Role of Vitamin D in the Metabolic Syndrome
Abstract
1. Introduction
2. Metabolic Syndrome
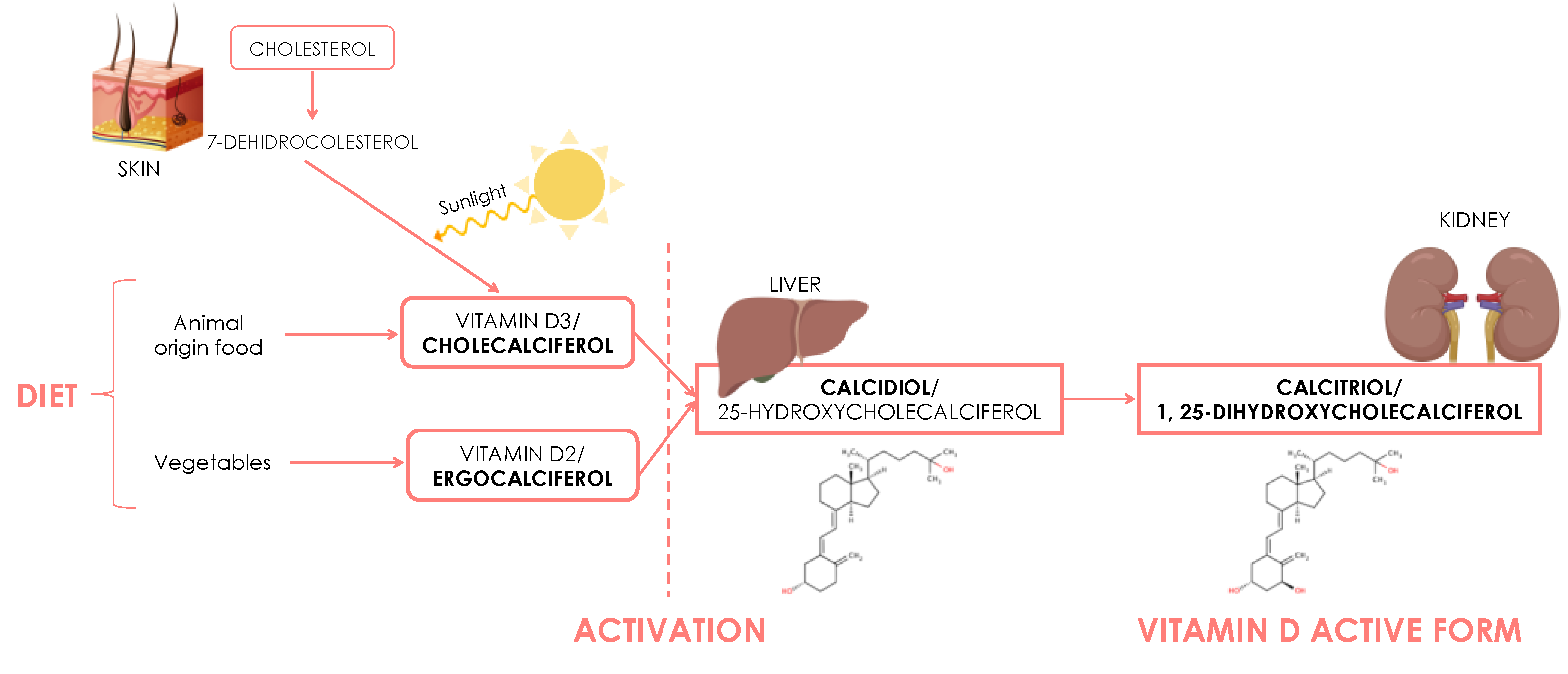
3. Vitamin D
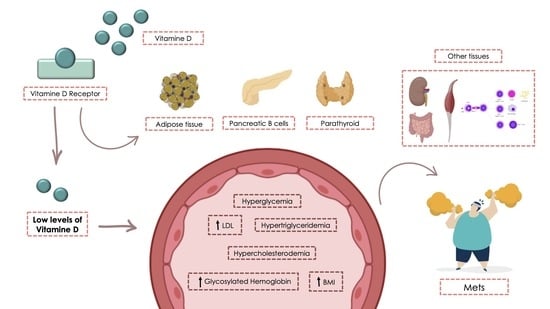
4. MetS and Vitamin D Deficiency
5. Effect of Vitamin D Supplementation on MetS
5.1. Vitamin D Supplementation and Insulin Resistance and Hyperglycemia
5.2. Vitamin D Supplementation and Dyslipidemia
5.3. Vitamin D Supplementation and Obesity
5.4. Vitamin D Supplementation and Hypertension
5.5. Vitamin D Supplementation Dosage
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the Provisional Report from the WHO Consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- García, M.J.; Sosa, L.; Adorno, R.; González, L.K.; Bataglia, V.; García, M.J.; Sosa, L.; Adorno, R.; González, L.K.; Bataglia, V. Prevalence of the Metabolic Syndrome in Patients Admitted to the Gynecology Service of the Central Hospital. Institute of Social Security, January–June 2017. Rev. Slud Publica Parag. 2018, 8, 40–43. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20. [Google Scholar] [CrossRef]
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of Metabolic Syndrome and Metabolic Syndrome Components in Young Adults: A Pooled Analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–17. ISBN 978-3-319-48382-5. [Google Scholar]
- Gradillas-García, A.; Álvarez, J.; Rubio, J.A.; de Abajo, F.J. Relationship between vitamin D deficiency and metabolic syndrome in adult population of the Community of Madrid. Endocrinol. Nutr. 2015, 62, 180–187. [Google Scholar] [CrossRef]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic Syndrome: A Closer Look at the Growing Epidemic and Its Associated Pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and Association Analysis of Obstructive Sleep Apnea with Gender and Age Differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef]
- Hooijschuur, M.C.E.; Ghossein-Doha, C.; Kroon, A.A.; de Leeuw, P.W.; Zandbergen, A.A.M.; van Kuijk, S.M.J.; Spaanderman, M.E.A. Metabolic Syndrome and Pre-Eclampsia. Ultrasound. Obstet. Gynecol. 2019, 54, 64–71. [Google Scholar] [CrossRef]
- Swarup, S.; Zeltser, R. Metabolic Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Raposo, L.; Martins, S.; Ferreira, D.; Guimarães, J.T.; Santos, A.C. Vitamin D, Parathyroid Hormone and Metabolic Syndrom—The PORMETS Study. BMC Endocr. Disord. 2017, 17, 71. [Google Scholar] [CrossRef]
- Valero-Zanuy, M.; Hawkins-Carranza, F. Metabolismo, fuentes endógenas y exógenas de vitamina D. Rev. Esp. Enferm. Metab. Oseas. 2007, 16, 63–70. [Google Scholar] [CrossRef]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Vitamin D and Autoimmune Diseases. Life Sci. 2019, 233, 116744. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alkharfy, K.M.; Khan, N.; Mohammed, A.K.; Vinodson, B.; Ansari, M.G.A.; Alenad, A.; Alokail, M.S. Association of VDR-Gene Variants with Factors Related to the Metabolic Syndrome, Type 2 Diabetes and Vitamin D Deficiency. Gene 2014, 542, 129–133. [Google Scholar] [CrossRef]
- Han, F.; Lv, Y.; Gong, L.; Liu, H.; Wan, Z.; Liu, L. VDR Gene Variation and Insulin Resistance Related Diseases. Lipids Health Dis. 2017, 16. [Google Scholar] [CrossRef]
- Karonova, T.; Grineva, E.; Belyaeva, O.; Bystrova, A.; Jude, E.B.; Andreeva, A.; Kostareva, A.; Pludowski, P. Relationship Between Vitamin D Status and Vitamin D Receptor Gene Polymorphisms with Markers of Metabolic Syndrome Among Adults. Front. Endocrinol. 2018, 9, 448. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Fang, T.-C. The Pleiotropic Effect of Vitamin, D. Nephrol. 2013, 2013. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Ghosh, S.; Pandit, K.; Chatterjee, P.; Mukherjee, P.S.; Chowdhury, S. Pandemic of Vitamin D Deficiency: Cardiometabolic Concern or Skeletal Biochemical Abnormality? Indian J. Endocrinol. Metab. 2019, 23, 215–221. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., del Valle, H.B., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Gani, L.U.; How, C.H. Vitamin D Deficiency. Singap. Med. J. 2015, 56, 433–437. [Google Scholar] [CrossRef]
- Mansouri, M.; Abasi, R.; Nasiri, M.; Sharifi, F.; Vesaly, S.; Sadeghi, O.; Rahimi, N.; Sharif, N.A. Association of Vitamin D Status with Metabolic Syndrome and Its Components: A Cross-Sectional Study in a Population of High Educated Iranian Adults. Diabetes Metab. Syndr. 2018, 12, 393–398. [Google Scholar] [CrossRef]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Poloni, P.F.; Orsatti, C.L.; Petri-Nahas, E.A. Vitamin D Deficiency Is Associated with Metabolic Syndrome in Postmenopausal Women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Mutt, S.J.; Jokelainen, J.; Sebert, S.; Auvinen, J.; Järvelin, M.-R.; Keinänen-Kiukaanniemi, S.; Herzig, K.-H. Vitamin D Status and Components of Metabolic Syndrome in Older Subjects from Northern Finland (Latitude 65° North). Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Navarro-Valverde, C.; Quesada-Gómez, J.M. Deficiencia de Vitamina D En España: Realidad o Mito? Rev. Osteoporos. Metab. Miner. 2014, 6, 5–10. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Zhou, Y.; Liu, J.; Wang, C.; Qu, Z.; Wei, Z.; Zhou, D. Genetically Increased Circulating 25(OH)D Level Reduces the Risk of Type 2 Diabetes in Subjects with Deficiency of Vitamin D. Medicine 2020, 99. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chang, H.-H.; Lu, C.-W.; Tseng, F.-Y.; Lee, L.-T.; Huang, K.-C. Vitamin D Status and Risk of Metabolic Syndrome among Non-Diabetic Young Adults. Clin. Nutr. 2015, 34, 484–489. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, E.Y.; Lee, J.H.; Kim, J.E.; Kim, K.J.; Rhee, Y.; Kim, H.C.; Youm, Y.; Kim, C.O. Associations of Serum 25-Hydroxyvitamin D with Metabolic Syndrome and Its Components in Elderly Men and Women: The Korean Urban Rural Elderly Cohort Study. BMC Geriatr. 2019, 19, 102. [Google Scholar] [CrossRef]
