Low Carb and Ketogenic Diets Increase Quality of Life, Physical Performance, Body Composition, and Metabolic Health of Women with Breast Cancer
Abstract
1. Introduction
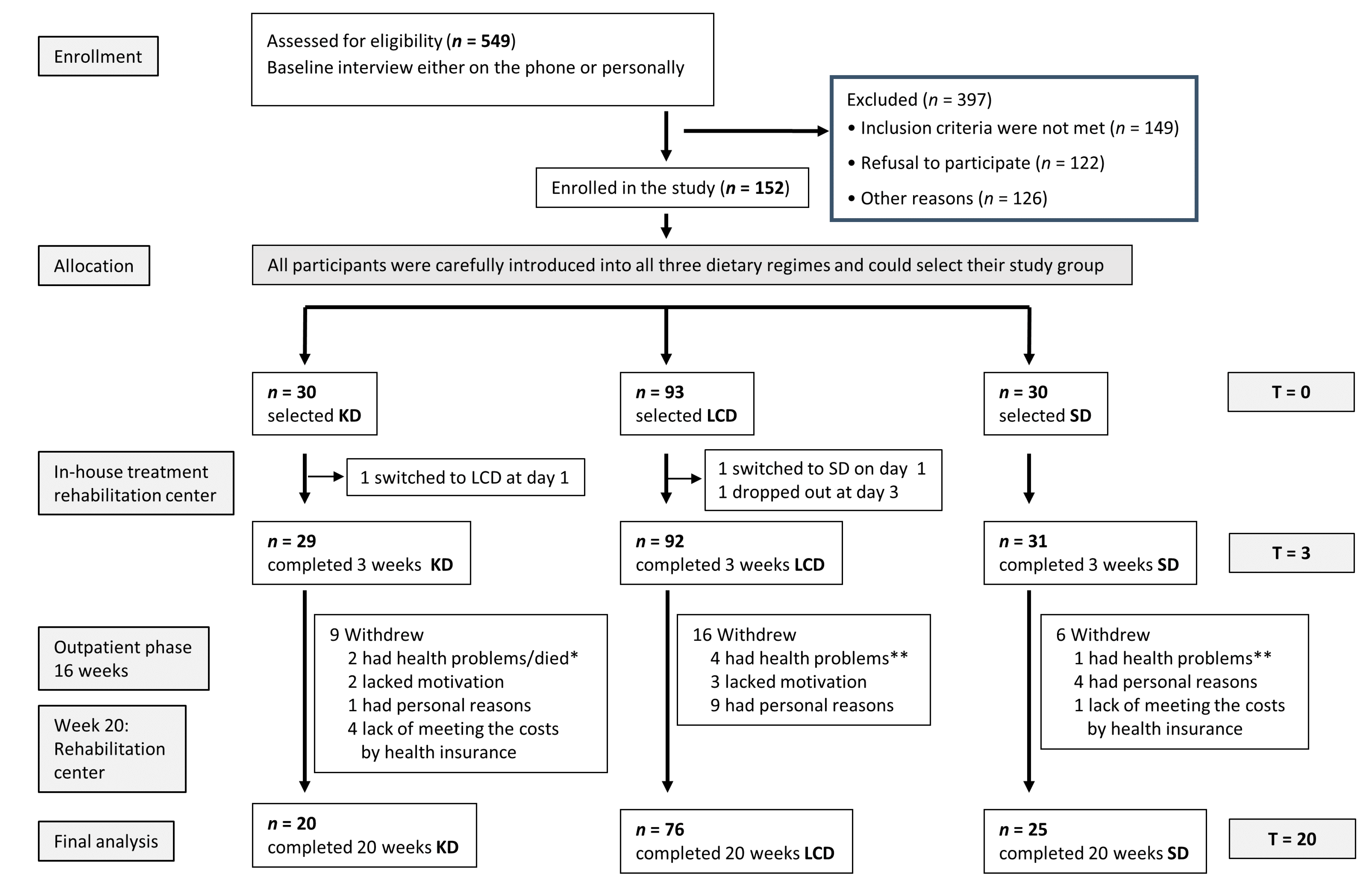
2. Materials and Methods
2.1. Study Design
- Three weeks of an inpatient multimodal intervention (for details, see Supplementary Materials) in the rehabilitation center (initial examination T0), followed by the implementation of the allocated diet and training of the patients in diet calculation, cooking, and realization of the diet regimen in routine daily life.
- Sixteen-week outpatient phase: continuing the selected nutritional regime under close contact and supervision of the study team, accompanied by food diaries and daily urine measurements of ketones (KD group).
- One closing week of intervention at the rehabilitation center (final examination: T20).
