The Potential Contribution of Fortified Maize Flour, Oil, Rice, Salt, and Wheat Flour to Estimated Average Requirements and Tolerable Upper Intake Levels for 15 Nutrients in 153 Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Population
2.3. Variables
- The EAR is the “nutrient intake value that is estimated to meet the requirements of half of healthy individuals in a group” [25]. It is the recommended indicator to estimate a population’s nutritional need [26], as well as fortification potential and safety [1]. The EAR was used for 13 of the 15 nutrients in the analysis. Since the EAR is lacking in fluoride, the Adequate Intake (AI) was used instead. The AI, which is conceptually more akin to a recommended dietary allowance than to an EAR, is “assumed to ensure nutritional adequacy” and in the case of fluoride, it is the amount “shown to maximize reductions in the incidence of dental caries without unwanted side effects, such as dental fluorosis” [27]. As fortifying with folic acid is recommended for the prevention of neural tube defects, the recommended intake of 400 µg of folic acid daily for women of reproductive age was used [28,29], instead of the lower EAR level, which is the amount recommended to normalize homocysteine levels. Lower doses of folic acid are not sufficient to prevent all folate-sensitive neural tube defects [30].
- The UL is “the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population” [25]; it refers to the chronic intake of nutrients. For the iodine UL, instead of the 1.1 mg/day value [25], a lower, 0.6 mg/day value was used [31]. The more conservative UL value was recommended as part of a harmonization exercise “to better identify any risk of potentially excessive intakes” [32].
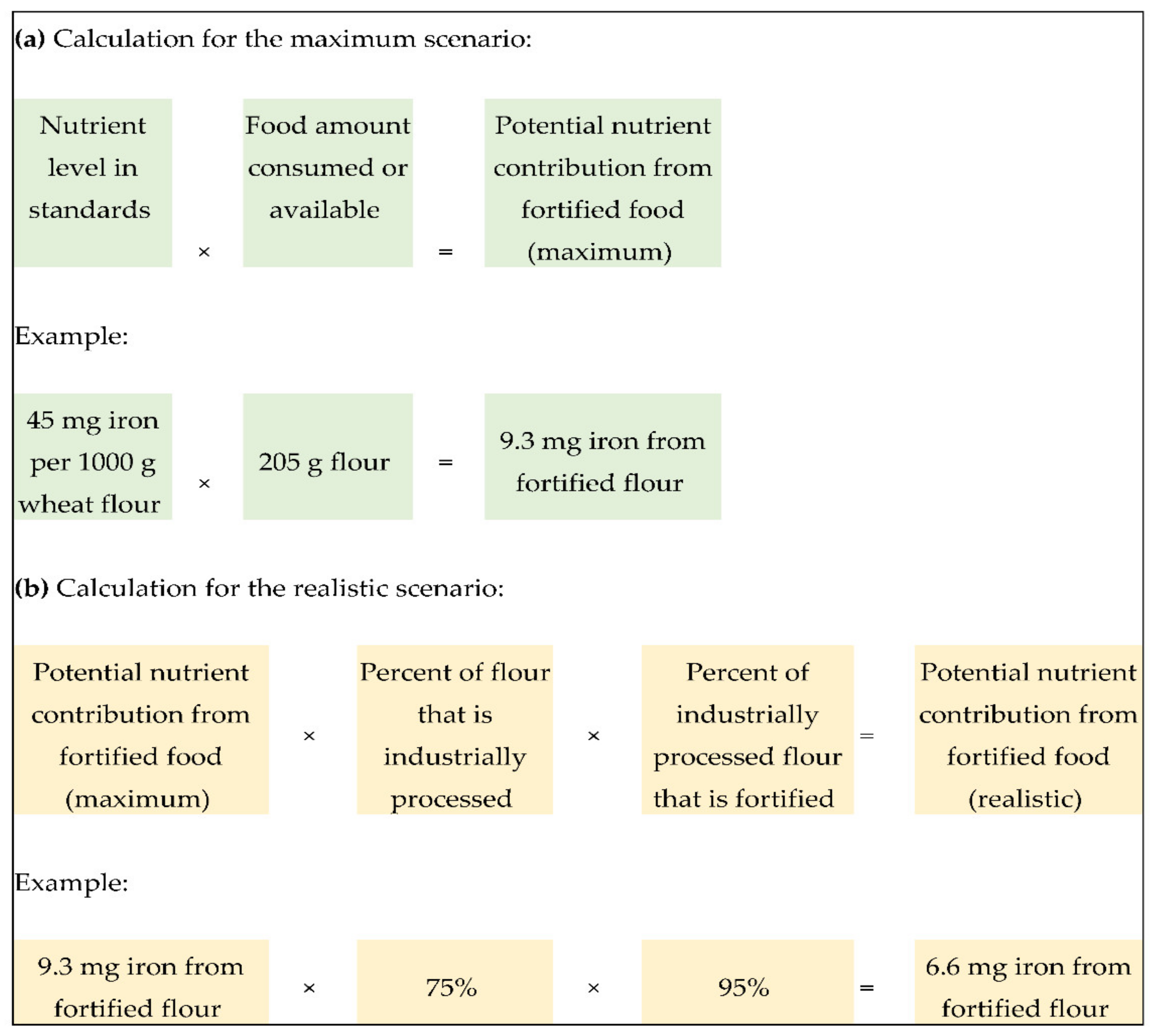
2.4. Calculation of Potential Nutrient Contribution under Two Scenarios
2.5. Comparison of Nutrient Contribution to Dietary Reference Intakes
2.6. Limitations of the Analyses
2.7. Descriptive Analyses
3. Results
4. Discussion
- If in the maximum scenario the potential nutrient contribution of any fortified food alone or in combination with others is substantially greater or lower than goals set for the country’s fortification program, then consider if the nutrient levels in standards might need to be changed.