- Zhu, W.; Heil, D.P. Associations of Vitamin D Status with Markers of Metabolic Health: A Community-Based Study in Shanghai, China. Diabetes Metab. Syndr. 2018, 12, 727–732. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Lu, F.; Liu, Y.; Lv, Y.; Qu, Y.; Gu, H.; Li, C.; Cai, J.; Ji, S.; et al. Vitamin D Deficiency and Metabolic Syndrome in Elderly Chinese Individuals: Evidence from CLHLS. Nutr. Metab. 2020, 17, 58. [Google Scholar] [CrossRef]
- Ganji, V.; Sukik, A.; Alaayesh, H.; Rasoulinejad, H.; Shraim, M. Serum Vitamin D Concentrations Are Inversely Related to Prevalence of Metabolic Syndrome in Qatari Women. Biofactors 2020, 46, 180–186. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Nascimento, C.M.C.; Costa-Guarisco, L.P.; Gomes, G.A.O.; Gramani-Say, K.; Orlandi, F.S.; Gratão, A.C.M.; Orlandi, A.A.S.; Pavarini, S.C.I.; Vasilceac, F.A.; et al. Vitamin D Deficient Older Adults Are More Prone to Have Metabolic Syndrome, but Not to a Greater Number of Metabolic Syndrome Parameters. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Tofano, R.J.; de Campos, A.L.; Rodrigues, A.S.; Quesada, K.; Bechara, M.D.; de Alvares-Goulart, R.; Oshiiwa, M. Association between Vitamin D Status and Metabolic Syndrome Risk Factors. Diabetes Metab. Syndr. 2018, 12, 501–507. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.L.; Tikkanen, E. Association of Vitamin D Status with Arterial Blood Pressure and Hypertension Risk: A Mendelian Randomisation Study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Luan, J.; Sofianopoulou, E.; Sharp, S.J.; Day, F.R.; Imamura, F.; Gundersen, T.E.; Lotta, L.A.; Sluijs, I.; Stewart, I.D.; et al. The Association between Circulating 25-Hydroxyvitamin D Metabolites and Type 2 Diabetes in European Populations: A Meta-Analysis and Mendelian Randomisation Analysis. PLoS Med. 2020, 17. [Google Scholar] [CrossRef]
- Wang, N.; Wang, C.; Chen, X.; Wan, H.; Chen, Y.; Chen, C.; Han, B.; Lu, Y. Vitamin D, Prediabetes and Type 2 Diabetes: Bidirectional Mendelian Randomization Analysis. Eur. J. Nutr. 2020, 59, 1379–1388. [Google Scholar] [CrossRef]
- Mehri, Z.; Salehi-Abargouei, A.; Shahvazi, S.; Samadi, M.; Zare, F.; Nadjarzadeh, A. The Association between Vitamin D Status and Metabolic Syndrome and Its Components among Female Teachers Residing in Yazd City. Endocrinol. Diabetes Nutr. 2019, 66, 628–638. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Bull-Ferreira-Campos, A.; Cordeiro, A.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Vitamin D Nutritional Status and Its Relationship with Metabolic Changes in Adolescents and Adults with Severe Obesity. Nutr. Hosp. 2018, 35, 847–853. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Weng, P.; Xia, F.; Li, Q.; Zhai, H.; Wang, N.; Lu, Y. Association of 25-Hydroxyvitamin D with Cardiometabolic Risk Factors and Metabolic Syndrome: A Mendelian Randomization Study. Nutr. J. 2019, 18. [Google Scholar] [CrossRef]
- Ferreira, P.P.; Cangussu, L.; Bueloni-Dias, F.N.; Orsatti, C.L.; Schmitt, E.B.; Nahas-Neto, J.; Nahas, E.A.P. Vitamin D Supplementation Improves the Metabolic Syndrome Risk Profile in Postmenopausal Women. Climacteric 2020, 23, 24–31. [Google Scholar] [CrossRef]
- Faraji, S.; Alizadeh, M. Mechanistic Effects of Vitamin D Supplementation on Metabolic Syndrome Components in Patients with or without Vitamin D Deficiency. J. Obes. Metab. Syndr. 2020, 29, 270–280. [Google Scholar] [CrossRef]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.-S.; Morisset, A.-S.; Carreau, A.-M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-Month Vitamin D Supplementation on Insulin Sensitivity and Secretion: A Randomised, Placebo-Controlled Trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef]
- Barbarawi, M.; Zayed, Y.; Barbarawi, O.; Bala, A.; Alabdouh, A.; Gakhal, I.; Rizk, F.; Alkasasbeh, M.; Bachuwa, G.; Manson, J.E. Effect of Vitamin D Supplementation on the Incidence of Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Safarpour, P.; Daneshi-Maskooni, M.; Vafa, M.; Nourbakhsh, M.; Janani, L.; Maddah, M.; Amiri, F.-S.; Mohammadi, F.; Sadeghi, H. Vitamin D Supplementation Improves SIRT1, Irisin, and Glucose Indices in Overweight or Obese Type 2 Diabetic Patients: A Double-Blind Randomized Placebo-Controlled Clinical Trial. BMC Fam. Pract. 2020, 21, 26. [Google Scholar] [CrossRef]
- Farrokhian, A.; Raygan, F.; Bahmani, F.; Talari, H.R.; Esfandiari, R.; Esmaillzadeh, A.; Asemi, Z. Long-Term Vitamin D Supplementation Affects Metabolic Status in Vitamin D-Deficient Type 2 Diabetic Patients with Coronary Artery Disease. J. Nutr. 2017, 147, 384–389. [Google Scholar] [CrossRef]