2.2. Patients
2.3. Procedure
2.4. Parameters Analyzed at T0 and T20
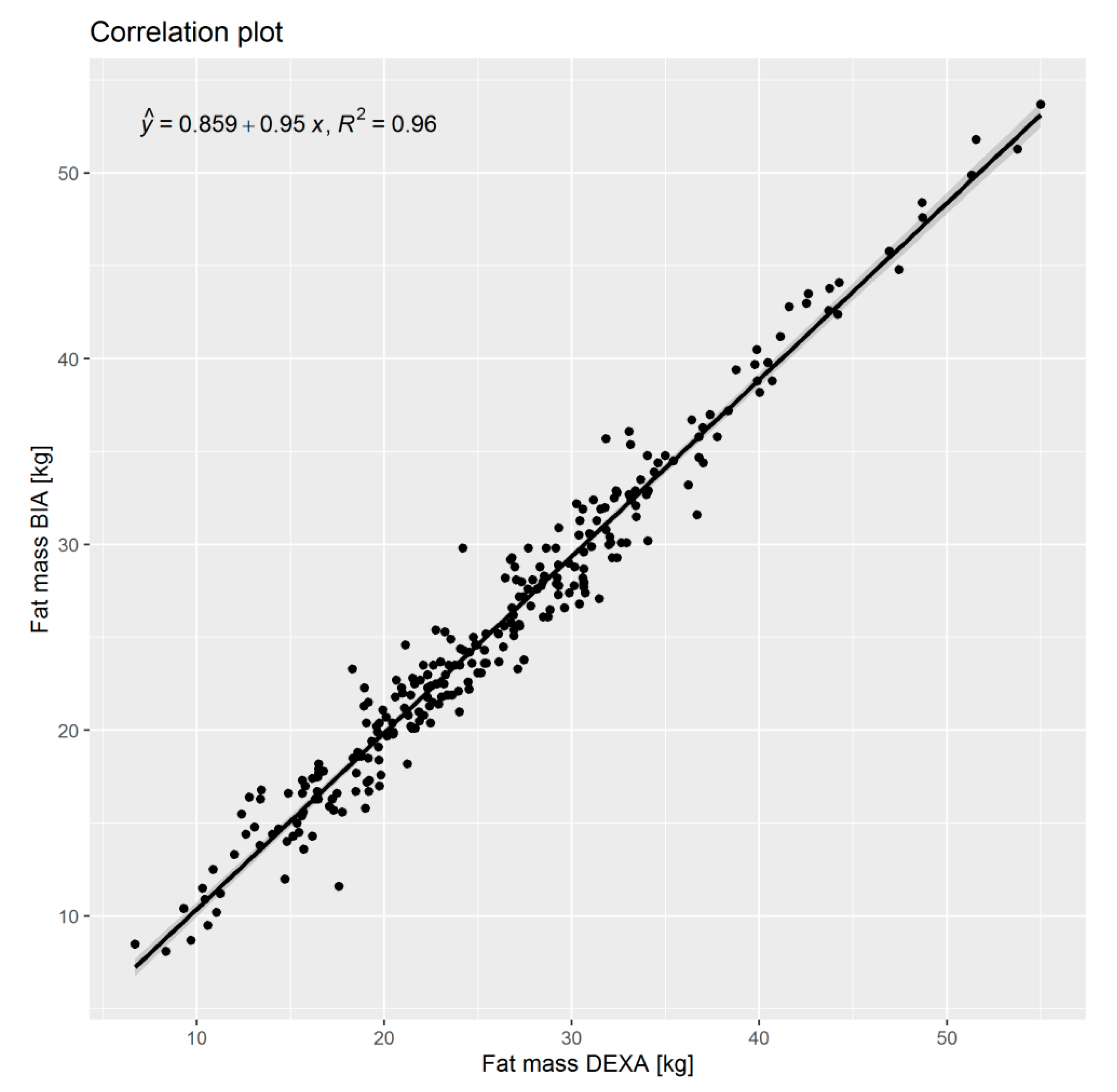
2.5. Diet Analysis
2.6. Data Collection and Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Choice of Diet Type
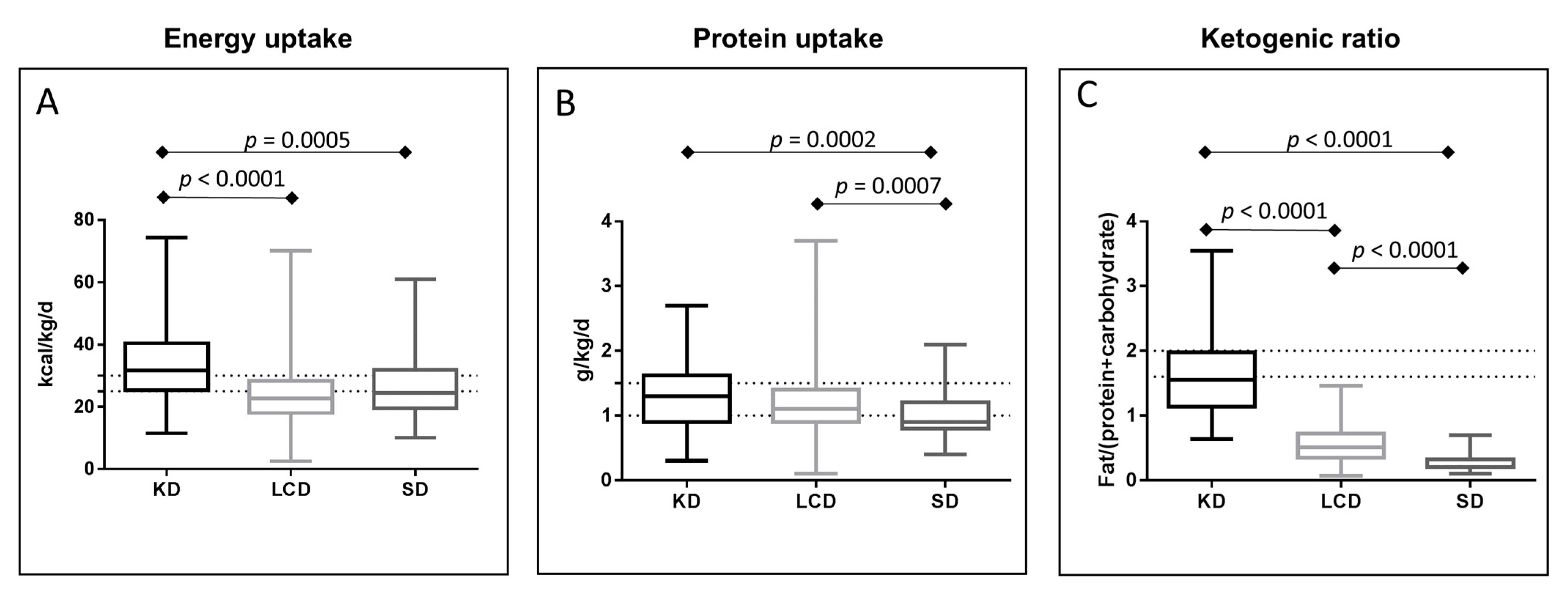
3.3. Energy and Protein Uptake
3.4. Physical Performance
3.5. Body Composition
3.6. Quality of Life (QoL)
3.7. Blood Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Ponziani, F.R.; Pompili, M.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; et al. Prognostic value of skeletal muscle mass during tyrosine kinase inhibitor (TKI) therapy in cancer patients: A systematic review and meta-analysis. Intern. Emerg. Med. 2020, 1–16. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Iwase, T.; Wang, X.; Shrimanker, T.V.; Kolonin, M.G.; Ueno, N.T. Body composition and breast cancer risk and treatment: Mechanisms and impact. Breast Cancer Res. Treat. 2021, 1–11. [Google Scholar] [CrossRef]
- Dev, R.; Bruera, E.; Dalal, S. Insulin resistance and body composition in cancer patients. Ann. Oncol. 2018, 29, ii18–ii26. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Bonanni, B. Relationship between Metabolic Disorders and Breast Cancer Incidence and Outcomes. Is There a Preventive and Therapeutic Role for Berberine? Anticancer Res. 2018, 38, 4393–4402. [Google Scholar] [CrossRef]
- Körber, J.; Pricelius, S.; Heidrich, M.; Müller, M.J. Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur. J. Clin. Nutr. 1999, 53, 740–745. [Google Scholar] [CrossRef]
- Hansell, D.T.; Davies, J.W.L.; Burns, H.J.G.; Shenkin, A. The Oxidation of Body Fuel Stores in Cancer Patients. Ann. Surg. 1986, 204, 637–642. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Cintoni, M.; Raoul, P.; Ianiro, G.; Salerno, L.; Pozzo, C.; Bria, E.; Muscaritoli, M.; Molfino, A.; et al. The Facts about Food after Cancer Diagnosis: A Systematic Review of Prospective Cohort Studies. Nutrients 2020, 12, 2345. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Norat, T.; Scoccianti, C.; Boutron-Ruault, M.-C.; Anderson, A.; Berrino, F.; Cecchini, M.; Espina, C.; Key, T.; Leitzmann, M.; Powers, H.; et al. European Code against Cancer 4th Edition: Diet and cancer. Cancer Epidemiol. 2015, 39, S56–S66. [Google Scholar] [CrossRef] [PubMed]
- Champ, C.E.; Mishra, M.V.; Showalter, T.N.; Ohri, N.; Dicker, A.P.; Simone, N.L. Dietary Recommendations During and After Cancer Treatment: Consistently Inconsistent? Nutr. Cancer 2013, 65, 430–439. [Google Scholar] [CrossRef]
- Wolfram, G.; Bechthold, A.; Boeing, H.; Ellinger, S.; Hauner, H.; Kroke, A.; Leschik-Bonnet, E.; Linseisen, J.; Lorkowski, S.; Schulze, M.B.; et al. Evidence-Based Guideline of the German Nutrition Society: Fat Intake and Prevention of Selected Nutrition-Related Diseases. Ann. Nutr. Metab. 2015, 67, 141–204. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Fearon, K.C.; Borland, W.; Preston, T.; Tisdale, M.J.; Shenkin, A.; Calman, K.C. Cancer cachexia: Influence of systemic ketosis on substrate levels and nitrogen metabolism. Am. J. Clin. Nutr. 1988, 47, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cancellara, P.; Pompei, P.; Moro, T. Ketogenic Diet and Skeletal Muscle Hypertrophy: A Frenemy Relationship? J. Hum. Kinet. 2019, 68, 233–247. [Google Scholar] [CrossRef]
- Breitkreutz, R.; Tesdal, K.; Jentschura, D.; Haas, O.; Leweling, H.; Holm, E. Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: A randomized controlled study. Wien. Klin. Wochenschr. 2005, 117, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Erickson, N.; Boscheri, A.; Linke, B.; Huebner, J. Systematic review: Isocaloric ketogenic dietary regimes for cancer patients. Med. Oncol. 2017, 34, 963. [Google Scholar] [CrossRef]
