Potential of Creatine in Glucose Management and Diabetes
Abstract
1. Introduction
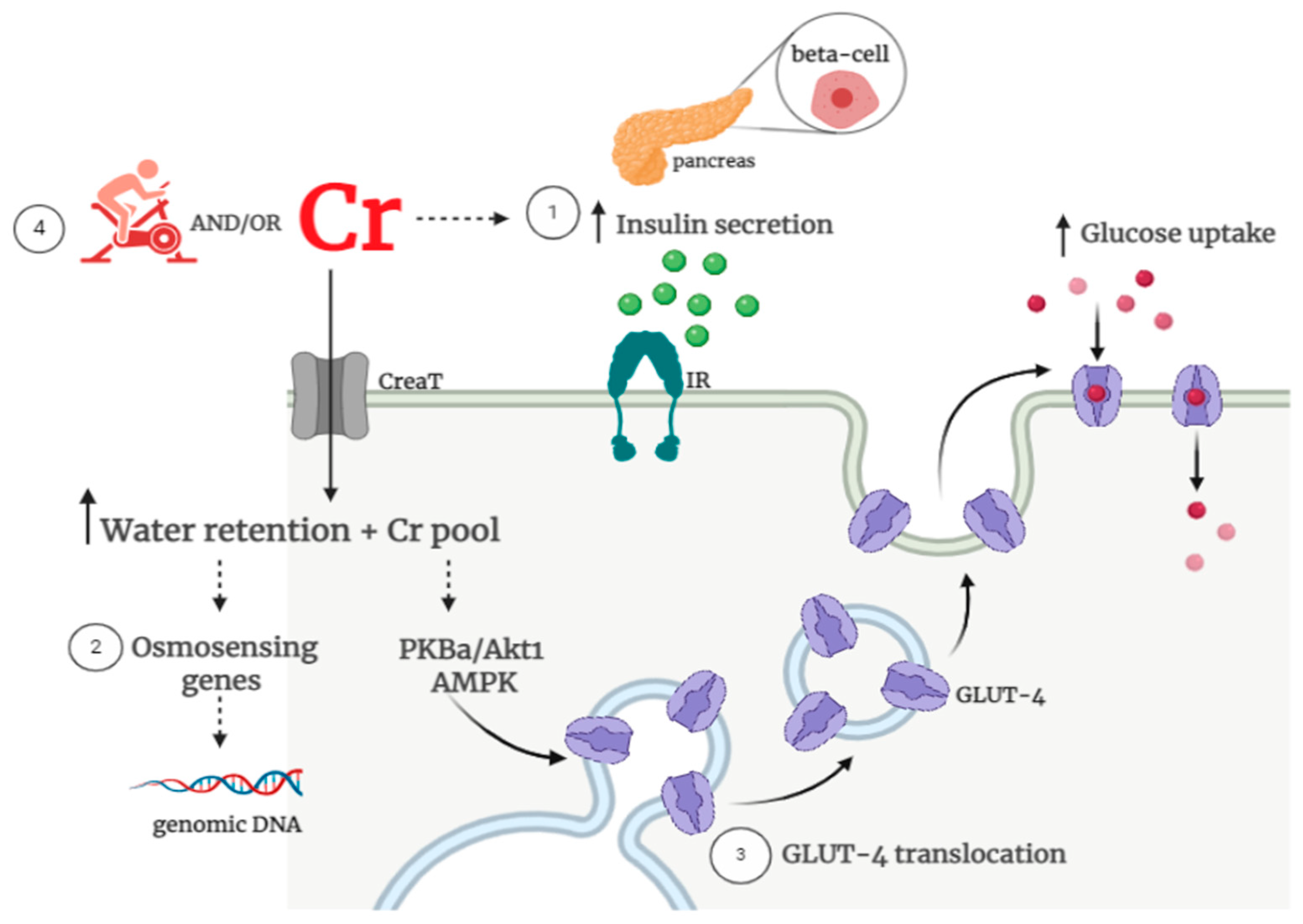
2. Insulin resistance in the Context of the Interplay between Creatine and Glucose Metabolism
3. Effects of Creatine Supplementation Alone on Glycemic Control
4. Effects of Creatine Supplementation Combined with Exercise on Glycemic Control
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S14–S31. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef]
- Op’t Eijnde, B.; Urso, B.; Richter, E.A.; Greenhaff, P.L.; Hespel, P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes 2001, 50, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.S.; Smith, J.L.; Oppelt, P.J.; Fisher, J.S. Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E347–E352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, A.G.; Arnall, D.A.; Kokkonen, J.; Day, R.; Evans, J. Muscle glycogen supercompensation is enhanced by prior creatine supplementation. Med. Sci. Sports Exerc. 2001, 33, 1096–1100. [Google Scholar] [CrossRef]
- Green, A.L.; Simpson, E.J.; Littlewood, J.J.; Macdonald, I.A.; Greenhaff, P.L. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol. Scand. 1996, 158, 195–202. [Google Scholar] [CrossRef]
- Marco, J.; Calle, C.; Hedo, J.A.; Villanueva, M.L. Glucagon-releasing activity of guanidine compounds in mouse pancreatic islets. FEBS Lett. 1976, 64, 52–54. [Google Scholar] [CrossRef]
- Alsever, R.N.; Georg, R.H.; Sussman, K.E. Stimulation of insulin secretion by guanidinoacetic acid and other guanidine derivatives. Endocrinology 1970, 86, 332–336. [Google Scholar] [CrossRef]
- Steenge, G.R.; Lambourne, J.; Casey, A.; Macdonald, I.A.; Greenhaff, P.L. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am. J. Physiol. 1998, 275, E974–E979. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.E.; Hargreaves, M.; Garnham, A.; Snow, R.J. Effect of creatine ingestion on glucose tolerance and insulin sensitivity in men. Med. Sci. Sports Exerc. 2003, 35, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Andreassen, O.A.; Jenkins, B.G.; Dedeoglu, A.; Kuemmerle, S.; Kubilus, J.K.; Kaddurah-Daouk, R.; Hersch, S.M.; Beal, M.F. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000, 20, 4389–4397. [Google Scholar] [CrossRef]
- Gualano, B.; Novaes, R.B.; Artioli, G.G.; Freire, T.O.; Coelho, D.F.; Scagliusi, F.B.; Rogeri, P.S.; Roschel, H.; Ugrinowitsch, C.; Lancha, A.H., Jr. Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids 2008, 34, 245–250. [Google Scholar] [CrossRef]
- Gualano, B.; Painneli, V.D.S.; Roschel, H.; Artioli, G.G.; Neves, M., Jr.; De Sa Pinto, A.L.; Da Silva, M.E.; Cunha, M.R.; Otaduy, M.C.; Leite Cda, C.; et al. Creatine in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef]