- Talari, H.R.; Najafi, V.; Raygan, F.; Mirhosseini, N.; Ostadmohammadi, V.; Amirani, E.; Taghizadeh, M.; Hajijafari, M.; Shafabakhsh, R.; Asemi, Z. Long-Term Vitamin D and High-Dose n-3 Fatty Acids’ Supplementation Improve Markers of Cardiometabolic Risk in Type 2 Diabetic Patients with CHD. Br. J. Nutr. 2019, 122, 423–430. [Google Scholar] [CrossRef]
- Wallace, H.J.; Holmes, L.; Ennis, C.N.; Cardwell, C.R.; Woodside, J.V.; Young, I.S.; Bell, P.M.; Hunter, S.J.; McKinley, M.C. Effect of Vitamin D3 Supplementation on Insulin Resistance and β-Cell Function in Prediabetes: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 1138–1147. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Trummer, C.; Theiler-Schwetz, V.; Kollmann, M.; Wölfler, M.; Pilz, S.; Obermayer-Pietsch, B. Effects of Vitamin D Supplementation on Body Composition and Metabolic Risk Factors in Men: A Randomized Controlled Trial. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Ghaderi, A.; Banafshe, H.R.; Motmaen, M.; Rasouli-Azad, M.; Bahmani, F.; Asemi, Z. Clinical Trial of the Effects of Vitamin D Supplementation on Psychological Symptoms and Metabolic Profiles in Maintenance Methadone Treatment Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 84–89. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Ebrahimi, F.A.; Hashemi, T.; Taghizadeh, M.; Razavi, M.; Sanami, M.; Asemi, Z. The Effects of Vitamin D and Omega-3 Fatty Acid Co-Supplementation on Glycemic Control and Lipid Concentrations in Patients with Gestational Diabetes. J. Clin. Lipidol. 2017, 11, 459–468. [Google Scholar] [CrossRef]
- Imga, N.N.; Karci, A.C.; Oztas, D.; Berker, D.; Guler, S. Effects of Vitamin D Supplementation on Insulin Resistance and Dyslipidemia in Overweight and Obese Premenopausal Women. Arch. Med. Sci. 2019, 15, 598–606. [Google Scholar] [CrossRef]
- Riek, A.E.; Oh, J.; Darwech, I.; Worthy, V.; Lin, X.; Ostlund, R.E.; Zhang, R.M.; Bernal-Mizrachi, C. Vitamin D3 Supplementation Decreases a Unique Circulating Monocyte Cholesterol Pool in Patients with Type 2 Diabetes. J. Steroid Biochem. Mol. Biol. 2018, 177, 187–192. [Google Scholar] [CrossRef]
- Liyanage, G.C.; Lekamwasam, S.; Weerarathna, T.P.; Liyanage, C.E. Effects of High-Dose Parenteral Vitamin D Therapy on Lipid Profile and Blood Pressure in Patients with Diabetic Nephropathy: A Randomized Double-Blind Clinical Trial. Diabetes Metab. Syndr. 2017, 11 (Suppl. 2), S767–S770. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Milajerdi, A.; Ghayour-Mobarhan, M.; Ferns, G.; Taghizadeh, M.; Badehnoosh, B.; Mirzaei, H.; Asemi, Z. The Effects of Vitamin D Supplementation on Glycemic Control, Lipid Profiles and C-Reactive Protein Among Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 201–210. [Google Scholar] [CrossRef]
- Hafez, M.; Musa, N.; Abdel-Atty, S.; Ibrahem, M.; Abdel-Wahab, N. Effect of Vitamin D Supplementation on Lipid Profile in Vitamin D-Deficient Children with Type 1 Diabetes and Dyslipidemia. Horm. Res. Paediatr. 2019, 91, 311–318. [Google Scholar] [CrossRef]
- Tamadon, M.R.; Soleimani, A.; Keneshlou, F.; Mojarrad, M.Z.; Bahmani, F.; Naseri, A.; Kashani, H.H.; Hosseini, E.S.; Asemi, Z. Clinical Trial on the Effects of Vitamin D Supplementation on Metabolic Profiles in Diabetic Hemodialysis. Horm. Metab. Res. 2018, 50, 50–55. [Google Scholar] [CrossRef]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2019, 55. [Google Scholar] [CrossRef]
- Golzarand, M.; Hollis, B.W.; Mirmiran, P.; Wagner, C.L.; Shab-Bidar, S. Vitamin D Supplementation and Body Fat Mass: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2018, 72, 1345–1357. [Google Scholar] [CrossRef]
- Lotfi-Dizaji, L.; Mahboob, S.; Aliashrafi, S.; Vaghef-Mehrabany, E.; Ebrahimi-Mameghani, M.; Morovati, A. Effect of Vitamin D Supplementation along with Weight Loss Diet on Meta-Inflammation and Fat Mass in Obese Subjects with Vitamin D Deficiency: A Double-Blind Placebo-Controlled Randomized Clinical Trial. Clin. Endocrinol. 2019, 90, 94–101. [Google Scholar] [CrossRef]
- Subih, H.S.; Zueter, Z.; Obeidat, B.M.; Al-Qudah, M.A.; Janakat, S.; Hammoh, F.; Sharkas, G.; Bawadi, H.A. A High Weekly Dose of Cholecalciferol and Calcium Supplement Enhances Weight Loss and Improves Health Biomarkers in Obese Women. Nutr. Res. 2018, 59, 53–64. [Google Scholar] [CrossRef]
- Duan, L.; Han, L.; Liu, Q.; Zhao, Y.; Wang, L.; Wang, Y. Effects of Vitamin D Supplementation on General and Central Obesity: Results from 20 Randomized Controlled Trials Involving Apparently Healthy Populations. Ann. Nutr. Metab. 2020, 76, 153–164. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The Effect of Vitamin D Supplementation on Serum 25OHD in Thin and Obese Women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef]
- Chandler, P.D.; Wang, L.; Zhang, X.; Sesso, H.D.; Moorthy, M.V.; Obi, O.; Lewis, J.; Prince, R.L.; Danik, J.S.; Manson, J.E.; et al. Effect of Vitamin D Supplementation Alone or with Calcium on Adiposity Measures: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2015, 73, 577–593. [Google Scholar] [CrossRef]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of Vitamin D3 Supplementation on Vascular and Metabolic Health of Vitamin D-Deficient Overweight and Obese Children: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef]