- If the potential nutrient contribution is substantially different between the maximum and realistic scenarios, then discuss strategies for addressing any factors that are affecting program implementation. For example, how can compliance be improved if the percent of food that is fortified is not as high as it should be? If the percent of food that is industrially processed is low, discuss whether current fortification of this food is benefiting any subpopulations and whether other food vehicle options should be assessed to increase the reach of fortified foods.
- If the potential nutrient contribution in the maximum or realistic scenarios exceeds 100% of the UL, then consider if the nutrient levels in standards might need to be changed.
- If the potential nutrient contribution was not calculated for the realistic scenario, then seek information from industry partners and government regulatory authorities on the percent of food that is industrially processed and the percent of food that is fortified, respectively.
- Given the limitations of this analysis in only presenting the contribution of nutrients added through fortification, seek or convene studies that estimate the totality of the diet. In that context, is fortification performing as planned? Does the fortification program need to be adjusted?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 978-92-4-159401-1. [Google Scholar]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The Epidemiology of Global Micronutrient Deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- UNICEF. Children, Food and Nutrition: Growing Well in a Changing World; UNICEF: New York, NY, USA, 2019; ISBN 978-92-806-5003-7. [Google Scholar]
- Centers for Disease Control and Prevention Micronutrient Facts. Available online: https://www.cdc.gov/nutrition/micronutrient-malnutrition/micronutrients/index.html (accessed on 7 December 2020).
- World Health Organization Micronutrients. Available online: https://www.who.int/health-topics/micronutrients#tab=tab_1 (accessed on 7 December 2020).