- Kruger, M.; Kratchmarova, I.; Blagoev, B.; Tseng, Y.H.; Kahn, C.R.; Mann, M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl. Acad. Sci. USA 2008, 105, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.M.; Martinez, V.B.; Granado, J.Q.; Juanatey, J.R. Advances in hypertension and diabetes in 2007. Rev. Esp. Cardiol. 2008, 61 (Suppl. 1), 58–71. [Google Scholar] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Bouzakri, K.; Karlsson, H.K.; Vestergaard, H.; Madsbad, S.; Christiansen, E.; Zierath, J.R. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 2006, 55, 785–791. [Google Scholar] [CrossRef]
- Palomino-Schatzlein, M.; Lamas-Domingo, R.; Ciudin, A.; Gutierrez-Carcedo, P.; Mares, R.; Aparicio-Gomez, C.; Hernandez, C.; Simo, R.; Herance, J.R. A Translational In Vivo and In Vitro Metabolomic Study Reveals Altered Metabolic Pathways in Red Blood Cells of Type 2 Diabetes. J. Clin. Med. 2020, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Groothof, D.; Schutten, J.C.; Flores-Guerrero, J.L.; Swarte, J.C.; Douwes, R.M.; Kema, I.P.; de Boer, R.A.; Garcia, E.; Connelly, M.A.; et al. Plasma creatine and incident type 2 diabetes in a general population-based cohort: The PREVEND study. Clin. Endocrinol. 2020. [Google Scholar] [CrossRef]
- Pinto, C.L.; Botelho, P.B.; Pimentel, G.D.; Campos-Ferraz, P.L.; Mota, J.F. Creatine supplementation and glycemic control: A systematic review. Amino Acids 2016, 48, 2103–2129. [Google Scholar] [CrossRef]
- Hill, R. The effect of the administration of creatine on the blood sugar (abstract). J. Biol. Chem. 1928, 78, iv. (In Abstract) [Google Scholar]
- Gempel, K.; Brdiczka, D.; Kaddurah-Daouk, R.; Wallimann, T.; Kaufhold, P.; Gerbitz, K.D. The creatine analogue cyclocreatine increases insulin secretion in INS-1 cells via a K+ channel independent mechanism. Diabetologia 1996, 39, 109. [Google Scholar]
- Rooney, K.; Bryson, J.; Phuyal, J.; Denyer, G.; Caterson, I.; Thompson, C. Creatine supplementation alters insulin secretion and glucose homeostasis in vivo. Metabolism 2002, 51, 518–522. [Google Scholar] [CrossRef]
- Op’t Eijnde, B.; Jijakli, H.; Hespel, P.; Malaisse, W.J. Creatine supplementation increases soleus muscle creatine content and lowers the insulinogenic index in an animal model of inherited type 2 diabetes. Int. J. Mol. Med. 2006, 17, 1077–1084. [Google Scholar] [CrossRef]
- van Loon, L.J.; Murphy, R.; Oosterlaar, A.M.; Cameron-Smith, D.; Hargreaves, M.; Wagenmakers, A.J.; Snow, R. Creatine supplementation increases glycogen storage but not GLUT-4 expression in human skeletal muscle. Clin. Sci. 2004, 106, 99–106. [Google Scholar] [CrossRef]
- Safdar, A.; Yardley, N.J.; Snow, R.; Melov, S.; Tarnopolsky, M.A. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol. Genom. 2008, 32, 219–228. [Google Scholar] [CrossRef]
- Low, S.Y.; Rennie, M.J.; Taylor, P.M. Modulation of glycogen synthesis in rat skeletal muscle by changes in cell volume. J. Physiol. 1996, 495 Pt 2, 299–303. [Google Scholar] [CrossRef]
- Baquet, A.; Hue, L.; Meijer, A.J.; van Woerkom, G.M.; Plomp, P.J. Swelling of rat hepatocytes stimulates glycogen synthesis. J. Biol. Chem. 1990, 265, 955–959. [Google Scholar] [CrossRef]