- Brzeziński, M.; Jankowska, A.; Słomińska-Frączek, M.; Metelska, P.; Wiśniewski, P.; Socha, P.; Szlagatys-Sidorkiewicz, A. Long-Term Effects of Vitamin D Supplementation in Obese Children During Integrated Weight-Loss Programme-A Double Blind Randomized Placebo-Controlled Trial. Nutrients 2020, 12, 1093. [Google Scholar] [CrossRef]
- Makariou, S.E.; Challa, A.; Siomou, E.; Tellis, C.; Tselepis, A.; Elisaf, M.; Liberopoulos, E. Vitamin D Status and Cardiometabolic Risk Factors in Greek Adolescents with Obesity—the Effect of Vitamin D Supplementation: A Pilot Study. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e64–e71. [Google Scholar] [CrossRef]
- Golzarand, M.; Shab-Bidar, S.; Koochakpoor, G.; Speakman, J.R.; Djafarian, K. Effect of Vitamin D3 Supplementation on Blood Pressure in Adults: An Updated Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 663–673. [Google Scholar] [CrossRef]
- Shu, L.; Huang, K. Effect of Vitamin D Supplementation on Blood Pressure Parameters in Patients with Vitamin D Deficiency: A Systematic Review and Meta-Analysis. J. Am. Soc. Hypertens. 2018, 12, 488–496. [Google Scholar] [CrossRef]
- Wu, S.H.; Ho, S.C.; Zhong, L. Effects of Vitamin D Supplementation on Blood Pressure. South. Med. J. 2010, 103, 729–737. [Google Scholar] [CrossRef]
- Hussin, A.M.; Ashor, A.W.; Schoenmakers, I.; Hill, T.; Mathers, J.C.; Siervo, M. Effects of Vitamin D Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Eur. J. Nutr. 2017, 56, 1095–1104. [Google Scholar] [CrossRef]
- Swart, K.M.; Lips, P.; Brouwer, I.A.; Jorde, R.; Heymans, M.W.; Grimnes, G.; Grübler, M.R.; Gaksch, M.; Tomaschitz, A.; Pilz, S.; et al. Effects of Vitamin D Supplementation on Markers for Cardiovascular Disease and Type 2 Diabetes: An Individual Participant Data Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2018, 107, 1043–1053. [Google Scholar] [CrossRef]
- Abboud, M. Vitamin D Supplementation and Blood Pressure in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1163. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sepidarkish, M.; Fazelian, S.; Rahimlou, M.; Omidi, A.; Ardehali, S.H.; Sanoobar, M.; Heshmati, J. Effect of Calcium and Vitamin D Co-Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis. Clin. Ther. 2020, 42, e45–e63. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Valenti, M.T.; del Forno, F.; Piacentini, G.; Pietrobelli, A. Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences. Nutrients 2018, 10, 1934. [Google Scholar] [CrossRef]
- Park, J.E.; Pichiah, P.B.T.; Cha, Y.-S. Vitamin D and Metabolic Diseases: Growing Roles of Vitamin D. J. Obes. Metab. Syndr. 2018, 27, 223–232. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Amer, O.E.; Khattak, M.N.K.; Sabico, S.; Ghouse-Ahmed-Ansari, M.; Al-Saleh, Y.; Aljohani, N.; Alfawaz, H.; Alokail, M.S. Effects of Different Vitamin D Supplementation Strategies in Reversing Metabolic Syndrome and Its Component Risk Factors in Adolescents. J. Steroid Biochem. Mol. Biol. 2019, 191, 105378. [Google Scholar] [CrossRef]
| Ref. | Year | MetS and Vit D | Objective | Methodology | Findings |
|---|---|---|---|---|---|
| Valero et al. [15] | 2007 | Endogenous and exogenous sources of vitamin D and its role in metabolism. | To investigate the sources where they can take vitamin D from requirements, intake, and effects on health. | Review | Physiological levels of 25(OH)D are required to keep the integrity of immune, bone, and muscular systems. Sunlight exposure is not enough for reaching and keeping acceptable levels of Vit D in some age groups. Artificial addition of Vit D in food has showed its efficacy for reaching these desirable levels, and most of the population would profit from it. For people over 65, calcium addition is also necessary. |
| Mansouri et al. [25] | 2018 | Nutrient deficit and metabolic disorders. | To determine the link between vitamin D and metabolic syndrome. | Cross-sectional study. A total of 352 faculty members. Blood samples for the determination of 25(OH)D concentrations, glycemic indicators, and lipid profile. | Reverse association of 25(OH)D serum levels and risk of abdominal obesity, hypertension, and abnormal glucose homeostasis. No significant association for metabolic syndrome. |
| Schmitt et al. [26] | 2018 | Vitamin D-deficiency and metabolic syndrome in postmenopausal women. | To study vitamin D-deficiency and its association with risk factors for metabolic syndrome in postmenopausal women. | Observational and cross-sectional cohort study. A total of 463 women. Levels of total cholesterol, HDL, LDL, triglycerides, glucose, insulin, and 25(OH)D were measured. | Deficiency of vitamin D in postmenopausal women is related to a higher prevalence of MetS, as well as hypertriglyceridemia and low HDL levels. |
| Wimalawansa et al. [28] | 2018 | Association between vitamin D, insulin resistance, obesity, type II diabetes, and metabolic syndrome. | To investigate the relationship between vitamin D and insulin resistance, obesity, type II diabetes, and metabolic syndrome. | Review | Large number of observational studies point out the improvement of type II diabetes, insulin resistance, obesity, and metabolic syndrome with adequate levels of vitamin D. |
