Preconditioning with Short-Term Dietary Restriction Attenuates Cardiac Oxidative Stress and Hypertrophy Induced by Chronic Pressure Overload
Abstract
1. Introduction
2. Materials and Methods
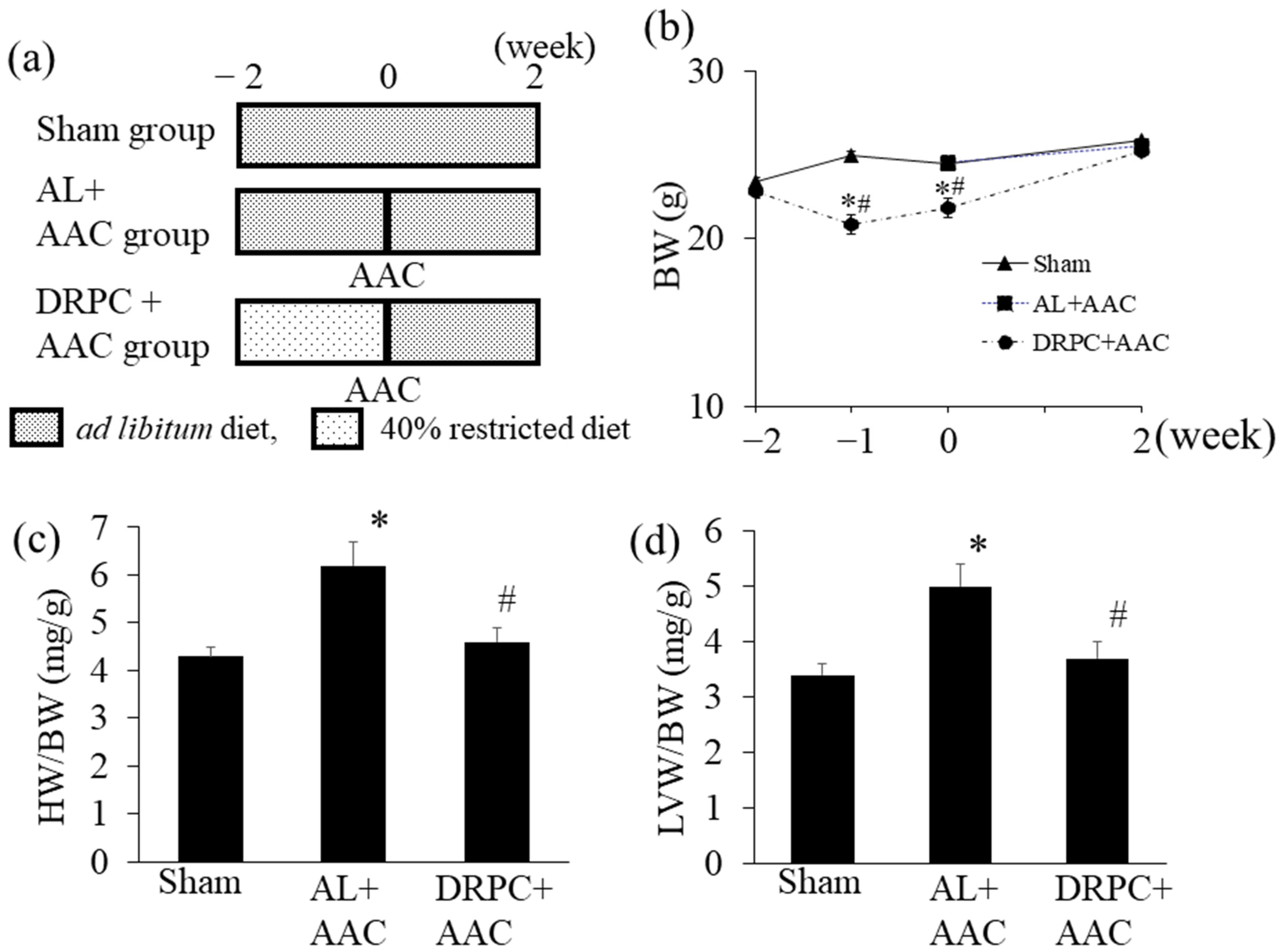
2.1. Animals and Experimental Protocols
2.2. Sample Collection and Histological Analysis
2.3. Echocardiography
2.4. Brain Natriuretic Peptide (BNP) mRNA Expression
2.5. Immunohistological Staining
2.6. In Situ Detection of Apoptosis
2.7. Mitochondrial Isolation and Measurement of Lipid Peroxide Levels
2.8. NADPH Oxidase-Derived Superoxide Production in Cardiac Tissue
2.9. Measurement of Superoxide Production from Isolated Heart Mitochondria
2.10. Measurement of Mitochondrial Permeability Transition (MPT)
2.11. Statistical Analysis
3. Results
3.1. Body and Organ Weights
3.2. Assessments of Cardiac Geometry and Function
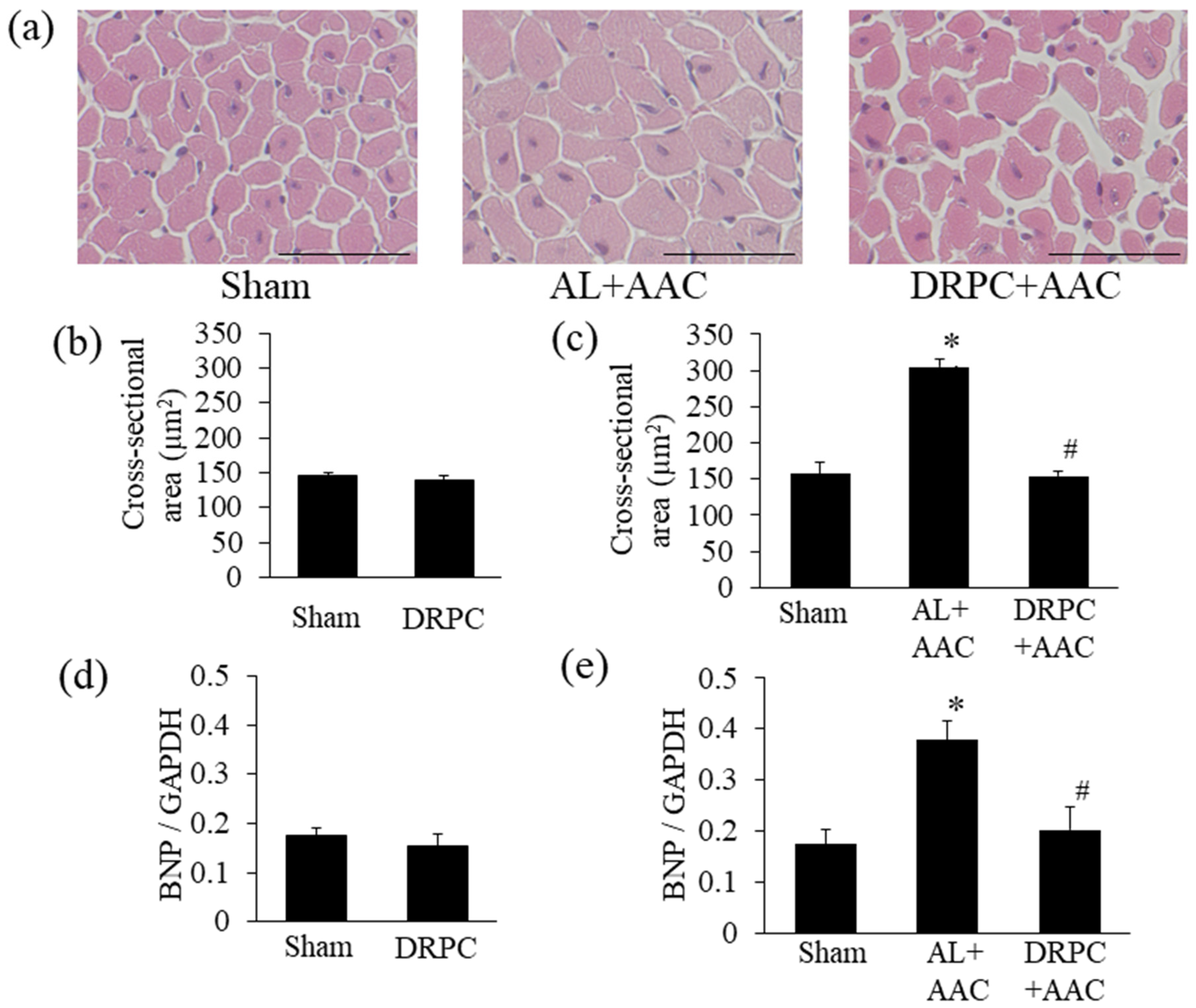
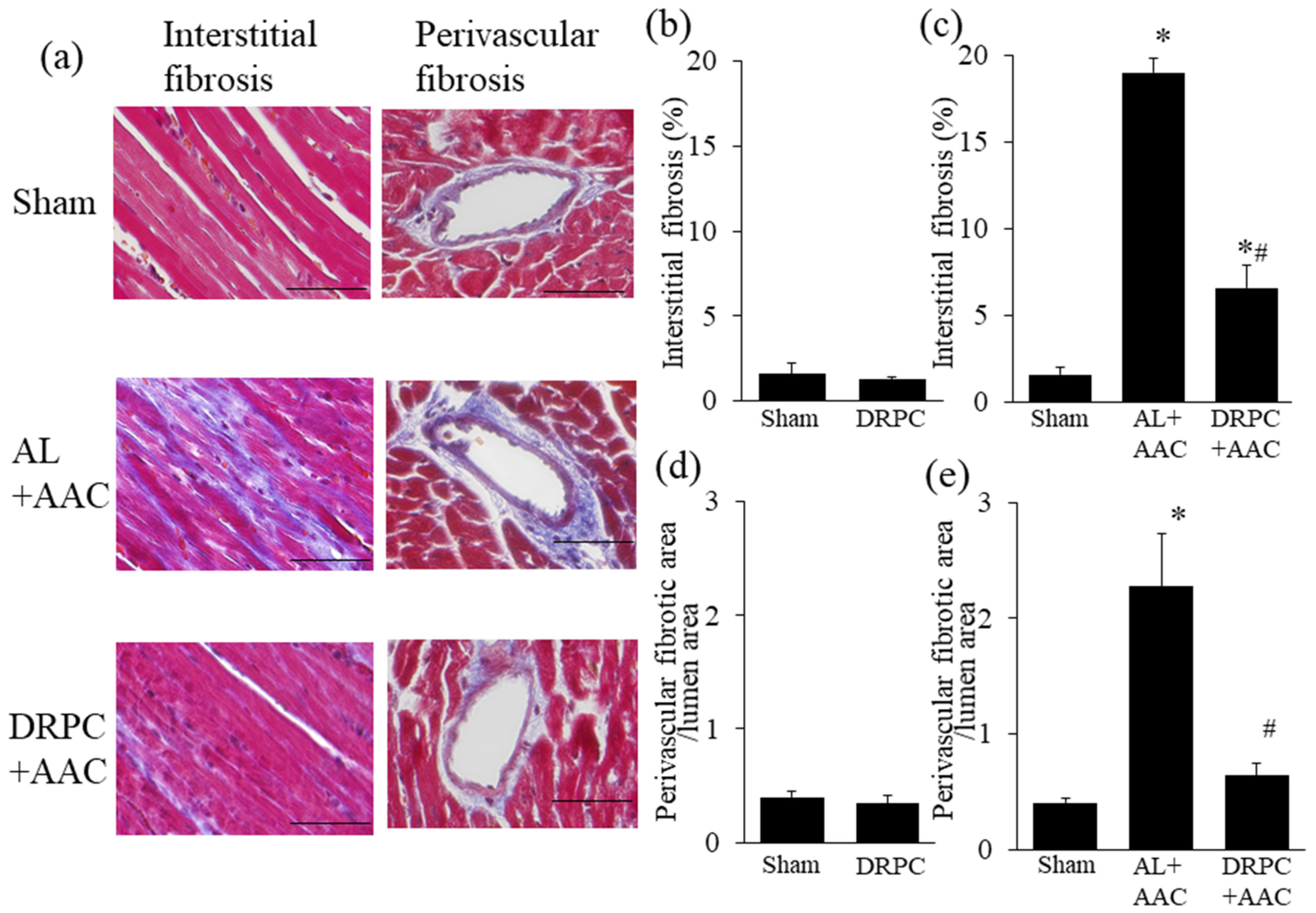
3.3. Histomorphometry
3.4. BNP mRNA Expression in the Hypertrophic Myocardium
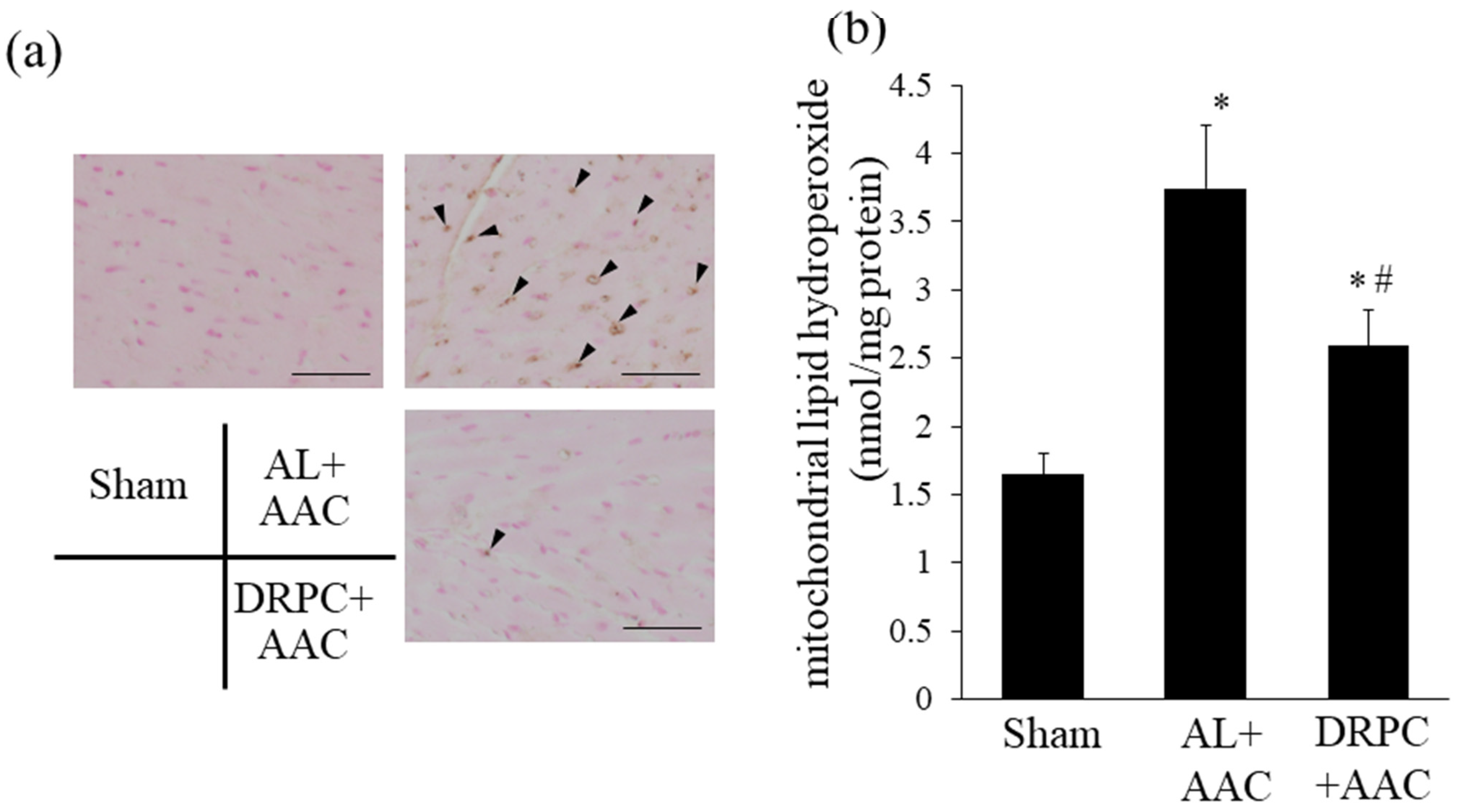
3.5. Oxidative Stress in the Hypertrophic Myocardium
3.6. Myocardial NADPH Oxidase-Dependent and Mitochondrial Superoxide Production
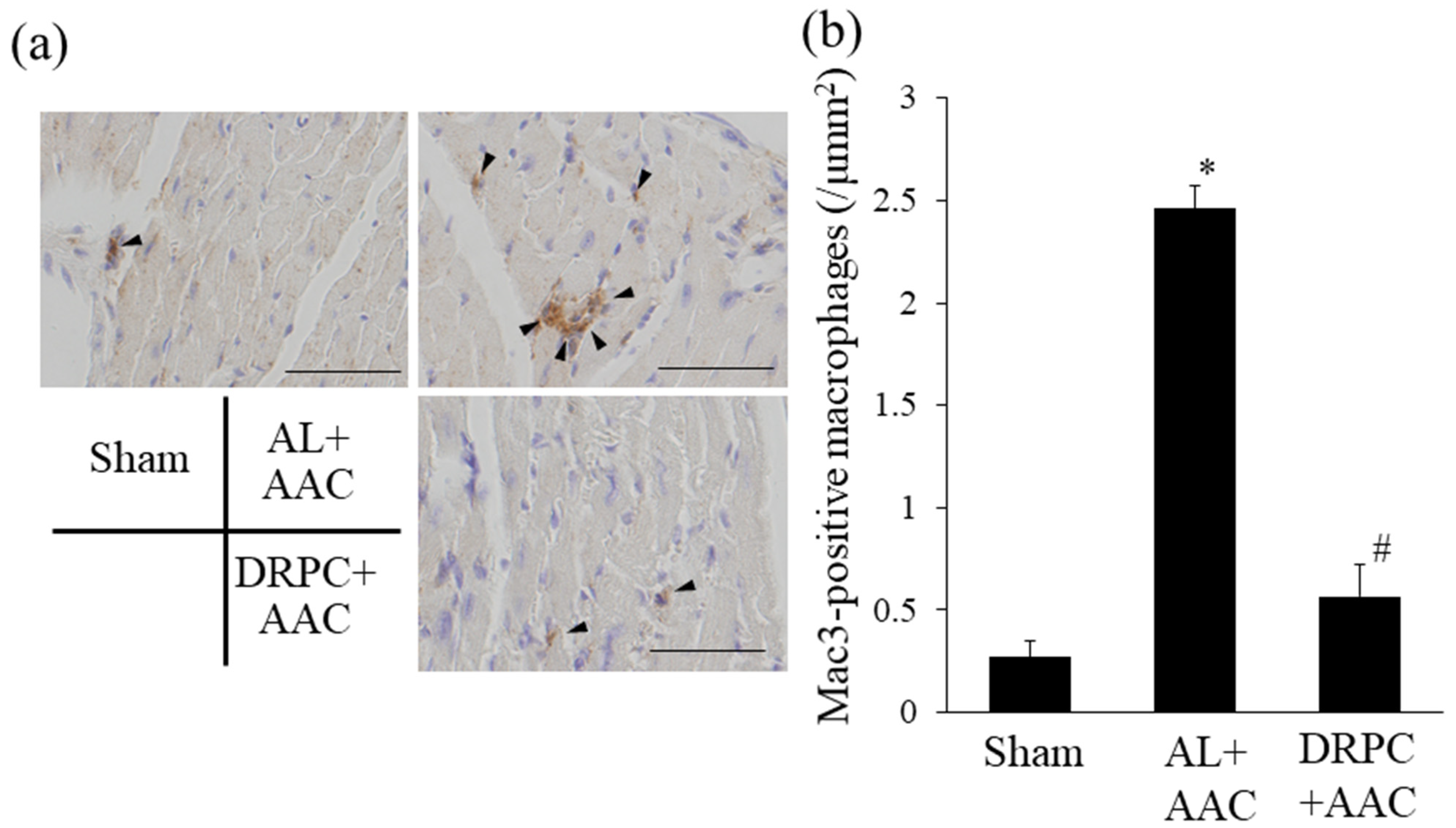
3.7. Infiltration of Macrophages in the Hypertrophic Myocardium
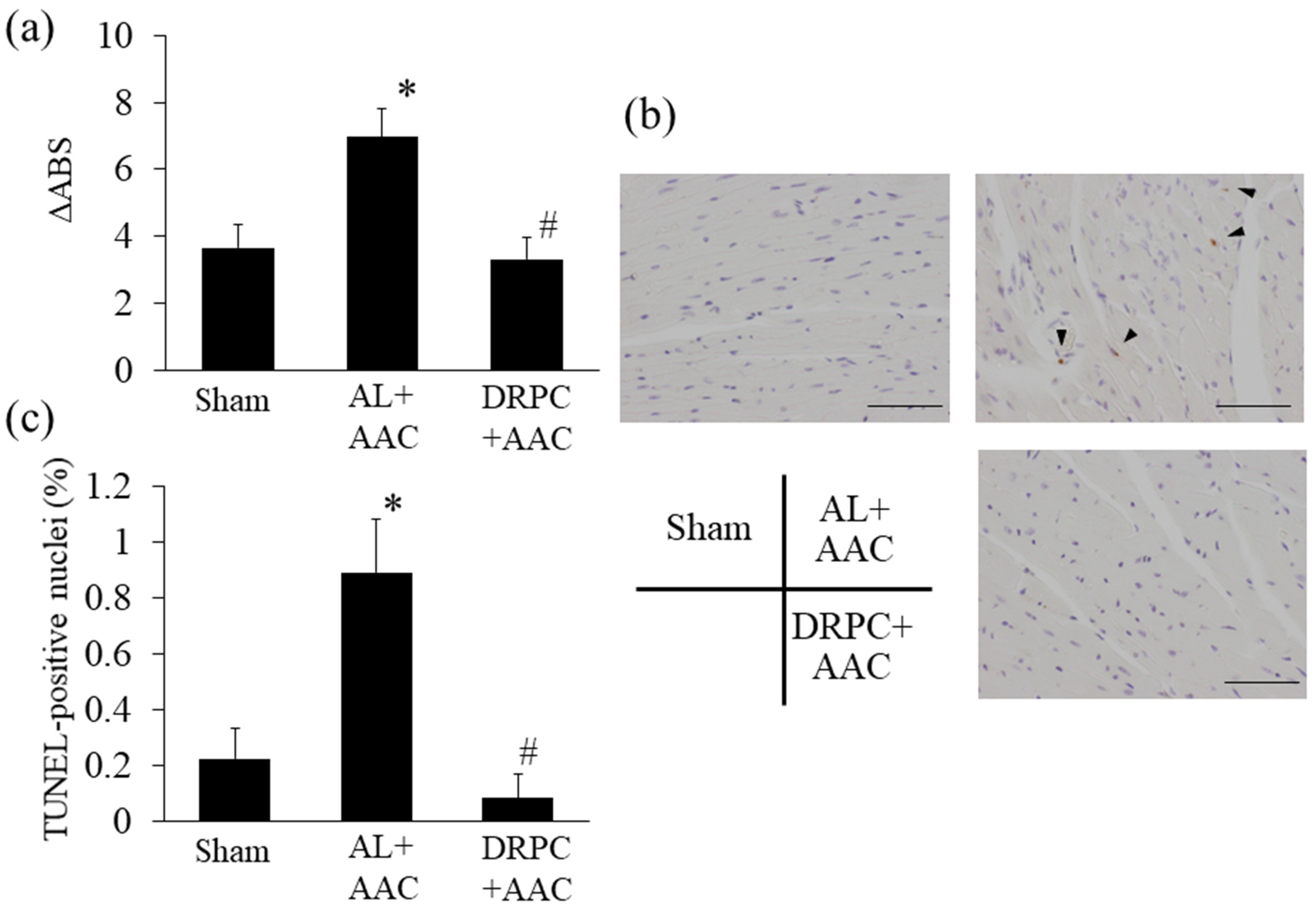
3.8. MPT and Apoptosis in the Hypertrophic Myocardium
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontana, L.; Klein, S. Aging, Adiposity, and Calorie Restriction. JAMA 2007, 297, 986–994. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span–From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef]