- Klassen, P.N.; Goldenberg, B.A.; Lambert, P.; Vagianos, K.; Kim, C.A. Ketogenic and low-sugar diets for patients with cancer: Perceptions and practices of medical oncologists in Canada. Support Care Cancer 2020, 28, 5243–5249. [Google Scholar] [CrossRef]
- Sremanakova, J.; Sowerbutts, A.M.; Burden, S. A systematic review of the use of ketogenic diets in adult patients with cancer. J. Hum. Nutr. Diet. 2018, 31, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, T.; Zeng, C.; Yang, M.; Li, G.; Han, J.; Wu, W. Association between metabolic syndrome and prognosis of breast cancer: A meta-analysis of follow-up studies. Diabetol. Metab. Syndr. 2020, 12, 10. [Google Scholar] [CrossRef]
- Oh, R.U. Low carbohydrate diet. In Statpearls; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ebbeling, C.B.; Feldman, H.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: Randomized trial. BMJ 2018, 363, k4583. [Google Scholar] [CrossRef]
- Heilmeyer, P.; Heilmeyer, B.; Knyrim, H.; Worm, N. Einfluss kohlenhydratreduzierter Ernährung auf die Hypertonie beim metabolischen Syndrom. AINS 2010, 25, 166–171. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of Dual-Energy X-Ray Absorptiometry Body Composition and Considerations Regarding Analysis and Repeatability of Measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.J.; Berger, J.O.; Johannesson, M.; Nosek, B.A.; Wagenmakers, E.-J.; Berk, R.; Bollen, K.A.; Brembs, B.; Brown, L.; Camerer, C.; et al. Redefine statistical significance. Nat. Hum. Behav. 2018, 2, 6–10. [Google Scholar] [CrossRef]
- Held, L. Reverse-Bayes analysis of two common misinterpretations of significance tests. Clin. Trials 2013, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Aapro, M. From guidelines to clinical practice: A roadmap for oncologists for nutrition therapy for cancer patients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919880084. [Google Scholar] [CrossRef] [PubMed]
- Gjuladin-Hellon, T.; Davies, I.G.; Penson, P.; Baghbadorani, R.A. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Deluche, E.; Leobon, S.; Desport, J.C.; Venat-Bouvet, L.; Usseglio, J.; Tubiana-Mathieu, N. Impact of body composition on outcome in patients with early breast cancer. Support. Care Cancer 2018, 26, 861–868. [Google Scholar] [CrossRef]
- Iwase, T.; Sangai, T.; Fujimoto, H.; Sawabe, Y.; Matsushita, K.; Nagashima, K.; Sato, Y.; Nakagawa, A.; Masuda, T.; Nagashima, T.; et al. Quality and quantity of visceral fat tissue are associated with insulin resistance and survival outcomes after chemotherapy in patients with breast cancer. Breast Cancer Res. Treat. 2019, 179, 435–443. [Google Scholar] [CrossRef]
- Klement, R.J.; Fink, M.K. Dietary and pharmacological modification of the insulin/IGF-1 system: Exploiting the full repertoire against cancer. Oncogenesis 2016, 5, e193. [Google Scholar] [CrossRef]
- Wright, C.; Simone, N.L. Obesity and tumor growth: Inflammation, immunity, and the role of a ketogenic diet. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 294–299. [Google Scholar] [CrossRef]