- Deldicque, L.; Louis, M.; Theisen, D.; Nielens, H.; Dehoux, M.; Thissen, J.P.; Rennie, M.J.; Francaux, M. Increased IGF mRNA in human skeletal muscle after creatine supplementation. Med. Sci. Sports Exerc. 2005, 37, 731–736. [Google Scholar] [CrossRef]
- Alves, C.R.; Ferreira, J.C.; de Siqueira-Filho, M.A.; Carvalho, C.R.; Lancha, A.H., Jr.; Gualano, B. Creatine-induced glucose uptake in type 2 diabetes: A role for AMPK-alpha? Amino Acids 2012, 43, 1803–1807. [Google Scholar] [CrossRef]
- Henriksen, E.J. Invited review: Effects of acute exercise and exercise training on insulin resistance. J. Appl. Physiol. 2002, 93, 788–796. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef]
- Kennedy, J.W.; Hirshman, M.F.; Gervino, E.V.; Ocel, J.V.; Forse, R.A.; Hoenig, S.J.; Aronson, D.; Goodyear, L.J.; Horton, E.S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999, 48, 1192–1197. [Google Scholar] [CrossRef]
- Ren, J.M.; Semenkovich, C.F.; Gulve, E.A.; Gao, J.; Holloszy, J.O. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J. Biol. Chem. 1994, 269, 14396–14401. [Google Scholar] [CrossRef]
- Charron, M.J.; Brosius, F.C., 3rd; Alper, S.L.; Lodish, H.F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc. Natl. Acad. Sci. USA 1989, 86, 2535–2539. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.Y.; Artioli, G.G.; Otaduy, M.C.G.; Leite, C.D.C.; Arruda, W.; Veiga, R.R.; Gualano, B. Effect of age, diet, and tissue type on PCr response to creatine supplementation. J. Appl. Physiol. 2017, 123, 407–414. [Google Scholar] [CrossRef]
- Gotshalk, L.A.; Kraemer, W.J.; Mendonca, M.A.; Vingren, J.L.; Kenny, A.M.; Spiering, B.A.; Hatfield, D.L.; Fragala, M.S.; Volek, J.S. Creatine supplementation improves muscular performance in older women. Eur. J. Appl. Physiol. 2008, 102, 223–231. [Google Scholar] [CrossRef]
- Rawson, E.S.; Clarkson, P.M. Acute creatine supplementation in older men. Int. J. Sports Med. 2000, 21, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Wehnert, M.L.; Clarkson, P.M. Effects of 30 days of creatine ingestion in older men. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 139–144. [Google Scholar] [CrossRef]
- Young, J.C.; Young, R.E. The effect of creatine supplementation on glucose uptake in rat skeletal muscle. Life Sci. 2002, 71, 1731–1737. [Google Scholar] [CrossRef]
- Nicastro, H.; Gualano, B.; de Moraes, W.M.; de Salles Painelli, V.; da Luz, C.R.; dos Santos Costa, A.; de Salvi Guimaraes, F.; Medeiros, A.; Brum, P.C.; Lancha, A.H., Jr. Effects of creatine supplementation on muscle wasting and glucose homeostasis in rats treated with dexamethasone. Amino Acids 2012, 42, 1695–1701. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Bourgeois, J.M.; Snow, R.; Keys, S.; Roy, B.D.; Kwiecien, J.M.; Turnbull, J. Histological assessment of intermediate- and long-term creatine monohydrate supplementation in mice and rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R762–R769. [Google Scholar] [CrossRef]
- Rooney, K.B.; Bryson, J.M.; Digney, A.L.; Rae, C.D.; Thompson, C.H. Creatine supplementation affects glucose homeostasis but not insulin secretion in humans. Ann. Nutr. Metab. 2003, 47, 11–15. [Google Scholar] [CrossRef]