- Kemi, M.; Keenan, K.P.; McCoy, C.; Hoe, C.M.; Soper, K.A.; Ballam, G.C.; van Zwieten, M.J. The Relative Protective Effects of Moderate Dietary Restriction versus Dietary Modification on Spontaneous Cardiomyopathy in Male Sprague-Dawley Rats. Toxicol. Pathol. 2000, 28, 285–296. [Google Scholar] [CrossRef]
- Yan, L.; Gao, S.; Ho, D.; Park, M.; Ge, H.; Chunbo, W.; Tian, Y.; Lai, L.; De Lorenzo, M.S.; Vatner, D.E.; et al. Calorie Restriction Can Reverse, as Well as Prevent, Aging Cardiomyopathy. Age 2013, 35, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, M.; Nakashima, C.; Takahashi, K.; Murase, T.; Hattori, T.; Ito, H.; Murohara, T.; Nagata, K. Calorie Restriction Attenuates Cardiac Remodeling and Diastolic Dysfunction in a Rat Model of Metabolic Syndrome. Hypertension 2013, 62, 957–965. [Google Scholar] [CrossRef]
- Shinmura, K.; Tamaki, K.; Ito, K.; Yan, X.; Yamamoto, T.; Katsumata, Y.; Matsuhashi, T.; Sano, M.; Fukuda, K.; Suematsu, M.; et al. Indispensable role of endothelial nitric oxide synthase in caloric restriction-induced cardioprotection against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H894–H903. [Google Scholar] [CrossRef]
- Finckenberg, P.; Eriksson, O.; Baumann, M.; Merasto, S.; Lalowski, M.M.; Levijoki, J.; Haasio, K.; Kytö, V.; Muller, D.N.; Luft, F.C.; et al. Caloric Restriction Ameliorates Angiotensin II-induced Mitochondrial Remodeling and Cardiac Hypertrophy. Hypertension 2012, 59, 76–84. [Google Scholar] [CrossRef]
- Kobara, M.; Furumori-Yukiya, A.; Kitamura, M.; Matsumura, M.; Ohigashi, M.; Toba, H.; Nakata, T. Short-Term Caloric Restriction Suppresses Cardiac Oxidative Stress and Hypertrophy Caused by Chronic Pressure Overload. J. Card. Fail. 2015, 21, 656–666. [Google Scholar] [CrossRef]