- Anderson, A.S.; Key, T.J.; Norat, T.; Scoccianti, C.; Cecchini, M.; Berrino, F.; Boutron-Ruault, M.-C.; Espina, C.; Leitzmann, M.F.; Powers, H.; et al. European Code against Cancer 4th Edition: Obesity, body fatness and cancer. Cancer Epidemiol. 2015, 39, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Astley, C.M.; Todd, J.N.; Salem, R.M.; Vedantam, S.; Ebbeling, C.B.; Huang, P.L.; Ludwig, D.S.; Hirschhorn, J.N.; Florez, J.C. Genetic Evidence That Carbohydrate-Stimulated Insulin Secretion Leads to Obesity. Clin. Chem. 2018, 64, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina. 2019, 55, 84. [Google Scholar] [CrossRef]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Alvarez, R.D., III; Huh, W.K.; Bevis, K.S.; Kim, K.H.; Straughn, J.M.; Gower, B. A Ketogenic Diet Reduces Central Obesity and Serum Insulin in Women with Ovarian or Endometrial Cancer. J. Nutr. 2018, 148, 1253–1260. [Google Scholar] [CrossRef]
- Klement, R.J.; Champ, C.E.; Kämmerer, U.; Koebrunner, P.S.; Krage, K.; Schäfer, G.; Weigel, M.; Sweeney, R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: III—final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Klement, R.J.; Koebrunner, P.S.; Krage, K.; Weigel, M.M.; Sweeney, R.A. Short-term effects of a Paleolithic lifestyle intervention in breast cancer patients undergoing radiotherapy: A pilot and feasibility study. Med. Oncol. 2021, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Allen, J.; Jarman, A.; Oyekunle, T.; Armstrong, A.J.; Moul, J.W.; Sandler, H.; Posadas, E.M.; Levin, D.; Wiggins, E.; et al. A randomized controlled trial of a 6-month low carbohydrate intervention on disease progression in men with recurrent prostate cancer: Carbohydrate and prostate study 2 (caps2). Clin. Cancer Res. 2020, 26, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Faivre-Finn, C.; Nackaerts, K.; Jordan, K.; Arends, J.; Douillard, J.; Ricardi, U.; Peters, S. Recommendation for supportive care in patients receiving concurrent chemotherapy and radiotherapy for lung cancer. Ann. Oncol. 2020, 31, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Buckley, J.D. Effects of a low carbohydrate weight loss diet on exercise capacity and tolerance in obese subjects. Obesity 2009, 17, 1916–1923. [Google Scholar] [CrossRef]
- Phinney, S.; Bistrian, B.; Evans, W.; Gervino, E.; Blackburn, G. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Loughney, L.A.; West, M.A.; Kemp, G.J.; Grocott, M.P.; Jack, S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst. Rev. 2018, 12, CD012280. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J. Age Dependency of Oxygen Uptake and Related Parameters in Exercise Testing: An Expert Opinion on Reference Values Suitable for Adults. Lung 2013, 191, 449–458. [Google Scholar] [CrossRef]
- Peters, E.; Schulz, L.M.; Reuss-Borst, M. Quality of life after cancer-How the extent of impairment is influenced by patient characteristics. BMC Cancer 2016, 16, 787. [Google Scholar] [CrossRef]
- Hinz, A.; Singer, S.; Brähler, E. European reference values for the quality of life questionnaire EORTC QLQ-C30: Results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014, 53, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Soleymani, T.; Gower, B.A. Favorable Effects of a Ketogenic Diet on Physical Function, Perceived Energy, and Food Cravings in Women with Ovarian or Endometrial Cancer: A Randomized, Controlled Trial. Nutrients 2018, 10, 1187. [Google Scholar] [CrossRef]
- Klement, R.J.; Weigel, M.M.; Sweeney, R.A. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Seyfried, T.N.; Kalamian, M.; Beheshti, M.; Davoodi, S.H. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: A randomized controlled clinical trial. Nutr. J. 2020, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Banwell, B.; Bergqvist, A.C.; Lehner-Gulotta, D.; Gampper, L.; Leytham, E.; Coleman, R.; Goldman, M.D. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol.-Neuroimmunol. Neuroinflammation 2019, 6, e565. [Google Scholar] [CrossRef] [PubMed]