| Mutt et al. [29] | 2019 | 25(OH)D levels and MetS in the elderly population from Northern latitudes. | To investigate the associations between serum 25(OH)D levels and prevalence of MetS and its components and assess the effects of vitamin D supplementation on MetS. | Cross-sectional study. A total of 636 subjects from Oulu45 cohort (263 male, 373 female). Determination of 25(OH)D plasmatic levels and assessment of vitamin D supplements usage. | Low vitamin D levels were associated with a higher prevalence of MetS. People under vitamin D supplementation had a lower incidence of MetS and its components. Low vitamin D levels are a risk factor for MetS among other lifestyle factors among older subjects in the Northern latitudes. |
| Navarro et al. [30] | 2014 | Status of vitamin D levels in the Spanish population. | To determine the levels of vitamin D in Spain. | Review | There is an insufficiency of vitamin D in the Spanish population. There are 50% of the population between 18 and 60 years with this deficiency, and up to 87% in the population over 65 years old. |
| Xu et al. [31] | 2020 | Genetically increased circulating 25(OH)D level and prevention of T2D. | To provide an updated estimate for the causality between vitamin D and T2D. | 2-sample multi-instrument variables MR | A higher genetically instrumented 25(OH)D was causally linked to a reduced risk of T2D risk. They confirm the causal role of vitamin D using 2 synthesis-related single-nucleotide polymorphisms (SNPs). |
| Huang et al. [32] | 2015 | Vitamin D and risk of metabolic syndrome. | To analyze vitamin D levels and the link with the risk of metabolic syndrome in non-diabetic adults. | Cross-sectional study. A total of 335 non-diabetic young adult individuals. Measurement of 25(OH)D, metabolic syndrome, and cardiometabolic parameters. | There is an inverse association between vitamin D and MetS. This link could be related to the joint effects of obesity and insulin resistance in individuals. |
| Lee et al. [33] | 2019 | 25(OH)D levels and MetS in the elderly population. | To evaluate the relationship between 25(OH)D levels and MetS in the elderly Korean urban and rural population. | Cohort study. A total of 2936 men and women. Measurement of 25(OH)D serum levels as well as diagnosis of MetS. | There is an association between low levels of 25(OH)D and MetS. Those levels achieved more association in these variables of MetS: high waist circumference, hypertriglyceridemia, as well as low high-density lipoprotein cholesterol. |
| Zhu et al. [34] | 2018 | Vitamin D and markers of metabolic health. | To investigate the link between vitamin D and markers of metabolic health. | Cross-sectional study. A total of 508 urban residents. Measurement of demographic and anthropometric data, as well as 25(OH)D serum levels, blood glucose, and lipid concentrations. | A higher serum 25(OH)D concentration was linked to a better metabolic profile and less risk for developing MetS. |
| Liu et al. [35] | 2020 | Vitamin D-deficiency and MetS criteria in the elderly population. | To analyze the association between serum 25(OH)D and MetS in elderly Chinese individuals. | Cross-sectional study. A total of 2493 elderly people from eight areas of China. 25(OH)D serum levels as well as antropometric and biochemical measurements were determined | High serum vitamin D concentrations were associated with a low prevalence of MetS according to the Adult Treatment Panel III criteria for adequate versus deficient vitamin D and inadequate versus deficient vitamin D levels. |
| Ganji et al. [36] | 2020 | Vitamin D deficiency and MetS prevalence and markers. | To study the relationship between serum vitamin D concentrations and prevalence of MetS and markers of MetS in Qatari women. | Cross-sectional study. A total of 700 women aged 20–80 years old. Independent variable:serum 25(OH)D concentration-dependent variables:MetS and indicators of MetS defined following the International Diabetes Federation criteria | The study showed an inverse relationship between the prevalence of MetS and serum 25(OH)D in Qatari women. No relationship was observed between serum 25(OH)D and waist circumference, blood pressure, HbA1C, blood glucose, HDL-cholesterol, and serum triglycerides. |
| Pott-Junior et al. [37] | 2020 | MetS parameters and low serum 25-hydroxyvitamin D (25(OH)D) levels. | To investigate the relationship between metabolic parameters and serum 25(OH)D levels in community-living older adults. | Cross-sectional study. n = 265. Adults aged 60 years were assessed for anthropometrics and metabolic measurements, including 25(OH)D, insulin, glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and inflammatory markers. | Subjects with 25(OH)D deficiency presented higher body weight, body mass index, waist circumference, triglycerides and TNF-α, and higher insulin resistance. MetS was more prevalent among 25(OH)D-deficient subjects. |