- Meyer, T.E.; Kovács, S.J.; Ehsani, A.A.; Klein, S.; Holloszy, J.O.; Fontana, L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 2006, 47, 398–402. [Google Scholar] [CrossRef]
- Holzenberger, M.; Dupont, J.; Ducos, B.; Leneuve, P.; Géloën, A.; Even, P.C.; Cervera, P.; Le Bouc, Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003, 421, 182–187. [Google Scholar] [CrossRef]
- López-Lluch, G.; Plácido, N. Calorie Restriction as an Intervention in Ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef]
- Walsh, M.E.; Shi, Y.; Van Remmen, H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med. 2014, 66, 88–99. [Google Scholar] [CrossRef]
- Gredilla, R.; Sanz, A.; Lopez-Torres, M.; Barja, G. Caloric Restriction Decreases Mitochondrial Free Radical Generation at Complex I and Lowers Oxidative Damage to Mitochondrial DNA in the Rat Heart. FASEB J. 2001, 15, 1589–1591. [Google Scholar] [CrossRef]
- Das, S.K.; Balasubramanian, P.; Weerasekara, Y.K. Nutrition Modulation of Human Aging: The Calorie Restriction Paradigm. Mol. Cell. Endocrinol. 2017, 455, 148–157. [Google Scholar] [CrossRef]
- Carlson, A.J.; Hoelzel, F. Apparent Prolongation of the Life Span of Rats by Intermittent Fasting. J. Nutr. 1946, 31, 363–375. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term Modified Alternate-Day Fasting: A Novel Dietary Strategy for Weight Loss and Cardioprotection in Obese Adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Dhahbi, J.M.; Kim, H.-J.; Mote, P.L.; Beaver, R.J.; Spindler, S.R. Temporal Linkage Between the Phenotypic and Genomic Responses to Caloric Restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 5524–5529. [Google Scholar] [CrossRef]