- Grigolon, R.B.; Gerchman, F.; Schöffel, A.C.; Hawken, E.R.; Gill, H.; Vazquez, G.H.; Mansur, R.B.; McIntyre, R.S.; Brietzke, E. Mental, emotional, and behavioral effects of ketogenic diet for non-epileptic neuropsychiatric conditions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109947. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the atkins, zone, ornish, and learn diets for change in weight and related risk factors among overweight premenopausal women: The a to z weight loss study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef]
- Champ, C.E.; Volek, J.S.; Siglin, J.; Jin, L.; Simone, N.L. Weight Gain, Metabolic Syndrome, and Breast Cancer Recurrence: Are Dietary Recommendations Supported by the Data? Int. J. Breast Cancer 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Kleckner, A.S.; Lin, P.-J.; Gilmore, N.J.; Culakova, E.; VanDerWoude, A.C.; Mustian, K.M.; Fernandez, I.D.; Dunne, R.F.; Deutsch, J.; et al. Excess Body Weight and Cancer-Related Fatigue, Systemic Inflammation, and Serum Lipids in Breast Cancer Survivors. Nutr. Cancer 2020, 1–11. [Google Scholar] [CrossRef]
- Fery, F.; Bourdoux, P.; Christophe, J.; Balasse, E. Hormonal and metabolic changes induced by an isocaloric isoproteinic ketogenic diet in healthy subjects. Diabete Metab. 1982, 8, 299–305. [Google Scholar]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Van Der Louw, E.J.; Reddingius, R.E.; Olieman, J.F.; Neuteboom, R.F.; Catsman-Berrevoets, C.E. Ketogenic diet treatment in recurrent diffuse intrinsic pontine glioma in children: A safety and feasibility study. Pediatr. Blood Cancer 2018, 66, e27561. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Akbari, M.E.; Mirzaei, H.R.; Mehrad-Majd, H.; Kalamian, M.; Davoodi, S.H. Feasibility, Safety, and Beneficial Effects of MCT-Based Ketogenic Diet for Breast Cancer Treatment: A Randomized Controlled Trial Study. Nutr. Cancer 2019, 72, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Prins, P.J.; Noakes, T.D.; Welton, G.L.; Haley, S.J.; Esbenshade, N.J.; Atwell, A.D.; Scott, K.E.; Abraham, J.; Raabe, A.S.; Buxton, J.D.; et al. High Rates of Fat Oxidation Induced by a Low-Carbohydrate, High-Fat Diet, Do Not Impair 5-km Running Performance in Competitive Recreational Athletes. J. Sports Sci. Med. 2019, 18, 738–750. [Google Scholar] [PubMed]
- Kaspar, M.B.; Austin, K.; Huecker, M.; Sarav, M. Ketogenic Diet: From the Historical Records to Use in Elite Athletes. Curr. Nutr. Rep. 2019, 8, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass Without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; LaFountain, R.A.; Volek, J.S.; Doyle, L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 2018, 81, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Frobel, T.; Albers, T.; Fikenzer, S.; Prinzhausen, J.; Kämmerer, U. A pilot case study on the impact of a self-prescribed ketogenic diet on biochemical parameters and running performance in healthy and physically active individuals. Nutr. Med. 2013, 1, 1–27. [Google Scholar]
- Phinney, S.D.; Horton, E.S.; Sims, E.A.H.; Hanson, J.S.; Danforth, E.; Lagrange, B.M. Capacity for Moderate Exercise in Obese Subjects after Adaptation to a Hypocaloric, Ketogenic Diet. J. Clin. Investig. 1980, 66, 1152–1161. [Google Scholar] [CrossRef]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J. Int. Soc. Sports Nutr. 2017, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, U.K.; Sütterlin, M.; Reuss-Borst, M. Low carb (lcd) and ketogenic (kd) diets increase quality of life, physical performance, body composition and metabolic health of women with breast cancer better than a standard diet (sd). Res. Sq. 2020. Preprint. [Google Scholar]