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Iron Review. J. Nutr. 2018, 148, 1001S–1067S. [Google Scholar] [CrossRef]
- Wiseman, E.M.; Bar-El Dadon, S.; Reifen, R. The Vicious Cycle of Vitamin A Deficiency: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Rohner, F.; Zimmermann, M.; Jooste, P.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of Nutrition for Development—Iodine Review. J. Nutr. 2014, 144, 1322S–1342S. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, A.; Luthringer, C.L.; Parvanta, I.; Garrett, G.S. Food Fortification Global Mapping Study 2016; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Mannar, M.G.V. Salt. In Food Fortification in a Globalized World; Elsevier Academic Press Inc.: San Diego, CA, USA, 2018; pp. 143–151. ISBN 978-0-12-802897-1. [Google Scholar]
- Chavasit, V.; Photi, J. Condiments and sauces. In Food Fortification in a Globalized World; Elsevier Academic Press Inc.: San Diego, CA, USA, 2018; pp. 153–158. ISBN 978-0-12-802897-1. [Google Scholar]
- Pachón, H. Wheat and maize flour fortification. In Food Fortification in a Globalized World; Elsevier Academic Press Inc.: San Diego, CA, USA, 2018; pp. 123–129. ISBN 978-0-12-802897-1. [Google Scholar]
- López de Romaña, D.; Olivares, M.; Pizarro, F. Milk and dairy products. In Food Fortification in a Globalized World; Elsevier Academic Press Inc.: San Diego, CA, USA, 2018; pp. 175–181. ISBN 978-0-12-802897-1. [Google Scholar]
- Marks, K.J.; Luthringer, C.L.; Ruth, L.J.; Rowe, L.A.; Khan, N.A.; De-Regil, L.M.; López, X.; Pachón, H. Review of Grain Fortification Legislation, Standards, and Monitoring Documents. Glob. Health Sci. Pract. 2018, 6, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.; Abudou, M.; Candeias, V.; Wu, T. Effect and Safety of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review with Meta-Analyses; WHO eLibrary of Evidence for Nutrition Actions (eLENA): Geneva, Switzerland, 2014; ISBN 978-92-4-150828-5. [Google Scholar]
- Keats, E.C.; Neufeld, L.M.; Garrett, G.S.; Mbuya, M.N.N.; Bhutta, Z.A. Improved Micronutrient Status and Health Outcomes in Low- and Middle-Income Countries Following Large-Scale Fortification: Evidence from a Systematic Review and Meta-Analysis. Am. J. Clin. Nutr 2019, 109, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.; Casas, J.; Taren, D.; Alverson, C.J.; Flores, A.; Frias, J. Neural Tube Defects in Latin America and the Impact of Fortification: A Literature Review. Public Health Nutr. 2014, 17, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Global Alliance for Improved Nutrition. Identifying Potential New Food Vehicles for Fortification in West Africa; Global Alliance for Improved Nutrition: Geneva, Switzerland, 2018. [Google Scholar]
- Pachón, H.; Dary, O. Consumption monitoring: Coverage, provision and utilization. In Food Fortification in a Globalized World; Elsevier Academic Press Inc.: San Diego, CA, USA, 2018; pp. 291–297. ISBN 978-0-12-802897-1. [Google Scholar]
- Neufeld, L.M.; Baker, S.; Garrett, G.S.; Haddad, L. Coverage and Utilization in Food Fortification Programs: Critical and Neglected Areas of Evaluation. J. Nutr. 2017, 147, 1015S–1019S. [Google Scholar] [CrossRef] [PubMed]
- Global Fortification Data Exchange Full GFDx Datasets. Available online: https://fortificationdata.org (accessed on 7 December 2020).
- Reynolds, B. The Potential Dietary Contribution of Food Fortification when Implemented According to National Standards. Master of Public Health Thesis Project, Johns Hopkins University, Baltimore, MD, USA. unpublished.
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, Regional and National Sodium Intakes in 1990 and 2010: A Systematic Analysis of 24 h Urinary Sodium Excretion and Dietary Surveys Worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organizations of the United Naitons New Food Balances. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 7 December 2020).
- Food and Nutrition Board. The National Academies of Sciences, Engineering, and Medicine Table: Dietary Reference Intake Values Summary. Available online: https://www.nationalacademies.org/our-work/summary-report-of-the-dietary-reference-intakes (accessed on 7 December 2020).