- Noyan, H.; El-Mounayri, O.; Isserlin, R.; Arab, S.; Momen, A.; Cheng, H.S.; Wu, J.; Afroze, T.; Li, R.-K.; Fish, J.E.; et al. Cardioprotective Signature of Short-Term Caloric Restriction. PLoS ONE 2015, 10, e0130658. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart Disease and Stroke statistics—2013 Update: A Report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef]
- Gerstenblith, G.; Frederiksen, J.; Yin, F.C.; Fortuin, N.J.; Lakatta, E.G.; Weisfeldt, M.L. Echocardiographic Assessment of a Normal Adult Aging Population. Circulation 1977, 56, 273–278. [Google Scholar] [CrossRef]
- Stewart, S.; MacIntyre, K.; Hole, D.J.; Capewell, S.; McMurray, J.J.; Stewart, S. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur. J. Heart Fail. 2001, 3, 315–322. [Google Scholar] [CrossRef]
- Sugden, P.H.; Clerk, A. Cellular Mechanisms of Cardiac Hypertrophy. J. Mol. Med. 1998, 76, 725–746. [Google Scholar] [CrossRef]
- Nakamura, K.; Fushimi, K.; Kouchi, H.; Mihara, K.; Miyazaki, M.; Ohe, T.; Namba, M. Inhibitory Effects of Antioxidants on Neonatal Rat Cardiac Myocyte Hypertrophy Induced by Tumor Necrosis Factor-Alpha and Angiotensin II. Circulation 1998, 98, 794–799. [Google Scholar] [CrossRef]
- Date, M.O.; Morita, T.; Yamashita, N.; Nishida, K.; Yamaguchi, O.; Higuchi, Y.; Hirotani, S.; Matsumura, Y.; Hori, M.; Tada, M.; et al. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J. Am. Coll. Cardiol. 2002, 39, 907–912. [Google Scholar] [CrossRef]
