White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Study Quality
2.3. Statistical Analysis
2.4. Subgroup Analyses and Meta-Regression Analyses
3. Results
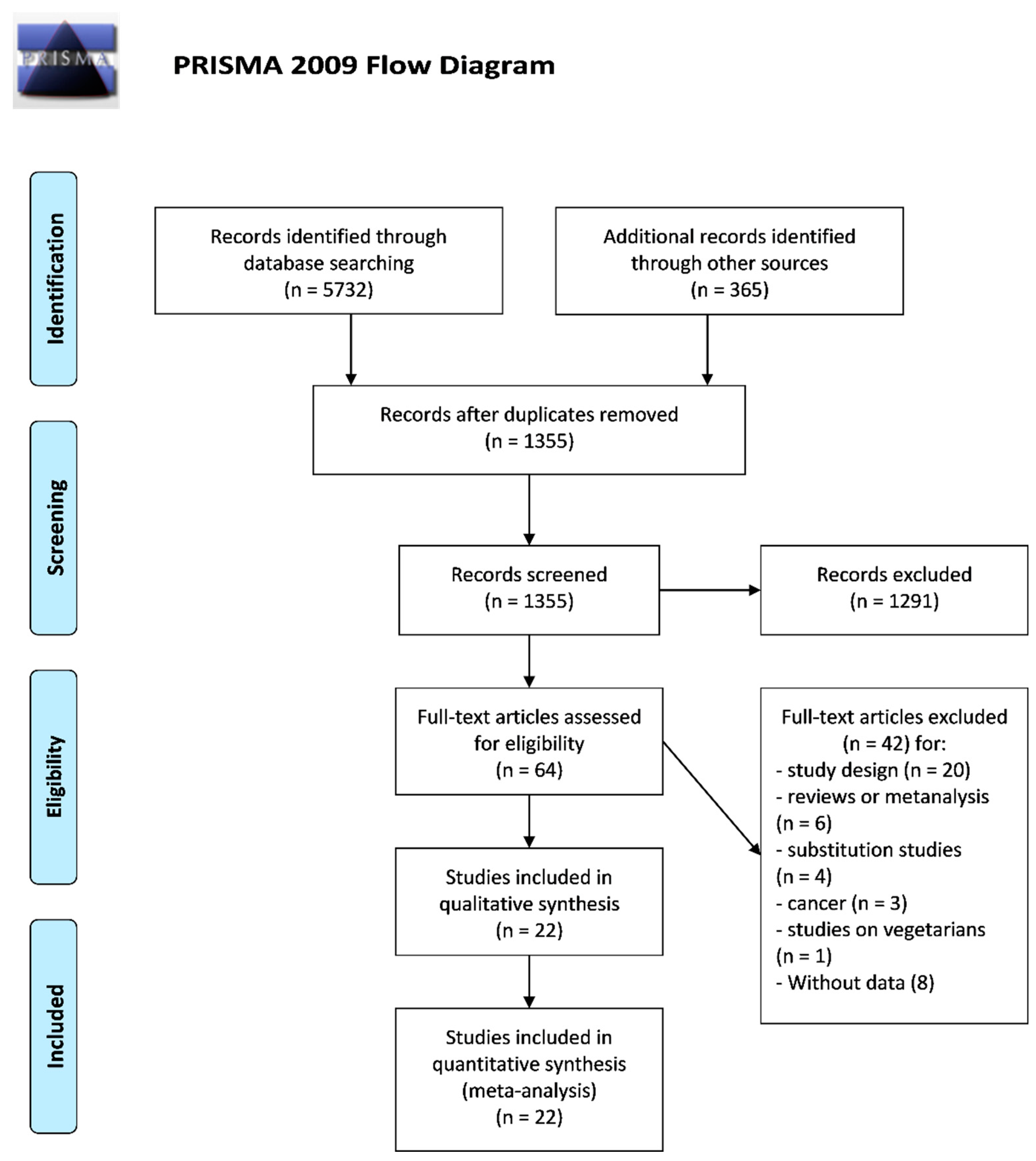
3.1. Study Selection and Main Characteristics
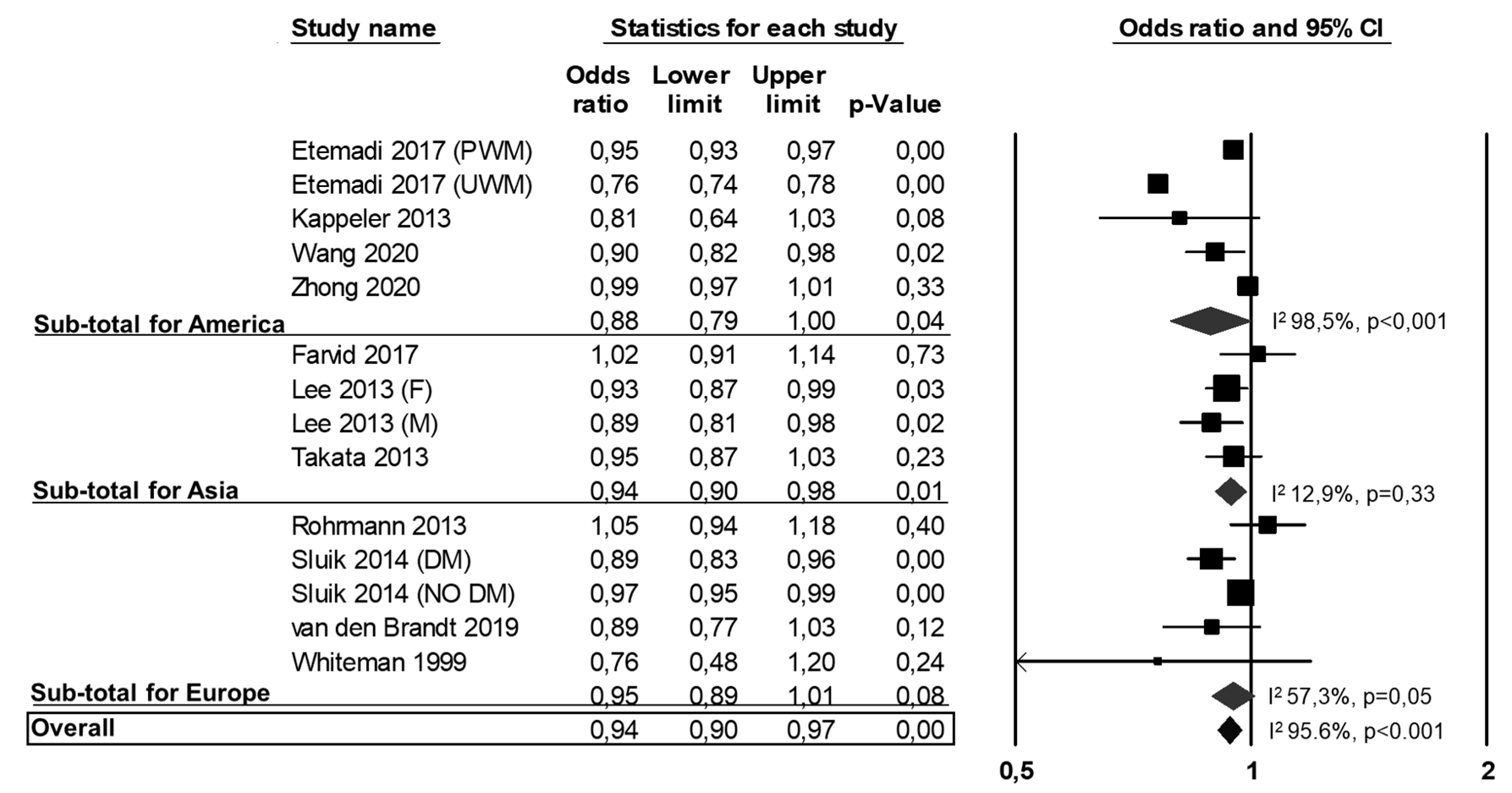
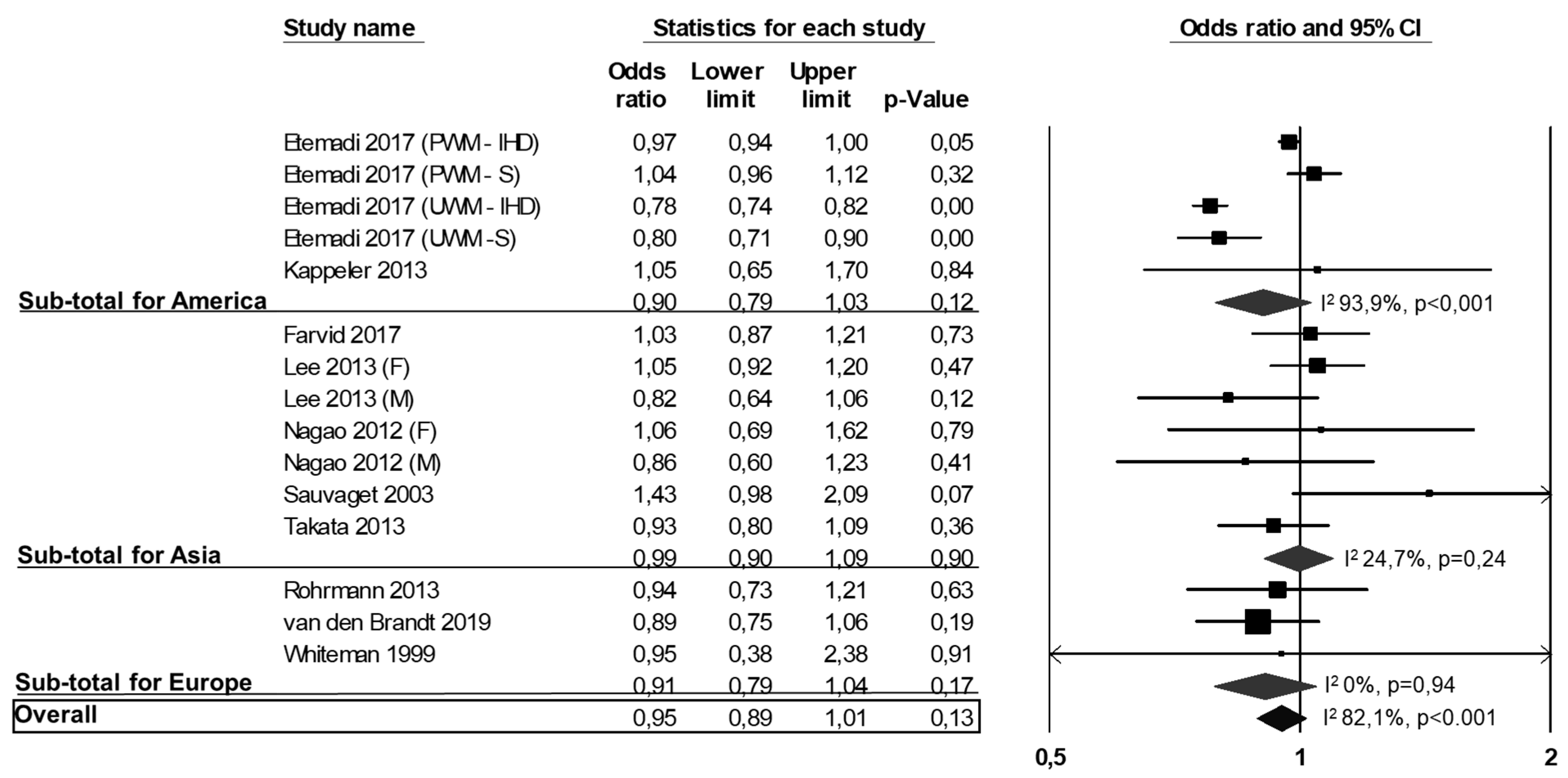
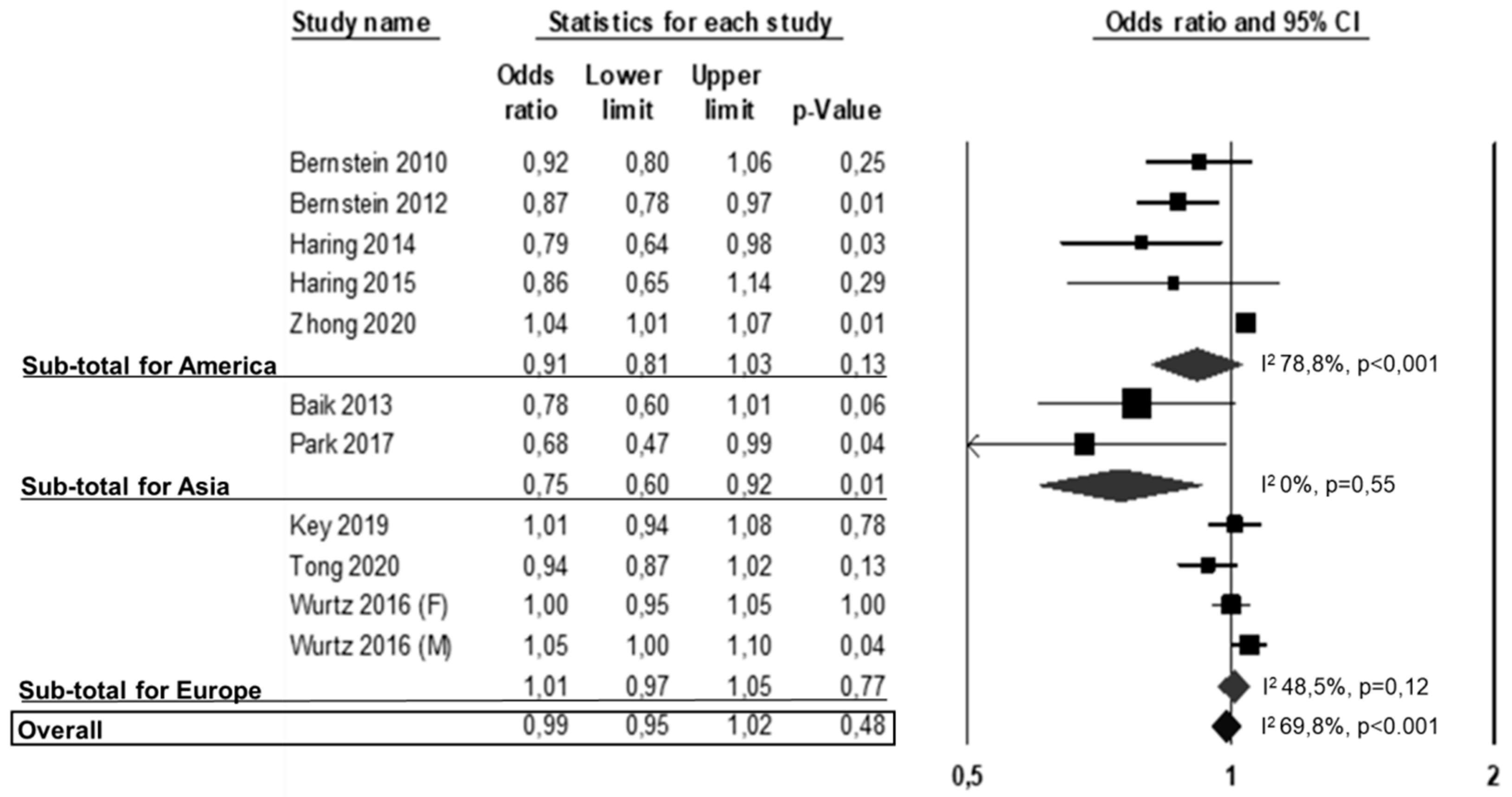
3.2. All-Cause Mortality, CVD Mortality and CVD Events
3.3. Publication Bias and Meta-Regressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Meier, T.; Gräfe, K.; Senn, F.; Sur, P.; Stangl, G.I.; Dawczynski, C.; März, W.; Kleber, M.E.; Lorkowski, S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: A systematic analysis of the Global Burden of Disease Study. Eur. J. Epidemiol. 2019, 34, 37–55. [Google Scholar] [CrossRef]
- Calabrese, I.; Riccardi, G. Effectiveness of changes in diet composition on reducing the incidence of cardiovascular disease. Curr. Cardiol. Rep. 2019, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, G.; Vaccaro, O.; Costabile, G.; Rivellese, A.A. How well can we control dyslipidemias through lifestyle modifications? Curr. Cardiol. Rep. 2016, 18, 66. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kühn, T.; Steur, M.; Weiderpass, E.; Wennberg, M.; et al. Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and me-ta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Alexander, L.W.G.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.T.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.-T.; Tsuji, I.; et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospective cohort studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat intake and mortality. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose–response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N. Red meat and processed meat consumption and all-cause mortality: A meta-analysis. Am. J. Epidemiol. 2014, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Han, M.A.; Guyatt, G.H.; Vernooij, R.W.; El Dib, R.; Cheung, K.; Milio, K.; Zworth, M.; Bartoszko, J.J.; Valli, C.; et al. Red and processed meat consumption and risk for all-cause mortality and cardiometabolic outcomes. Ann. Intern. Med. 2019, 171, 703. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; De Munain, A.L.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Ursin, G.; Veierød, M.B. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia 2009, 52, 2277–2287. [Google Scholar] [CrossRef]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—An updated review of the evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Michas, G.; Lajous, M.; Mozaffarian, D. Processing of meats and cardiovascular risk: Time to focus on preservatives. BMC Med. 2013, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Babio, N.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Serra-Majem, L.; Salaverria, I.; Lamuela-Raventós, R.M.; et al. Replacing red meat and processed red meat for white meat, fish, legumes or eggs is associated with lower risk of incidence of metabolic syndrome. Clin. Nutr. 2016, 35, 1442–1449. [Google Scholar] [CrossRef]
- Hu, F.B. Protein, body weight, and cardiovascular health. Am. J. Clin. Nutr. 2005, 82, 242S–247S. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Sinha, R.; Ward, M.H.; Graubard, B.I.; Inoue-Choi, M.; Dawsey, S.M.; Abnet, C.C. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: Population based cohort study. BMJ 2017, 357, j1957. [Google Scholar] [CrossRef]
- Sluik, D.; Boeing, H.; Li, K.; Kaaks, R.; Johnsen, N.F.; Tjønneland, A.; Arriola, L.; Barricarte, A.; Masala, G.; Grioni, S.; et al. Lifestyle factors and mortality risk in individuals with diabetes mellitus: Are the associations different from those in individuals without diabetes? Diabetologia 2013, 57, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Son, J.; Jang, J.; Kang, R.; Chung, H.-K.; Lee, K.W.; Lee, S.-M.; Lim, H.; Shin, M.-J. Unprocessed meat consumption and incident cardiovascular diseases in Korean adults: The Korean Genome and Epidemiology study (KoGES). Nutrients 2017, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, C.; Nagano, J.; Allen, N.; Grant, E.J.; Beral, V. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span study. Int. J. Epidemiol. 2003, 32, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; Katzke, V.; Kühn, T.; Boeing, H.; et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: A prospective study of 418,329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobs, E.J.; Shah, R.A.; Stevens, V.L.; Gansler, T.; McCullough, M.L. Red and processed meat, poultry, fish, and egg intakes and cause-specific and all-cause mortality among men with nonmetastatic prostate cancer in a U.S. cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.; Cho, N.H.; Kim, S.H.; Shin, C. Dietary information improves cardiovascular disease risk prediction models. Eur. J. Clin. Nutr. 2012, 67, 25–30. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada, 2011. University of Ottawa, Canada. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 19 February 2021).