| Parameter | Ketogenic Diet KD (n = 29) | Low Carb Diet LCD (n = 92) | Standard Diet SD (n = 31) | p-Value |
|---|---|---|---|---|
| Age (year) | 53 (38–64) | 52 (26–66) | 53 (37–60) | n.s. |
| Karnofsky index | 100 (90–100) | 100 (80–100) | 100 (80–100) | n.s. |
| -Body composition | ||||
| Body mass index (kg/m2) | 23.4 (18.1–35.4) | 27.2 (18.0–41.0) | 26.6 (17.6–40.2) | 0.0010 * |
| Fat mass (kg) | 20.1 (10.3–41.5) | 27.8 (6.7–55) | 23.4 (9.3–40.7) | 0.0006 * |
| Visceral fat mass (kg) | 8.2 (4.0–24.0) | 13.6 (2.9–28.4) | 13.1 (4.2–23.4) | 0.0006 * |
| Skeletal muscle mass (kg) | 41.7 (35–49.4) | 42.2 (32.5–56.7) | 38.8 (29.9–54) | 0.365 |
| Phase angle (°) | 5.68 (4.36–6.94) | 5.55 (4.43–6.61) | 5.8 (4.51–6.53) | 0.25 |
| Menopause | n.s. | |||
| Premenopause | 5 (17.2%) | 22 (23.9%) | 10 (32.3%) | |
| Postmenopause | 14 (48.3%) | 54 (58.7%) | 16 (51.6%) | |
| Unknown | 10 (34.5%) | 16 (17.4&) | 5 (16.1%) | |
| Neoadjuvant chemotherapy | n.s. | |||
| No | 20 (69.0%) | 75 (81.5%) | 26 (83.9%) | |
| Yes | 9 (31.0%) | 17 (18.5%) | 5 (16.1%) | |
| Metastases | 0.0031 * | |||
| No | 19 65.5%) | 86 (93.4%) | 27 (87.1%) | |
| Yes | 6 (20.7%) | 3 (3.3%) | 2 (6.45%) | |
| Unknown | 4 (13.8%) | 3 (3.3%) | 2 (6.45%) | |
| Estrogen receptor status | n.s. | |||
| Negative | 8 (27.6%) | 14 (15.2%) | 3 (9.7%) | |
| Positive | 20 (69.0%) | 78 (84.8%) | 28 (90.3%) | |
| Unknown | 1 (3.4%) | 0 | 0 | |
| Progesterone receptor status | n.s. | |||
| Negative | 9 (31.0%) | 15 (16.3%) | 4 (12.9%) | |
| Positive | 19 (65.5%) | 77 (83.7%) | 27 (87.1%) | |
| Unknown | 1 (3.4%) | 0 | 0 | |
| HER2/neu status | n.s. | |||
| Negative | 23 (79.3%) | 77 (83.7%) | 23 (74.2%) | |
| Positive | 6 (20.7%) | 15 (16.3%) | 8 (15.8%) | |
| Anti-Hormone Therapy | n.s. | |||
| Tamoxifen | 10 (34.5%) | 54 (58.7%) | 19 (61.3%) | |
| Aromatase Inhibitor | 6 (20.7%) | 23 (25.0%) | 6 (19.35%) | |
| None | 13 (44.8%) | 15 (16.3%) | 6 (19.35%) | |
| Herceptin | n.s. | |||
| Yes | 5 (17.2%) | 13 (14.1%) | 6 (19.4%) | |
| No | 24 (82.8%) | 79 (85.9%) | 25 (80.6%) |
| KD (n = 19) | LCD (n = 70) | SD (n = 23) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter and Unit | T0 | T20 | p-Value | T0 | T20 | p-Value | T0 | T20 | p-Value |
| RQ | 0.82 (0.63–0.91) | 0.75 (0.65–0.80) | n.s. | 0.83 (0.70–1.07) | 0.85 (0.68–1.06) | n.s. | 0.90 (0.74–1.01) | 0.90 (0.77–1.07) | n.s. |
| VO2/kg(max) (mL/(kg × min)) | 22.4 (13.7–36.0) | 27.9 (16.7–38.3) | n.s. | 20.0 (8.4–34.6) | 22.2 (13.0–36.8) | 0.0003 * | 19.8 (11.6–28.9) | 22.5 (10.2–38.2) | 0.0013 * |
| Threshold power (Watt) | 117 (75–178) | 121 (87–185) | n.s. | 111 (50–175) | 113 (65–161) | n.s. | 93 (50–148) | 90 (49–160) | n.s. |
| Maximum power (Watt) | 140 (75–218) | 139 (105–209) | n.s. | 126 (68–178) | 130 (70–186) | n.s. | 121 (65–166) | 128 (72–170) | n.s. |
| TTE (min) | 8.33 (4.17–13.7) | 8.17 (5.83–13.0) | n.s. | 7.59 (3.67–11.0) | 7.67 (3.67–11.5) | n.s. | 7.17 (3.0–10.2) | 7.67 (3.83–10.3) | n.s. |
| Lactate (mmol/L) | 3.77 (1.66–6.04) | 4.47 (2.20–8.04) | n.s. | 3.94 (0.58–6.30) | 4.03 (0.91–8.90) | n.s. | 3.55 (1.60–5.09) | 4.28 (1.88–6.34) | n.s. |
| KD | LCD | SD | T0 Intergroup | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T20 | p-Value | T0 | T20 | p-Value | T0 | T20 | p-Value | p-Value | |
| Weight (kg) | 65.2 (49.8–94) | 62.9 (49.1–86.1) | 0.009 | 74.1 (48.9–116.2) | 69.2 (46.4–115.1) | <0.0001 * | 68.6 (42.8–97.9) | 65.8 (43–93) | 0.012 | n.s. |