- Murdoch, C.E.; Zhang, M.; Cave, A.C.; Shah, A.M. NADPH Oxidase-Dependent Redox Signalling in Cardiac Hypertrophy, Remodelling and Failure. Cardiovasc. Res. 2006, 71, 208–215. [Google Scholar] [CrossRef]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative Stress Regulates Collagen Synthesis and Matrix Metalloproteinase Activity in Cardiac Fibroblasts. Am. J. Physiol. Cell Physiol. 2001, 280, C53–C60. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef]
- Dai, D.-F.; Hsieh, E.J.; Liu, Y.; Chen, T.; Beyer, R.P.; Chin, M.T.; MacCoss, M.J.; Rabinovitch, P.S. Mitochondrial Proteome Remodelling in Pressure Overload-Induced Heart Failure: The Role of Mitochondrial Oxidative Stress. Cardiovasc. Res. 2012, 93, 79–88. [Google Scholar] [CrossRef]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and Ageing: Role in Heart, Skeletal Muscle and Adipose Tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Kobara, M.; Hamada, J.; Yoshifuji, Y.; Shiraishi, T.; Tanaka, T.; Wang, J.; Toba, H.; Nakata, T. Additive Amelioration of Oxidative Stress and Cardiac Function by Combined Mineralocorticoid and Angiotensin Receptor Blockers in Postinfarct Failing Hearts. J. Cardiovasc. Pharmacol. 2012, 60, 140–149. [Google Scholar] [CrossRef]
- Weiss, E.P.; Fontana, L. Caloric Restriction: Powerful Protection for the Aging Heart and Vasculature. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1205–H1219. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Wang, Z.Q.; Bell-Farrow, A.D.; Collins, J.; Morgan, T.; Wagner, J.D. Caloric Restriction and Cardiovascular Aging in Cynomolgus Monkeys (Macaca Fascicularis): Metabolic, Physiologic, and Atherosclerotic Measures From a 4-year Intervention Trial. J. Gerontol. Biol. Sci. Med. Sci. 2004, 59, 1007–1014. [Google Scholar] [CrossRef]