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of fried food consumption with all cause, cardiovascular, and cancer mortality: Prospective cohort study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.A.; Iso, H.; Yamagishi, K.; Date, C.; Tamakoshi, A. Meat consumption in relation to mortality from cardiovascular disease among Japanese men and women. Eur. J. Clin. Nutr. 2012, 66, 687–693. [Google Scholar] [CrossRef]
- Würtz, A.M.L.; Hansen, M.D.; Tjønneland, A.; Rimm, E.B.; Schmidt, E.B.; Overvad, K.; Jakobsen, M.U. Substitution of meat and fish with vegetables or potatoes and risk of myocardial infarction. Br. J. Nutr. 2016, 116, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Gronroos, N.; Nettleton, J.A.; Von Ballmoos, M.C.W.; Selvin, E.; Alonso, A. Dietary protein intake and coronary heart disease in a large community based cohort: Results from the Atherosclerosis Risk in Communities (ARIC) study. PLoS ONE 2014, 9, e109552. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Misialek, J.R.; Rebholz, C.M.; Petruski-Ivleva, N.; Gottesman, R.F.; Mosley, T.H.; Alonso, A. Association of dietary protein consumption with incident silent cerebral infarcts and stroke. Stroke 2015, 46, 3443–3450. [Google Scholar] [CrossRef]
- Kappeler, R.; Eichholzer, M.; Rohrmann, S. Meat consumption and diet quality and mortality in NHANES III. Eur. J. Clin. Nutr. 2013, 67, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Overvad, K.; Bueno-De-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjønneland, A.; Nailler, L.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat consumption and mortality—Results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Shu, X.-O.; Gao, Y.-T.; Li, H.; Zhang, X.; Gao, J.; Cai, H.; Yang, G.; Xiang, Y.-B.; Zheng, W. Red meat and poultry intakes and risk of total and cause-specific mortality: Results from cohort studies of Chinese adults in Shanghai. PLoS ONE 2013, 8, e56963. [Google Scholar] [CrossRef] [PubMed]
- Van den Brandt, P.A. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur. J. Epidemiol. 2019, 34, 351–369. [Google Scholar] [CrossRef]
- Whiteman, D.; Muir, J.; Jones, L.; Murphy, M.; Key, T. Dietary questions as determinants of mortality: The OXCHECK experience. Public Health Nutr. 1999, 2, 477–487. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Pan, A.; Rexrode, K.M.; Stampfer, M.; Hu, F.B.; Mozaffarian, D.; Willett, W.C. Dietary protein sources and the risk of stroke in men and women. Stroke 2012, 43, 637–644. [Google Scholar] [CrossRef]
- Farvid, M.S.; Malekshah, A.F.; Pourshams, A.; Poustchi, H.; Sepanlou, S.G.; Sharafkhah, M.; Khoshnia, M.; Farvid, M.; Abnet, C.C.; Kamangar, F.; et al. Dietary protein sources and all-cause and cause-specific mortality: The Golestan Cohort study in Iran. Am. J. Prev. Med. 2017, 52, 237–248. [Google Scholar] [CrossRef]
- Durante, A.; Bronzato, S. A contemporary review of the relationship between red meat consumption and cardiovascular risk. Int. J. Prev. Med. 2017, 8, 40. [Google Scholar] [CrossRef]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary intake of heme iron and risk of cardiovascular disease: A dose–response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35. [Google Scholar] [CrossRef]
- Han, M.; Guan, L.; Ren, Y.; Zhao, Y.; Liu, D.; Zhang, D.; Liu, L.; Liu, F.; Chen, X.; Cheng, C.; et al. Dietary iron intake and risk of death due to cardiovascular diseases: A systematic review and dose-response meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr 2020, 29, 309–321. [Google Scholar] [PubMed]
- Rosi, A.; Mena, P.; Pellegrini, N.; Turroni, S.; Neviani, E.; Ferrocino, I.; Di Cagno, R.; Ruini, L.; Ciati, R.; Angelino, D.; et al. Environmental impact of omnivorous, ovo-lacto-vegetarian, and vegan diet. Sci. Rep. 2017, 7, 6105. [Google Scholar] [CrossRef] [PubMed]
- Carlsson-Kanyama, A.; González, A.D. Potential contributions of food consumption patterns to climate change. Am. J. Clin. Nutr. 2009, 89, 1704S–1709S. [Google Scholar] [CrossRef] [PubMed]
| Author, Publication Year, Location | Participants | Dietary Intake Assessment Method | Total Cases | Highest vs. Lowest Intake | Outcome | HR for the Highest vs. Lowest Intake | Adjusted Variables |
|---|---|---|---|---|---|---|---|