| BMI (kg/m2) | 23.4 (18.1–35.4) | 22.1 (17.8–32.4) | 0.009 | 27.2 (18.0–41) | 25.1 (17.5–42) | <0.0001 * | 26.6 (17.6–40.2) | 25 (17.7–36.8) | 0.0098 | 0.001 * |
| FM (kg) | 20.1 (10.3–41.5) | 17.5 (11.3–36.8) | n.s. | 27.8 (6.7–55) | 24.2 (8.4–41.6) | <0.0001 * | 26.9 (10.4–40) | 23.4 (9.3–40.7) | 0.017 | 0.0006 * |
| Visceral FM (kg) | 8.2 (4.0–24.0) | 6.9 (4.1–20.2) | n.s. | 13.6 (2.9–28.4) | 11.3 (3.6–31.0) | <0.0001 * | 13.1 (4.2–23.4) | 11.7 (4.1–22.8) | 0.031 | 0.0006 * |
| SMM (kg) | 41.7 (35–49.4) | 40.8 (34.2–47.8) | n.s. | 42.2 (32.5–56.7) | 41.3 (33.3–58.8) | 0.0011 * | 38.8 (29.9–54) | 38.5 (31.2–54.6) | 0.200 | n.s. |
| SMM/FM | 2.0 (1.2–4.3) | 2.4 (1.3–3.7) | n.s. | 1.5 (0.9–6.4) | 1.6 (1.0–5.2) | <0.0001 * | 1.6 (1.1–2.9) | 1.7 (1.1–3.4) | 0.037 | <0.0001 * |
| BIA (BCM) (kg) | 23.1 (18.1–26.2) | 21.7 (18.7–26.8) | n.s. | 22.7 (16.0–31.2) | 22.4 (16.0–31.2) | <0.0001 * | 22.7 (15.6–30.3) | 21.8 (15.6–30.5) | 0.217 | n.s. |
| BIA PA (°) | 5.68 (4.36–6.94) | 5.62 (4.61–6.91) | n.s. | 5.55 (4.43–6.61) | 5.59 (4.36–6.73) | n.s. | 5.8 (4.51–6.56) | 5.7 (4.61–6.53) | 0.726 | n.s. |
| BMD (g/cm2) | 1.10 (0.88–1.43) | 1.10 (0.87–1.28) | n.s. | 1.07 (0.88–1.49) | 1.06 (0.87–1.52) | n.s. | 1.04 (0.84–1.31) | 1.05 (0.92–1.26) | 0.361 | n.s. |
| KD (n = 20) | LCD (n = 75) | SD (n = 24) | T0 Inter-Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T20 | p-Value | T0 | T20 | p-Value | T0 | T20 | p-Value | p-Value | |
| Glob. health/QoL | 66.7 (25–92) | 75 (16.7–100) | 0.004 * | 50 (0–83,3) | 66.7 (16.7–100) | <0.0001 * | 54.2 (16.7–83.3) | 66.7 (25–91.7) | 0.008 | n.s. |
| Physical funct. | 80 (40–100) | 93.3 (20–100) | n.s. | 73.3 (20–100) | 80 (46.7–100) | <0.0001 * | 73.3 (40–100) | 83.4 (46.7–100) | 0.002 * | 0.0005 * |
| Emotional funct. | 58.3 (0–100) | 79.2 (0–100) | 0.006 | 50 (0–100) | 66.7 (0–100) | <0.0001 * | 50 (0–100) | 58.3 (0–100) | n.s. | n.s. |
| Cognitive funct. | 66.7 (0–100) | 75 (16.7–100) | n.s. | 66.7 (0–100) | 66.7 (0–100) | n.s. | 58.4 (0–100) | 66.7 (16.7–100) | n.s. | n.s. |
| Social funct. | 66.7 (0–100) | 66.7 (0–100) | n.s. | 66.7 (0–100) | 66.7 (0–100) | 0.002 * | 66.7 (0–100) | 66.7 (33.3–100) | 0.002 * | n.s. |
| Role funct. | 66.7 (0–100) | 75 (0–100) | n.s. | 50 (0–100) | 66.7 (0–100) | <0.0001 * | 66.7 (0–100) | 66.7 (16.7–100) | n.s. | n.s. |
| Fatigue | 33.3 (0–100) | 11.1 (0–88.9) | n.s. | 66.7 (0–100) | 33.3 (0–100) | <0.0001 * | 55.6 (0–100) | 50 (0–88.9) | n.s. | <0.0001 * |
| Pain | 33.3 (0–100) | 25 (0–100) | n.s. | 66.7 (0–100) | 33.3 (0–100) | 0.0001 * | 50 (0–100) | 33.3 (0–83.3) | n.s. | n.s. |
| Dyspnea | 33.3 (0–100) | 33.3 (0–100) | n.s. | 66.7 (0–100) | 33.3 (0–100) | <0.0001 * | 33.3 (0–100) | 33.3 (0–66.7) | n.s. | n.s. |
| Insomnia | 66.7 (0–100) | 33.3 (0–100) | 0.010 | 66.7 (0–100) | 66.7 (0–100) | n.s. | 66.7 (0–100) | 33.3 (0–100) | n.s. | 0.005 |
| Nausea/ Vomiting | 0 (0–100) | 0 (0–50) | n.s. | 0 (0–50) | 0 (0–50) | n.s. | 0 (0–83.3) | 0 (0–66.7) | n.s. | n.s. |
| Appetite loss | 0 (0–100) | 0 (0–66.7) | n.s. | 0 (0–66.7) | 0 (0–66.7) | n.s. | 0 (0–100) | 0 (0–66.7) | n.s. | n.s. |
| Consti- pation | 0 (0–100) | 0 (0–66.7) | n.s. | 0 (0–100) | 0 (0–100) | n.s. | 0 (0–66.7) | 0 (0–66.7) | n.s. | n.s. |
| Diarrhea | 0 (0–100) | 0 (0–66.7) | n.s. | 0 (0–100) | 0 (0–100) | n.s. | 0 (0–66.7) | 0 (0–66.7) | n.s. | n.s. |
| KD | LCD | SD | Intra-Group Diff. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter and Dimension | T0 | T20 | p-Value | T0 | T20 | p-Value | T0 | T20 | p-Value | T0 p-Value |
| TG (mg/dL) | 70 (45–177) | 78 (42–184) | n.s. | 104 (42–489) | 84 (43–330) | <0.0001 * | 91 (48–265) | 85 (50–261) | n.s. | <0.0001 * |