- Institute of Medicine. DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000; ISBN 978-0-309-07311-0. [Google Scholar]
- National Institutes of Health Fluoride. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Fluoride-HealthProfessional (accessed on 7 December 2020).
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. In The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- World Health Organization. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150199-6. [Google Scholar]
- Crider, K.S.; Qi, Y.P.; Devine, O.; Tinker, S.C.; Berry, R.J. Modeling the Impact of Folic Acid Fortification and Supplementation on Red Blood Cell Folate Concentrations and Predicted Neural Tube Defect Risk in the United States: Have We Reached Optimal Prevention? Am. J. Clin. Nutr. 2018, 107, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Parma, Italy, 2006; ISBN 978-92-9199-014-6. [Google Scholar]
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed Harmonized Nutrient Reference Values for Populations. Adv. Nutr. 2020, 11, 469–483. [Google Scholar] [CrossRef] [PubMed]
- García-Casal, M.N.; Mowson, R.; Rogers, L.; Grajeda, R.; Consultation Working Groups. Risk of Excessive Intake of Vitamins and Minerals Delivered through Public Health Interventions: Objectives, Results, Conclusions of the Meeting, and the Way Forward. Ann. N. Y. Acad. Sci. 2019, 1446, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Jayatissa, R.; Fernando, D.N. Supplementation of Micronutrients in Children and Food Fortification Initiatives in Sri Lanka: Benefits versus Risks. Ann. N. Y. Acad. Sci. 2019, 1446, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, H.; Andersen, R.; Antoine, J.-M.; Finglas, P.; Hoekstra, J.; Kardinaal, A.; Nordmann, H.; Pekcan, G.; Pentieva, K.; Sanders, T.A.; et al. Application of the BRAFO Tiered Approach for Benefit-Risk Assessment to Case Studies on Dietary Interventions. Food Chem. Toxicol. 2012, 50 (Suppl. 4), S710–S723. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.L.; Lividini, K.; Guyondet, C.; Bermudez, O.I. Assessing Alternative Industrial Fortification Portfolios: A Bangladesh Case Study. Food Nutr. Bull. 2015, 36, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Friesen, V.M.; Mbuya, M.N.N.; Aaron, G.J.; Pachón, H.; Adegoke, O.; Noor, R.A.; Swart, R.; Kaaya, A.; Wieringa, F.T.; Neufeld, L.M. Fortified Foods Are Major Contributors to Apparent Intakes of Vitamin A and Iodine, but Not Iron, in Diets of Women of Reproductive Age in 4 African Countries. J. Nutr. 2020, 150, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Micha, R.; Golden, C.D.; Mozaffarian, D.; Myers, S.S. Global Expanded Nutrient Supply (GENuS) Model: A New Method for Estimating the Global Dietary Supply of Nutrients. PLoS ONE 2016, 11, e0146976. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, T.; Carey, E.; Sharma, S.; Biadgilign, S. Trends in Energy and Nutrient Supply in Ethiopia: A Perspective from FAO Food Balance Sheets. Nutr. J. 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed]