- Das, S.K.; Gilhooly, C.H.; Golden, J.K.; Pittas, A.G.; Fuss, P.J.; Cheatham, R.A.; Tyler, S.; Tsay, M.; McCrory, M.A.; Lichtenstein, A.H.; et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: A 1-y randomized controlled trial. Am. J. Clin. Nutr. 2007, 85, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative Stress, Caloric Restriction, and Aging. Science 1996, 3, 59–63. [Google Scholar] [CrossRef]
- Il’yasova, D.; Fontana, L.; Bhapkar, M.; Pieper, C.F.; Spasojevic, I.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kraus, W.E.; CALERIE Study Investigators. Effects of 2 Years of Caloric Restriction on Oxidative Status Assessed by Urinary F2-isoprostanes: The CALERIE 2 Randomized Clinical Trial. Aging Cell 2018, 17, e12719. [Google Scholar]
- Lee, J.; Yu, B.P.; Herlihy, J.T. Modulation of Cardiac Mitochondrial Membrane Fluidity by Age and Calorie Intake. Free Radic. Biol. Med. 1999, 26, 260–265. [Google Scholar] [CrossRef]
- López-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie Restriction Induces Mitochondrial Biogenesis and Bioenergetic Efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef]
- Judge, S.; Judge, A.; Grune, T.; Leeuwenburgh, C. Short-term CR decreases cardiac mitochondrial oxidant production but increases carbonyl content. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R254–R259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.W.; Bai, F.; Wang, J.; Zheng, R.H.; Yang, L.W.; James, E.A.; Zhao, Z.Q. Edaravone inhibits pressure overload-induced cardiac fibrosis and dysfunction by reducing expression of angiotensin II AT1 receptor. Drug Des. Devel. Ther. 2017, 11, 3019–3033. [Google Scholar] [CrossRef]