| Chol (mg/dL) | 243 (167–352) | 242 (142–357) | n.s. | 224 (157–373) | 219 (127–338) | <0.0001 * | 213 (152–328) | 207 (149–256) | n.s. | n.s. |
| HDL (mg/dL) | 76 (39–129) | 74 (39–121) | n.s. | 65 (37–103) | 67 (39–104) | 0.008 | 66 (33–94) | 64 (44–94) | n.s. | 0.0003 * |
| LDL (mg/dL) | 141 (73–226) | 157 (67–205) | n.s. | 145 (87–266) | 140 (76–234) | <0.0001 * | 137 (93–234) | 122 (88–174) | n.s. | n.s. |
| LDL/HDL | 1.7 (0.6–4.6) | 1.8 (0.7–4.6) | n.s. | 2.3 (1.0–5.0) | 2.0 (0.9–3.7) | <0.0001 * | 2.1 (1.1–3.7) | 1.9 (1.0–3.4) | n.s. | 0.002 * |
| TG/HDL | 0.9 (0.4–4.5) | 0.9 (0.4–3.5) | n.s. | 1.7 (0.4–9.8) | 1.3 (0.4–8.1) | <0.0001 * | 1.4 (0.6–6.1) | 1.3 (0.6–5.2) | n.s. | <0.0001 * |
| Glucose (mg/dL) | 85 (67–114) | 86 (64–104) | n.s. | 89 (72–161) | 87 (74–132) | 0.004 * | 84 (68–100) | 86 (74–109) | n.s. | n.s. |
| Crea (mg/dL) | 0.81 (0.59–1.1) | 0.80 (0.6–1.0) | n.s. | 0.76 (0.51–1.1) | 0.72 (0.5–1.0) | 0.0002 * | 0.77 (0.56–1.2) | 0.80 (0.51–1.1) | n.s. | n.s. |
| GFR (mL/min) | 76.6 (55.4–110) | 85.1 (64–106) | n.s. | 91.2 (53.6–125) | 95.6 (60–113) | 0.0002 * | 90.9 (49.4–115) | 84.8 (56.7–122) | n.s. | n.s. |
| Uric acid (mg/dL) | 4.8 (2.7–7.6) | 4.8 (3.6–6.6) | n.s. | 5.2 (2.8–8.8) | 4.9 (2.8–8.0) | 0.008 | 5.1 (3.4–8.7) | 4.9 (3.2–9.2) | n.s. | n.s. |
| BUN (mg/dL) | 30 (24–47) | 32 (25–46) | n.s. | 28 (16–47) | 31 (19–61) | <0.0001 * | 29 (13–41) | 31 (18–40) | n.s. | n.s. |
| AP (U/L) | 62 (26–163) | 63 (29–111) | n.s. | 66 (26–120) | 62 (32–156) | 0.003 * | 55 (24–125) | 64 (24–123) | n.s. | n.s. |
| AST (U/L) | 20 (10–126) | 21 (10–85) | n.s. | 23 (9–160) | 22 (12–96) | 0.0049 * | 22 (10–64) | 22 (13–60) | n.s. | n.s. |
| CRP (mg/L) | 0.53 (0.06–6.7) | 0.65 (0.2–10.3) | n.s. | 1.7 (0.02–10.3) | 1.0 (0.1–10.4) | 0.007 | 2.0 (0.03–12.2) | 1.2 (0.2–7.7) | n.s. | n.s. |
| TSH (mU/L) | 1.3 (0.4–3.7) | 1.4 (0.4–5.2) | n.s. | 1.4 (0.1–8.9) | 1.5 (0.06–4.5) | n.s. | 0.8 (0.05–3.6) | 0.9 (0.3–3.7) | n.s. | 0.004 * |
| Insulin (µU/L) | 10.2 (5–33.3) | 9.6 (6.3–31.6) | n.s. | 14.6 (4.0–45.1) | 13.0 (5–45.) | 0.001 * | 13.9 (7.3–60.4) | 12.8 (5.9–41.6) | n.s. | n.s. |
| IGF-1 | 9.1 (4.1–50) | 9.0 (2.7–48) | n.s. | 7.0 (1.5–19.4) | 6.5 (1.2–18) | n.s. | 5.7 (1.7–22.4) | 6.2 (2.3–21.9) | n.s. | n.s. |
| HOMA-IR | 2.1 (0.8–7.6) | 2.0 (1.3–6.8) | n.s. | 3.3 (0.8–10.5) | 2.7 (1.0–10.2) | <0.0001 * | 2.7 (1.5–14.2) | 2.8 (1.1–9.1) | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kämmerer, U.; Klement, R.J.; Joos, F.T.; Sütterlin, M.; Reuss-Borst, M. Low Carb and Ketogenic Diets Increase Quality of Life, Physical Performance, Body Composition, and Metabolic Health of Women with Breast Cancer. Nutrients 2021, 13, 1029. https://doi.org/10.3390/nu13031029
Kämmerer U, Klement RJ, Joos FT, Sütterlin M, Reuss-Borst M. Low Carb and Ketogenic Diets Increase Quality of Life, Physical Performance, Body Composition, and Metabolic Health of Women with Breast Cancer. Nutrients. 2021; 13(3):1029. https://doi.org/10.3390/nu13031029
Chicago/Turabian StyleKämmerer, Ulrike, Rainer J. Klement, Fabian T. Joos, Marc Sütterlin, and Monika Reuss-Borst. 2021. "Low Carb and Ketogenic Diets Increase Quality of Life, Physical Performance, Body Composition, and Metabolic Health of Women with Breast Cancer" Nutrients 13, no. 3: 1029. https://doi.org/10.3390/nu13031029
APA StyleKämmerer, U., Klement, R. J., Joos, F. T., Sütterlin, M., & Reuss-Borst, M. (2021). Low Carb and Ketogenic Diets Increase Quality of Life, Physical Performance, Body Composition, and Metabolic Health of Women with Breast Cancer. Nutrients, 13(3), 1029. https://doi.org/10.3390/nu13031029