| Countries with Fortification Requirements for 1 | Number of Countries |
|---|---|
| Any food | 153 |
| Specific food 2 | |
| Maize flour | 19 |
| Oil | 32 |
| Rice | 14 |
| Salt | 137 |
| Wheat flour | 93 |
| Specific nutrient 3 | |
| Vitamin A | 67 |
| Vitamin B1 (thiamine) | 98 |
| Vitamin B2 (riboflavin) | 86 |
| Vitamin B3 (niacin) | 95 |
| Vitamin B6 (pyridoxine) | 29 |
| Vitamin B12 | 39 |
| Vitamin D | 22 |
| Vitamin E | 4 |
| Folic acid | 102 |
| Calcium | 28 |
| Fluoride | 20 |
| Iodine | 137 |
| Iron | 125 |
| Selenium | 2 |
| Zinc | 53 |
| Nutrient | In the Maximum Scenario 1, Median: | In the Realistic Scenario 1, Median: | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Contribution 2 in mg/capita/day | % EAR Met 3 | % UL Met 4 | n | Median Contribution 2 in mg/capita/day | % EAR Met 3 | % UL Met 4 | |
| Vitamin A | 64 | 0.2 | 48.3 | 8.1 | 38 | 0.02 | 4.7 | 0.8 |
| Vitamin B1 (thiamine) | 92 | 0.7 | 75.4 | -- 5 | 92 | 0.2 | 27.1 | -- 5 |
| Vitamin B2 (riboflavin) | 81 | 0.4 | 46.1 | -- 5 | 81 | 0.2 | 22.4 | -- 5 |
| Vitamin B3 (niacin) | 89 | 5.4 | 49.2 | 15.5 | 89 | 1.9 | 17.0 | 5.4 |
| Vitamin B6 (pyridoxine) | 26 | 0.6 | 51.6 | 0.6 | 26 | 0.1 | 4.8 | 0.1 |
| Vitamin B12 | 36 | 0.001 | 63.8 | -- 5 | 36 | 0.0001 | 8.5 | -- 5 |
| Vitamin D | 22 | 0.003 | 33.7 | 3.4 | 10 | 0.001 | 5.1 | 0.5 |
| Vitamin E | 4 | 2.6 | 21.9 | 0.3 | 1 | 2.3 | 19.6 | 0.2 |
| Folic acid | 96 | 0.2 | 49.7 6 | 19.9 | 96 | 0.1 | 19.7 6 | 7.9 |
| Calcium | 27 | 203.5 | 25.4 | 8.1 | 27 | 187.0 | 23.4 | 7.5 |
| Fluoride | 20 | 1.6 | 53.9 7 | 16.2 | 1 | 0.9 | 31.5 7 | 9.4 |
| Iodine | 136 | 0.3 | 338.3 | 53.6 8 | 13 | 0.3 | 180.5 | 24.6 8 |
| Iron | 117 | 5.2 | 63.6 | 11.5 | 115 | 2.2 | 27.0 | 4.9 |
| Selenium | 2 | 0.03 | 60.4 | 67.9 | 2 | 0.0002 | 20.6 | 2.3 |
| Zinc | 50 | 3.8 | 56.4 | 9.6 | 50 | 0.6 | 8.2 | 1.4 |
| Nutrient | Maximum Scenario 1,2 | Realistic Scenario 1,2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <50% Met | 50–150% Met | >150% Met | Total | <50% Met | 50–150% Met | >150% Met | Total | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Vitamin A | 32 | 50.0 | 26 | 40.6 | 6 | 9.4 | 64 | 100.0 | 32 | 84.2 | 6 | 15.8 | 0 | 0.0 | 38 | 100.0 |
| Vitamin B1(thiamine) | 28 | 30.4 | 49 | 53.3 | 15 | 16.3 | 92 | 100.0 | 56 | 60.9 | 29 | 31.5 | 7 | 7.6 | 92 | 100.0 |
| Vitamin B2 (riboflavin) | 44 | 54.3 | 36 | 44.4 | 1 | 1.2 | 81 | 100.0 | 62 | 76.5 | 19 | 23.5 | 0 | 0.0 | 81 | 100.0 |
| Vitamin B3 (niacin) | 46 | 51.7 | 40 | 44.9 | 3 | 3.4 | 89 | 100.0 | 66 | 74.2 | 23 | 25.8 | 0 | 0.0 | 89 | 100.0 |
| Vitamin B6 (pyridoxine) | 12 | 46.2 | 13 | 50.0 | 1 | 3.8 | 26 | 100.0 | 23 | 88.5 | 3 | 11.5 | 0 | 0.0 | 26 | 100.0 |
| Vitamin B12 | 16 | 44.4 | 13 | 36.1 | 7 | 19.4 | 36 | 100.0 | 31 | 86.1 | 5 | 13.9 | 0 | 0.0 | 36 | 100.0 |