- Izem-Meziane, M.; Djerdjouri, B.; Rimbaud, S.; Caffin, F.; Fortin, D.; Garnier, A.; Veksler, V.; Joubert, F.; Ventura-Clapier, R. Catecholamine-induced Cardiac Mitochondrial Dysfunction and mPTP Opening: Protective Effect of Curcumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H665–H674. [Google Scholar] [CrossRef]
- Hafner, A.V.; Dai, J.; Gomes, A.P.; Xiao, C.-Y.; Palmeira, C.M.; Rosenzweig, A.; Sinclair, D.A. Regulation of the mPTP by SIRT3-mediated Deacetylation of CypD at Lysine 166 Suppresses Age-Related Cardiac Hypertrophy. Aging 2010, 2, 914–923. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart: Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Tsujimoto, I.; Hikoso, S.; Yamaguchi, O.; Kashiwase, K.; Nakai, A.; Takeda, T.; Watanabe, T.; Taniike, M.; Matsumura, Y.; Nishida, K.; et al. The Antioxidant Edaravone Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy. Hypertension 2005, 45, 921–926. [Google Scholar] [CrossRef]
- Grieve, D.J.; Byrne, J.A.; Siva, A.; Layland, J.; Johar, S.; Cave, A.C.; Shah, A.S. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J. Am. Coll. Cardiol. 2006, 47, 817–826. [Google Scholar] [CrossRef]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef]
- Meydani, M.; Das, S.; Band, M.; Epstein, S.; Roberts, S. The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: Results from the CALERIE Trial of Human Caloric Restriction. J. Nutr. Health Aging 2011, 15, 456–460. [Google Scholar] [CrossRef]