| Barbalho et al. [38] | 2018 | Vitamin D and markers of metabolic health. | To investigate the link between vitamin D and markers of metabolic health. | Cross-sectional study. A total of 200 patients (89 men, 111 women). Determination of anthropometric and biochemical parameters, blood pressure, atherogenic indices, and presence of MetS. | Patients with altered values for this vitamin presented significantly higher values for glycemia, HbA1c, total cholesterol, LDL-c, triglycerides, BMI, waist circumference, and atherogenic indices. |
| Vimaleswaran et al. [39] | Vitamin D and arterial blood pressure and hypertension risk. | To test whether 25(OH)D concentration is causally associated with blood pressure and hypertension risk. | MR study | Increased plasma concentrations of 25(OH)D might reduce the risk of hypertension. Each 25(OH)D-increasing allele of the synthesis score was associated with a change of −0.10 mm Hg in systolic blood pressure and a change of −0.08 mm Hg in diastolic blood pressure. | |
| Vimaleswaran et al. [40] | Vitamin D status and obesity. | To explore the causality and direction of the relationship between body mass index (BMI) and 25-hydroxyvitamin D [25(OH)D]. | Bidirectional MR study | Higher BMI leads to lower 25(OH)D, whereas any effects of lower 25(OH)D-increasing BMI are likely to be small. | |
| Zheng et al. [41] | Circulating 25-hydroxyvitamin D metabolites and T2D. | To examine the potential causality of these associations using Mendelian randomization (MR) analysis. | MR study | The findings based on MR analysis in a large sample of European ancestry do not support a causal association of a total of 25(OH)D or 25(OH)D metabolites with T2D and argue against the use of vitamin D supplementation for the prevention of T2D. | |
| Wang et al. [42] | Vitamin D, prediabetes, and T2D. | To explore the causal relationship between 25-hydroxyvitamin D (25(OH)D) and glycemic status and indices. | Biredictional MR | The MR-derived odds ratios of genetically determined 25(OH)D for risk of T2D and prediabetes were 0.985 and 0.982, respectively. Fasting glucose and HbA1c were not significant either. | |
| Mehri et al. [43] | 2019 | Vitamin D and MetS and its components in females. | To determine the relationship between MetS and its components with vitamin D status. | Observational case-control study. Participants were 276 Iranian female teachers (124 in the case group and 152 in the control group). | Authors did not find an association between vitamin D status and MetS. There is a necessity of using prospective studies to link the vitamin D effects in the development of MetS. |
| Teixeira et al. [44] | 2018 | Association between vitamin D-deficiency and obesity. | To analyze vitamin D and metabolic profile in adolescents and adults and their relationship with severe obesity complications. | Observational comparative study. Population: adolescents and adults with severe obesity. Measurement of circumference and BMI. | There was a high prevalence of deficiency and insufficiency of vitamin D and its association with metabolic changes in the adult and adolescent population with obesity. |
| Chen et al. [45] | 25-hydroxyvitamin D cardiometabolic risk factors and MetS. | To test whether genetically lowered vitamin D levels were associated with MS and its metabolic traits. | MR study | Lower measured 25(OH)D levels were associated with MetS after multivariable adjustment. However, the MR-derived odds ratio of genetically determined 25(OH) D for risk of MS was 0.977. |
| Ref. | Year | Supplementation Characteristics | Findings |
|---|---|---|---|
| Lemieux et al. [48] | 2019 | Vitamin D3 5000 IU daily, 6 months | In pre-diabetic patients, vitamin D supplementation for 6 months significantly increased peripheral insulin sensitivity and β-cell function. |
| Babarawi et al. [49] | 2020 | In patients with prediabetes, vitamin D supplementation at moderate to high doses (≥1000 IU/day) significantly reduced the incidence risk of T2DM compared with placebo. | |
| Safarpour et al. [50] | 2020 | 50,000 IU/week vitamin D (Zahravi Co® pearls), 8 weeks | Vitamin D supplementation improved T2D by decreasing HbA1c and increasing SIRT1. |
| Farrokhian et al. [51] | 2017 | 50,000-IU vitamin D supplements every 2 weeks, 6 months | Compared with placebo, vitamin D supplementation resulted in significant reductions in fasting plasma glucose, serum insulin, homeostasis model assessment of insulin resistance, and β cell function. |
| Talari et al. [52] | 2019 | 50,000 IU vitamin D supplements every 2 weeks + 2 × 1000 mg/day n-3 fatty acids from flaxseed oil, 6 months | Vitamin D and n-3 fatty acids’ co-supplementation led to a significant reduction in fasting plasma glucose, insulin, insulin resistance and LDL, and a significant increase in insulin sensitivity and HDL compared with the placebo. |
| Wallace et al. [53] | 2019 | 3000 IU (75 µg) vitamin D3 daily, 26 weeks | There was no difference between treatment and placebo group in measures of whole-body, peripheral, or hepatic IR or in any measure of glycemic control or β-cell function. |
| Lerchbaum et al. [54] | 2019 | 20,000 IU of vitamin D3/week, 12 weeks | In healthy middle-aged men, vitamin D treatment had a negative effect on insulin sensitivity. |