| Baik, 2013, Asia | n 9026 (M 4694, F 4332) Age 52 years Follow-up 8 years * | FFQ (103 items) | CVD: 352 | Predefined categories ≥ 1 serving/week vs. 0 | Incident CVD | 0.78 (95% CI 0.60, 1.01) | sex, age, systolic blood pressure, antihypertensive treatment, total cholesterol in serum, HDL cholesterol in serum, smoking status, diabetes mellitus, BMI, legumes intake, carbonated soft drink intake and green tea intake |
| Bernstein, 2010, America | n 84136 (F) Age 58 years Follow-up 26 years | FFQ | IHD: 3162 | 0.56 serving/day vs. 0.07 serving/day 1 | Incident IHD | 0.92 (95% CI 0.8, 1.06) | age, time period, total energy, cereal fiber, alcohol, trans fat, BMI, cigarette smoking, menopausal status, parental history of early myocardial infarction, multivitamin use, vitamin E supplement use, aspirin use at least once per week, physical exercise |
| Bernstein, 2012, America | n 127160 (M 43150, F 84010) Age 59 years Follow-up 24.6 years | FFQ (61–131 items) | Stroke: 4030 Ischemic stroke: 2212 Hemorrhagic stroke: 693 | 0.72 serving/day vs. 0.14 serving/day (M) 2 0.54 serving/day vs. 0.14 serving/day (F) 3 | Incident Stroke Incident Ischemic stroke Incident Hemorrhagic stroke | 0.87 (95% CI 0.78, 0.97) 0.89 (95% CI 0.76, 1.03) 0.74 (95% CI 0.52, 1.06) | BMI, cigarette smoking, physical exercise, parental history of early myocardial infartcion (<60 y), menopausal status (only women), multivitamine use, vitamin E supplement use, aspirin use at least once per wk, total energy intake, cereal fiber, alcohol, transfat, fruit and vegetables and other protein sources |
| Etemadi, 2017, America | n 536 969 (M 316505, F 220464) Age 62 years Follow-up 15.6 years * | FFQ (124 items) | All-cause mortality: 128524 IHD mortality: 34723 Stroke mortality: 5837 | All-cause mortality IHD mortality Stroke mortality | 0.95 (95% CI 0.93, 0.96) (PWM) 0.76 (95% CI 0.74, 0.78) (UWM) 0.97 (95% CI 0.94, 1.00) (PWM) 0.78 (95% CI 0.74, 0.81) (UWM) 1.04 (95% CI 0.96, 1.12) (PWM) 0.8 (95% CI 0.71, 0.89) (UWM) | sex, age, marital status, ethnicity, education, fifths of composite deprivation index, perceived health at baseline, history of heart disease, stroke, diabetes, cancer, smoking status, BMI, vigorous physical activity, usual activity throughout day, alcohol consumption, fruit and vegetable intakes, total energy intake and total meat intake | |
| Farvid, 2017, Asia | n 42 403 (M 18318, F 24085) Age 51.6 years Follow-up 8.1 years * | FFQ (116 items) | All-cause mortality: 3291 CVD mortality: 1467 IHD mortality: 764 Stroke mortality: 507 | 1.33 serving/day vs. 0.11 serving/day 4 | All-cause mortality CVD mortality IHD mortality Stroke mortality | 1.02 (95% CI 0.91, 1.14) 1.03 (95% CI 0.87, 1.21) 0.97 (95% CI 0.77, 1.22) 1.06 (95% CI 0.8, 1.39) | age, ethnicity, education, marital status, residency, smoking status, opium use, alcohol, BMI, systolic blood pressure, occupational physical activity, family history of cancer, wealth score, medication and energy intake |
| Haring, 2014, America | n 12 066 (M 5333, F 6733) Age 53.8 years Follow-up 22 years * | FFQ (66 items) | IHD: 1147 | 0.8 serving/day vs. 0.1 serving/day | Incident IHD | 0.79 (95% CI 0.64, 0.98) | age, sex, race, study center, total energy intake, smoking, education, systolic blood pressure, use of antihypertensive medicatione, HDLcholesterol, total cholesterol, use of lipid lowering medication, BMI, waist to hip ratio, alcohol intake, sports related physical activity, leisure related physical activity, CHO intake, fiber intake and magnesium intake |
| Haring, 2015, America | n 11 601 (M 5116, F 6485) Age 53.8 years Follow-up 22.7 years * | FFQ (66 items) | Stroke: 699 Ischemic stroke: 598 Hemorrhagic stroke: 114 | 0.8 serving/day vs. 0.07 serving/day | Incident Stroke Incident Ischemic stroke Incident Hemorrhagic stroke | 0.86 (95% CI 0.65, 1.14) 0.94 (95% CI 0.7, 1.27) 0.56 (95% CI 0.26, 1.2) | age, sex, race, study center, total energy intake, cigarette years, education, systolic blood pressure, use of antihypertensive medicatione, HDLcholesterol, total cholesterol, use of lipid lowering medication, BMI, waist to hip ratio, alcohol intake, sports related physical activity, leisure related physical activity, CHO intake, fiber intake and magnesium intake |