- Müller, M.J.; Enderle, J.; Pourhassan, M.; Braun, W.; Eggeling, B.; Lagerpusch, M.; Glüer, C.-C.; Kehayias, J.J.; Kiosz, D.; Bosy-Westphal, A. Metabolic Adaptation to Caloric Restriction and Subsequent Refeeding: The Minnesota Starvation Experiment Revisited. Am. J. Clin. Nutr. 2015, 102, 807–819. [Google Scholar] [CrossRef]


| Sham | AL+AAC | DRPC+AAC | |
|---|---|---|---|
| LVDd (mm) | 2.58 ± 0.19 | 2.38 ± 0.12 | 2.32 ± 0.17 |
| IVST (mm) | 0.80 ± 0.03 | 1.33 ± 0.03 * | 0.92 ± 0.06 # |
| PWT (mm) | 0.74 ± 0.04 | 1.28 ± 0.05 * | 0.98 ± 0.04 *# |
| LVFS (%) | 61.5 ± 0.4 | 67.7 ± 0.3 | 61.3 ± 0.4 |
| E/A | 2.80 ± 0.16 | 1.64 ± 0.05 * | 2.04 ± 0.07 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobara, M.; Naseratun, N.; Toba, H.; Nakata, T. Preconditioning with Short-Term Dietary Restriction Attenuates Cardiac Oxidative Stress and Hypertrophy Induced by Chronic Pressure Overload. Nutrients 2021, 13, 737. https://doi.org/10.3390/nu13030737
Kobara M, Naseratun N, Toba H, Nakata T. Preconditioning with Short-Term Dietary Restriction Attenuates Cardiac Oxidative Stress and Hypertrophy Induced by Chronic Pressure Overload. Nutrients. 2021; 13(3):737. https://doi.org/10.3390/nu13030737
Chicago/Turabian StyleKobara, Miyuki, Nessa Naseratun, Hiroe Toba, and Tetsuo Nakata. 2021. "Preconditioning with Short-Term Dietary Restriction Attenuates Cardiac Oxidative Stress and Hypertrophy Induced by Chronic Pressure Overload" Nutrients 13, no. 3: 737. https://doi.org/10.3390/nu13030737
APA StyleKobara, M., Naseratun, N., Toba, H., & Nakata, T. (2021). Preconditioning with Short-Term Dietary Restriction Attenuates Cardiac Oxidative Stress and Hypertrophy Induced by Chronic Pressure Overload. Nutrients, 13(3), 737. https://doi.org/10.3390/nu13030737