| Vitamin D | 16 | 72.7 | 4 | 18.2 | 2 | 9.1 | 22 | 100.0 | 10 | 100.0 | 0 | 0.0 | 0 | 0.0 | 10 | 100.0 |
| Vitamin E | 4 | 100.0 | 0 | 0.0 | 0 | 0.0 | 4 | 100.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
| Folic acid 3 | 48 | 50.0 | 40 | 41.7 | 8 | 8.3 | 96 | 100.0 | 70 | 72.9 | 24 | 25.0 | 2 | 2.1 | 96 | 100.0 |
| Calcium | 20 | 74.1 | 7 | 25.9 | 0 | 0.0 | 27 | 100.0 | 22 | 81.5 | 5 | 18.5 | 0 | 0.0 | 27 | 100.0 |
| Fluoride 4 | 7 | 35.0 | 12 | 60.0 | 1 | 5.0 | 20 | 100.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
| Iodine | 0 | 0.0 | 3 | 2.2 | 133 | 97.8 | 136 | 100.0 | 2 | 15.4 | 2 | 15.4 | 9 | 69.2 | 13 | 100.0 |
| Iron | 43 | 36.8 | 60 | 51.3 | 14 | 12.0 | 117 | 100.0 | 72 | 62.6 | 38 | 33.0 | 5 | 4.3 | 115 | 100.0 |
| Selenium | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 2 | 100.0 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 2 | 100.0 |
| Zinc | 20 | 40.0 | 22 | 44.0 | 8 | 16.0 | 50 | 100.0 | 43 | 86.0 | 6 | 12.0 | 1 | 2.0 | 50 | 100.0 |
| Food | Industrially Processed | Fortified | ||
|---|---|---|---|---|
| n3 | Median (%) | n3 | Median (%) | |
| Maize flour | 19 | 20.0 | 19 | 5.0 |
| Oil | 5 | 85.0 | 11 | 59.7 |
| Rice | 14 | 39.5 | 14 | 0.1 |
| Salt | 25 | 100.0 | 34 | 77.1 |
| Wheat flour | 93 | 100.0 | 93 | 95.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachón, H.; Reynolds, B.; Duong, M.; Tsang, B.L.; Childs, L.; Luthringer, C.L.; Kang, Y.; Vasta, F.C.; Codling, K. The Potential Contribution of Fortified Maize Flour, Oil, Rice, Salt, and Wheat Flour to Estimated Average Requirements and Tolerable Upper Intake Levels for 15 Nutrients in 153 Countries. Nutrients 2021, 13, 579. https://doi.org/10.3390/nu13020579
Pachón H, Reynolds B, Duong M, Tsang BL, Childs L, Luthringer CL, Kang Y, Vasta FC, Codling K. The Potential Contribution of Fortified Maize Flour, Oil, Rice, Salt, and Wheat Flour to Estimated Average Requirements and Tolerable Upper Intake Levels for 15 Nutrients in 153 Countries. Nutrients. 2021; 13(2):579. https://doi.org/10.3390/nu13020579
Chicago/Turabian StylePachón, Helena, Bethany Reynolds, Michelle Duong, Becky L. Tsang, Lana Childs, Corey L. Luthringer, Yunhee Kang, Florencia C. Vasta, and Karen Codling. 2021. "The Potential Contribution of Fortified Maize Flour, Oil, Rice, Salt, and Wheat Flour to Estimated Average Requirements and Tolerable Upper Intake Levels for 15 Nutrients in 153 Countries" Nutrients 13, no. 2: 579. https://doi.org/10.3390/nu13020579
APA StylePachón, H., Reynolds, B., Duong, M., Tsang, B. L., Childs, L., Luthringer, C. L., Kang, Y., Vasta, F. C., & Codling, K. (2021). The Potential Contribution of Fortified Maize Flour, Oil, Rice, Salt, and Wheat Flour to Estimated Average Requirements and Tolerable Upper Intake Levels for 15 Nutrients in 153 Countries. Nutrients, 13(2), 579. https://doi.org/10.3390/nu13020579