| Kappeler, 2013, America | n 17 611 (M 8239, F 9372) Age 41 years Follow-up 22 years | FFQ (81 items) | All- cause mortality: 3683 CVD mortality: 1554 | ≥13 times/months vs. 0 | All- cause mortality CVD mortality | 0.81 (95% CI 0.64, 1.03) 1.05 (95% CI 0.65, 1.71) | age, race, sex, smoking status, alcohol consumption, physical activity, socioeconomic status, BMI, marital status, fruit and vegetable intake, history of hypertension, diabetes, hypercolesterolemia, use of aspririn and ibuprofen, use of mineral and vitamin supplements, family history of diabetes, or hypercholesterolemia and hormone replacement therapy and oral contraceptive use (only women) |
| Key, 2019, Europe | n 409 885 (M 106751, F 303134) Age 51.7 years Follow-up 12.6 years | FFQ (EPIC study) | IHD: 7198 | 46 g/die vs. 0 g/die | Incident IHD | 1.01 (95% CI 0.94, 1.1) | age, smoking status and number of cigarettes-day, diabetes mellitus, hypertension, hyperlipidemia, physical activity level, employment status, educational level, BMI, alcohol intake, energy intake, fruit and vegetable intake, sugars intake and fiber from cereals intake |
| Lee, 2013, Asia | n 296 721 (M 112310, F 184411) Age n.a. Follow-up 6.6–15.6 years | FFQ (6–17 items) | All- cause mortality: 14326 (M) and 9957 (F) CVD mortality: 3579 (M) and 2794 (F) | Mean intake: 4.6–22.3 g/day (M) 2.8–15.4 g/day (F) | All-cause mortality CVD mortality | 0.89 (95% CI 0.81, 0.98) (M) 0.93 (95% CI 0.86, 0.99) (F) 0.82 (95% CI 0.64, 1.06) (M) 1.05 (95% CI 0.92, 1.18) (F) | age, BMI, education level, smoking status, rural/urban residence, alcohol intake, fruit and vegetable intake and total energy intake |
| Nagao, 2012, Asia | n 51 638 (M 20466, F 31217) Age 55.7 (M) and 56.1 (F) years Follow-up 18.4 years * | FFQ (40 items) | IHD mortality: 301 (M) and 236 (F) | 27.3 g/day vs.1.9 g/day (M) 22.4 g/day vs. 1.5 g/day (F) | IHD mortality | 0.86 (95% CI 0.6, 1.23) (M) 1.06 (95% CI 0.69, 1.62) (F) | age, BMI, ethanol intake, perceived mental stress, walking time, sports participation time, education years, history of hypertension and diabetes, total energy and energy-adjusted food (rice, fish, soy, vegetables and fruits) intakes |
| Park, 2017, Asia | n 9311(M 4461, F 4850) Age 52.1 years Follow-up 7.8 years * | FFQ (110 items) | CVD: 486 | 1.41 serving/week vs. 0 | Incident CVD | 0.68 (95% CI 0.47, 0.99) | age, sex, educational level, household income, residential area, smoking status, alcohol intake, BMI, physical activity, total energy intake and total fruit and vegetable intake |
| Rohrmann, 2013, Europe | n 448 568 (M 127321, F 321247) Age 51.3 years Follow-up 12.7 years | FFQ (EPIC study) | All-cause mortality:26344 CVD mortality: 5556 | 50.3 g/day vs. 9.7 g/day (M) 35.6 g/day vs.10.5 g/day (F) | All-cause mortality CVD mortality | 1.05 (95% CI 0.94, 1.18) 0.94 (95% CI 0.73, 1.21) | education, body weight, body height, total energy intake, alcohol consumption, physical activity, smoking status, smoking duration and other meat intake |
| Sauvaget, 2003, Asia | n 32049 Age 56 years * Follow-up 16 years | FFQ (22 items) | Stroke mortality: 1462 | 17.9 ± 39.61 g/day vs. 4.72 ± 24 g/day | Stroke mortality | 1.43 (95% CI 0.98, 2.1) | city, radiation dose, self-reported BMI, smoking status, alcohol habits, education level, history of diabetes or hypertension |
| Sluik, 2014, Europe | n 265 295 (M 107011, F 158284) Age 57.4 (with DM) and 51.8 (w/o DM) Follow-up 9.9 years * | FFQ (300–500 items) | All-cause mortality: 830 (with DM) and 12135 (w/o DM) | 10 g/day vs. 0 | All-cause mortality | 0.89 (95% CI 0.83, 0.96) (with DM) 0.97 (95%CI 0.95, 1.00) (w/o DM) | sex, prevalence of heart disease, cancer or stroke, educational attainment, diabetes medication use (only for DM) and the following when there were no exposure variables (alcohol consumption, smoking behaviour, physical activity and underlying dietary patterns) |
| Takata, 2013, Asia | n 134 290 (M 61128, F 73162) Age 55.5 (M) and 52.9 (F) years Follow-up 8.6 years * | Gender specific FFQ (81 items for M and 77 items for F) | All- cause mortality: 6943 CVD mortality: 2163 IHD mortality: 590 Ischemic stroke mortality: 504 Hemorrhagic stroke mortality: 530 | 37.9 g/day vs. 0.9 g/day (M) 33.8 g/day vs. 1.4 g/day (F) | All-cause mortality CVD mortality IHD mortality Ischemic stroke mortality Hemorrhagic stroke mortality | 0.95 (95% CI 0.87, 1.03) 0.93 (95% CI 0.79, 1.08) 1.08 (95% CI 0.81, 1.44) 0.99 (95%CI 0.72, 1.37) 1.05 (95%CI 0.77, 1.42) | age, total energy intake, income, occupation, education level, comorbidity index, physical activity level, total vegetable intake, total fruit intake, fish intake, red meat intake, smoking history and alcohol consumption (only men) |