| Ghaderi et al. [55] | 2017 | 50,000 IU vitamin D supplements every 2 weeks, 12 weeks | Patients who received vitamin D supplements had significantly decreased fasting plasma glucose, serum insulin levels, homeostasis model of assessment-insulin resistance, serum triglycerides total, and LDL compared with the placebo. |
| Jamilian et al. [56] | 2017 | 50,000 IU vitamin D every 2 weeks + 1000 mg omega-3 fatty acids twice a day, 6 weeks | Overall, vitamin D and omega-3 fatty acids co-supplementation for 6 weeks among gestational diabetes patients had beneficial effects on fasting plasma glucose, serum insulin levels, the homeostatic model of assessment for insulin resistance, quantitative insulin sensitivity check index, serum triglycerides, and very-low-density lipoprotein cholesterol levels. |
| Imga et al. [57] | 2019 | 100,000 IU/week as a loading dose for 8 weeks following a maintenance dose of 3000 IU/day | After the sixth month of supplementation in both overweight and obese subjects, a significant reduction was detected in HOMA-IR, low-density lipoprotein cholesterol, and parathyroid hormone levels. |
| Riek et al. [58] | 2018 | Vitamin D3 4000 IU/day for 4 months | Assessment of oxidized LDL uptake in monocytes cultured in the patient’s own serum before vs. after treatment resulted in >50% reduction in the vitamin D group with no change in the placebo group. The reduction in monocyte cholesterol uptake was reflected in a 19% decrease in total monocyte cholesterol content. |
| Liyanage et al. [59] | 2017 | 50,000 IU of vitamin D3 intramuscularly, monthly for 6 months | There was a significant improvement of serum HDL level with six months of therapy of high-dose vitamin D in patients with early diabetic nephropathy. |
| Ostadmohammadi et al. [60] | 2019 | This meta-analysis demonstrated the beneficial effects of vitamin D supplementation on improving glycemic control, HDL, and C-reactive protein levels among patients with cardiovascular diseases. | |
| Hafez et al. [61] | 2019 | VD3 in the form of 4000 IU/day for a period of 4 months | After 4 months of vitamin D supplementation, the mean difference (at 0 and 4 months) in LDL and HbA1c was statistically significant. |
| Tamadon et al. [62] | 2018 | oral vitamin D3 supplements at a dosage of 50,000 IU every 2 weeks for 12 weeks | No significant effect of vitamin D supplementation on lipid profiles and other biomarkers of inflammation and oxidative stress compared with the placebo was observed. |
| Perna [63] | 2019 | Vitamin D3 supplementation, from 25,000 to 600,000 IU/monthly of cholecalciferol. Duration of the treatment: from 1 to 12 months. | Cholecalciferol supplementation decreases the BMI by −0.32 kg/m2 and the waist circumference by −1.42 cm but does not statistically affect weight loss −0.43 kg. |
| Golzarand et al. [64] | 2018 | Supplementation from 400 to 7000 IU of daily cholecalciferol. Duration of the treatment: range from 3 to 12 months. | Cholecalciferol supplementation increased in individuals after the intervention. A decrease in body fat percentage was found (−0.31%, 95% CI: −1.07 to 0.44), although this was not significant. |
| Lotfi-Dizaji et al. [65] | 2019 | Weekly bolus dose of 50,000 UI od vitamin D. Duration of the treatment: 12 weeks. | An increase in serum 25(OH)D levels was found after vitamin D administration. The analysis showed a significant decrease in weight and body fat percentage after comparison between groups. |
| Subih et al. [66] | 2020 | Supplementation consisted of vitamin D3, with doses of 50,000 IU weekly, or vitamin D3 combined with calcium, with doses of 50,000 IU weekly of vitamin D and 1,200 mg/dL daily of calcium. Duration of the treatment: 3 months. | Compared to the control group, those women supplemented with vitamin D did not show significant changes related to weight loss or improvement of obesity biomarkers. However, after co-supplementation of calcium and vitamin D, a decrease in weight was reported, as well as an improvement in some of the blood metabolic profiles. |
| Duan et al. [67] | 2018 | Daily doses of vitamin D supplementation from 100 to 8571 UI. Duration of the treatment: range from 1.5 to 36 months. | Comparisons with the placebo or control group determined that neither BMI nor waist circumference decreased significantly after vitamin D administration. |
| Gallagher et al. [68] | 2013 | Daily doses of vitamin D ranged from 400 to 4800 IU. Duration of the treatment: 12 months. | Although an inverse relationship was found between serum vitamin D levels and fat mass, between-group analyses found no significant reductions in BMI or fat mass after supplementation. |
| Chandler et al. [69] | 2015 | When compared with placebo, vitamin D supplementation had no significant effect on BMI, weight, or fat mass. Likewise, no significant reduction in BMI, weight, or fat mass was observed in participants who received vitamin D plus calcium compared with those who received calcium control. | |
| Rajakumar et al. [70] | 2020 | Daily vitamin D oral doses containing 600, 1000, and 2000 UI. Duration of the treatment: 6 months. | Vitamin D supplementation showed no effect on BMI, waist circumference, waist-to-hip ratio, and percentage of fat tissue in children. In contrast, a significant decrease in SBP and DBP levels was observed after daily administration of 1000 IU. |
