; Achasova, K.M.; Alperina, E.L.; Kozhevnikova, E.N. Dietary Fucose Affects Macrophage Polarization and Reproductive Performance in Mice. Nutrients 2021, 13, 855. https://doi.org/10.3390/nu13030855
Litvinova EA, Bets VD, Feofanova NA, Gvozdeva OV, Achasova KM, Alperina EL, Kozhevnikova EN. Dietary Fucose Affects Macrophage Polarization and Reproductive Performance in Mice. Nutrients. 2021; 13(3):855. https://doi.org/10.3390/nu13030855
Chicago/Turabian StyleLitvinova, Ekaterina A., Victoria D. Bets, Natalya A. Feofanova, Olga V. Gvozdeva, Kseniya M. Achasova, Elizaveta L. Alperina, and Elena N. Kozhevnikova. 2021. "Dietary Fucose Affects Macrophage Polarization and Reproductive Performance in Mice" Nutrients 13, no. 3: 855. https://doi.org/10.3390/nu13030855
APA StyleLitvinova, E. A., Bets, V. D., Feofanova, N. A., Gvozdeva, O. V., Achasova, K. M., Alperina, E. L., & Kozhevnikova, E. N. (2021). Dietary Fucose Affects Macrophage Polarization and Reproductive Performance in Mice. Nutrients, 13(3), 855. https://doi.org/10.3390/nu13030855