| Tong, 2020, Europe | n 418 329 (M 140117, F 278212) Age 50.9 years Follow-up 12.7 years | FFQ (EPIC study) | Stroke: 7378 Ischemic stroke: 4281 Hemorrhagic stroke: 1430 | 44.6 g/day vs. 0 * | Stroke Ischemic stroke Hemorrhagic stroke | 0.94 (95%CI 0.87, 1.02) 0.97 (95%CI 0.88, 1.07) 0.97 (95%CI 0.82, 1.16) | age, smoking status and number of cigarettes per day, history of diabetes, prior hypertension, prior hyperlipidaemia, Cambridge physical activity index, employment status, level of education completed, current alcohol consumption, BMI, and observed intake of energy, and stratified by sex and EPIC centre. |
| van den Brandt, 2019, Europe | n 120 852 Age 61.4 years Follow-up 10 years | FFQ | All-cause mortality: 8823 CVD mortality: 2985 | 22.8 g/day vs. 0 | All-cause mortality CVD mortality | 0.89 (95% CI 0.77, 1.03) 0.89 (95%CI 0.75, 1.06) | age, sex, cigarette smoking status, number of cigarettes smoked per day, years of smoking, diabetes, body height, BMI, non-occupational physical activity, highest level of education, intake of alcohol, vegetable and fruit, energy, use of nutritional supplements and postmenopausal HRT (only women) |
| Wang, 2020, America | n 9286 (M) Age 72.1 years Follow-up 23 years | FFQ (68 items) | All-cause mortality: 4682 | 3.5 serving/week * vs. 0.6 serving/week * | All-cause mortality | 0.9 (95% CI 0.82, 0.98) | age, calendar year of prostate cancer diagnosis, tumor extent, Gleason score, nodal involvement, education, family history of prostate cancer, history of PSA testing, BMI, smoking status, physical activity, history of diabetes, CVD history and other cancer, total fruit and vegetable intake, energy intake, egg intake, fish intake, processed and unprocessed meat intake and red meat intake. |
| Whiteman, 1999, UK | n 10 055 Age n.a. Follow-up 9 years | FFQ | All-cause mortality: 472 IHD mortality: 96 | 4–7 days/week vs. <1 day/week | All-cause mortality IHD mortality | 0.76 (95% CI 0.48, 1.19) 0.95 (95% CI 0.38, 2.38) | gender, smoking and age group |
| Wurtz, 2016, Europe | n 55 171 (M 26029, F 29142) Age 55 (M) * and 56 (F) * Follow-up 13.5 (M) * and 13.6 (F) * | FFQ (192 items) | IHD: 1694 (M) and 656 (F) | 21.4 g/day vs. 0 | Incident IHD | 1.05 (95% CI 1.00, 1.11) (M) 1.0 (95% CI 0.9, 1.1) (F) | age, total energy intake, alcohol abstinence, alcohol intake, BMI, waist circumference, smoking status and amount, physical activity, duration of schooling, menopausal status, use of hormone replacement therapy (only women), investigated food items, fruits, sweets, soft drinks, lean dairy products, fatty dairy products, potato chips, refined cereals, wholegrain cereals, nuts |
| Zhong, 2020, America | n 29 682 (M 13168 F 16514) Age 53.7 years Follow-up 19 years * | FFQ | All-cause mortality: 8875 CVD: 6963 | 0.29 serving/day vs. 0 5 | All-cause mortality Incident CVD | 0.99 (95% CI 0.97, 1.02) 1.04 (95% CI 1.01, 1.06) | age, sex, race/ethnicity, educational level, total energy, smoking status, smoking pack-years, cohort-specific physical activity z score, alcohol intake, hormone therapy, fruits, legumes, potatoes, other vegetables, excluding legumes and potatoes, nuts and seeds, whole grains, refined grains, low-fat dairy products, high-fat dairy products, sugar-sweetened beverages, eggs, and 3 of the 4 food types (processed meat, unprocessed red meat, poultry, and fish |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupoli, R.; Vitale, M.; Calabrese, I.; Giosuè, A.; Riccardi, G.; Vaccaro, O. White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 676. https://doi.org/10.3390/nu13020676
Lupoli R, Vitale M, Calabrese I, Giosuè A, Riccardi G, Vaccaro O. White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies. Nutrients. 2021; 13(2):676. https://doi.org/10.3390/nu13020676
Chicago/Turabian StyleLupoli, Roberta, Marilena Vitale, Ilaria Calabrese, Annalisa Giosuè, Gabriele Riccardi, and Olga Vaccaro. 2021. "White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies" Nutrients 13, no. 2: 676. https://doi.org/10.3390/nu13020676
APA StyleLupoli, R., Vitale, M., Calabrese, I., Giosuè, A., Riccardi, G., & Vaccaro, O. (2021). White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies. Nutrients, 13(2), 676. https://doi.org/10.3390/nu13020676