| Brzeziński et al. [71] | 2020 | Daily supplementation of 1200 UI of vitamin D. Duration of the treatment: 26 weeks. | There showed no significant decrease in either BMI or weight of obese children in comparison with the placebo group. |
| Makariou et al. [72] | 2020 | The vitamin D administration regimen was 2000 IU daily for a duration of 3 months. | The results did not show a relationship between vitamin D supplementation and BMI, blood pressure, lipids, glucose, and insulin levels, among others. |
| Golzarand et al. [73] | 2016 | The dose of vitamin D administration ranged from 200 to 12,000 IU per day. The mean duration of treatment was 5.6 ± 4.0 months. | The results showed that the daily administration of vitamin D supplementation in doses higher than 800 IU in subjects older than 50 years significantly reduced both SBP and DBP (p < 0.001). Moreover, hypotensive effects were observed in both the group of healthy subjects and the group of hypertensive subjects. |
| Shu & Huang [74] | 2018 | The daily dose of vitamin D administered was from 2800 UI to more than 5000 UI. Duration of the treatment: from 8 to 20 weeks. | Results show a significant effect of vitamin D supplementation on peripheral DBP in vitamin D-deficient participants, but not the same with peripheral SBP. Subgroup analysis showed a significant decrease in peripheral SBP and DBP in Asia, 8 weeks of intervention, and more than 5000 IU of daily vitamin D supplementation subgroups. |
| Wu et al. [75] | 2010 | The vitamin D dose administered varied in a range between 200 IU and 400 UI per day. Duration of the treatment: from 5 to 15 weeks. | The analysis concluded that vitamin D supplementation reduced SBP by 2.44 mmHg, but no effect on DBP was observed compared to the placebo groups. |
| Hussin et al. [76] | 2017 | The daily vitamin D administration regimen varied between 1000 UI and 5000 UI. Treatment duration ranged from 4 to 52 weeks. | The effects of vitamin D supplementation showed no improvement in endothelial function. Specifically, no significant changes in blood pressure, post-occlusive vasodilation of the brachial artery, among others, were observed. |
| Swart et al. [77] | 2018 | Vitamin D supplementation varies between 200 IU and 7000 IU daily. The duration of treatment ranged from 8 weeks to 1 year. | All the studies analyzed found a significant increase in serum vitamin D levels. However, vitamin D supplementation had no effect on SBP and DBP. |
| Abboud et al. [78] | 2020 | Vitamin D administration regimens were in a daily range between 400 IU and 43,000 IU, with a duration between 12 and 24 weeks. | The results showed that vitamin D supplementation was ineffective in reducing SBP and DBP in children and adolescent populations. |
| Morvaridzadeh et al. [79] | 2020 | Vitamin D was administered daily between 200 IU and 7142 IU. Calcium dosage ranged from 500 to 1200 mg daily. Treatment duration ranged from 6 weeks to 7 years. | Vitamin D and calcium co-supplementation did not show a reduction in SBP compared to the control group, although a significant decrease in DBP was found. |
| Carbonare et al. [80] | 2018 | Vitamin D3 | Two regimens were equivalent; most of the patients reached the normal range of vitamin D after six months of treatment with increased calcium levels and decreased bone turnover. Waist circumference also decreased. The relationship between serum 25(OH)vitamin D3 concentration and waist circumference supports vitamin D having a protective role in the current setting since waist size is directly associated with the risk of cardiovascular and metabolic diseases. |
| Al-Daghri et al. [82] | 2019 | Vitamin D3vitamin D-fortified milk | There was an increase in 25(OH)D levels in all groups. It is also revealed a clinically significant decrease in triglycerides, glucose, and systolic blood pressure, as well as a clinically significant increase in HDL over time, all in favor of the Vit D group. MetS incidence did decrease in the Vit. D group only. Consequently, oral vitamin D supplementation is superior to vitamin D-fortified milk in improving vitamin D status. Reduction in the incidence of MetS in the Arab adolescent population secondary to vitamin D correction may be dose-dependent. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. https://doi.org/10.3390/nu13030830
Melguizo-Rodríguez L, Costela-Ruiz VJ, García-Recio E, De Luna-Bertos E, Ruiz C, Illescas-Montes R. Role of Vitamin D in the Metabolic Syndrome. Nutrients. 2021; 13(3):830. https://doi.org/10.3390/nu13030830
Chicago/Turabian StyleMelguizo-Rodríguez, Lucía, Víctor J. Costela-Ruiz, Enrique García-Recio, Elvira De Luna-Bertos, Concepción Ruiz, and Rebeca Illescas-Montes. 2021. "Role of Vitamin D in the Metabolic Syndrome" Nutrients 13, no. 3: 830. https://doi.org/10.3390/nu13030830
APA StyleMelguizo-Rodríguez, L., Costela-Ruiz, V. J., García-Recio, E., De Luna-Bertos, E., Ruiz, C., & Illescas-Montes, R. (2021). Role of Vitamin D in the Metabolic Syndrome. Nutrients, 13(3), 830. https://doi.org/10.3390/nu13030830