- Rocic, B.; Bajuk, N.B.; Rocic, P.; Weber, D.S.; Boras, J.; Lovrencic, M.V. Comparison of antihyperglycemic effects of creatine and metformin in type II diabetic patients. Clin. Investig. Med. 2009, 32, E322. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.M.; Sewell, D.A.; Hultman, E.; Greenhaff, P.L. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J. Appl. Physiol. 1999, 87, 598–604. [Google Scholar] [CrossRef]
- King, D.S.; Dalsky, G.P.; Staten, M.A.; Clutter, W.E.; Van Houten, D.R.; Holloszy, J.O. Insulin action and secretion in endurance-trained and untrained humans. J. Appl. Physiol. 1987, 63, 2247–2252. [Google Scholar] [CrossRef]
- Gan, S.K.; Kriketos, A.D.; Ellis, B.A.; Thompson, C.H.; Kraegen, E.W.; Chisholm, D.J. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 2003, 26, 1706–1713. [Google Scholar] [CrossRef]
- Devlin, J.T.; Hirshman, M.; Horton, E.D.; Horton, E.S. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 1987, 36, 434–439. [Google Scholar] [CrossRef]
- Bruce, C.R.; Kriketos, A.D.; Cooney, G.J.; Hawley, J.A. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia 2004, 47, 23–30. [Google Scholar] [CrossRef]
- Rocic, B.; Vucic, M.; Mesic, R.; Rocic, P.; Coce, F. Hypoglycemic effect of creatine in insulin dependent diabetic patients. Diabetol. Croatica 1995, 24, 117–120. [Google Scholar]
- Souza, R.A.; Santos, R.M.; Osório, R.A.L.; Cogo, J.C.; Prianti-Júnior, A.C.G.; Martins, R.A.B.L.; Ribeiro, W. Influence of the short and long term supplementation of creatine on the plasmatic concentrations of glucose and lactate in Wistar rats. Rev. Bras. Med. Esporte 2006, 12, 361–365. [Google Scholar] [CrossRef][Green Version]
- Araújo, M.B.D.; Vieira Junior, R.C.; Moura, L.P.D.; Costa Junior, M.; Dalia, R.A.; Sponton, A.C.D.S.; Ribeiro, C.; Mello, M.A.R.D. Influence of creatine supplementation on indicators of glucose metabolism in skeletal muscle of exercised rats. Motriz 2013, 19, 709–716. [Google Scholar] [CrossRef]
- Freire, T.O.; Gualano, B.; Leme, M.D.; Polacow, V.O.; Lancha, A.H., Jr. Effects of creatine supplementation on glucose uptake in rats submitted to exercise training. Rev. Bras. Med. Esporte 2008, 14, 431–435. [Google Scholar] [CrossRef][Green Version]
- Vaisy, M.; Szlufcik, K.; De Bock, K.; Eijnde, B.O.; Van Proeyen, K.; Verbeke, K.; Van Veldhoven, P.; Hespel, P. Exercise-induced, but not creatine-induced, decrease in intramyocellular lipid content improves insulin sensitivity in rats. J. Nutr. Biochem. 2011, 22, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Eijnde, B.O.; Verbessem, P.; Ramaekers, M.; Van Leemputte, M.; Richter, E.A.; Hespel, P. Combined creatine and protein supplementation in conjunction with resistance training promotes muscle GLUT-4 content and glucose tolerance in humans. J. Appl. Physiol. 2003, 94, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.L.P.; Antunes, B.M.M.; Gomes, A.C.; Lira, F.S.; Pimentel, G.D.; Boule, N.G.; Mota, J.F. Creatine supplementation does not promote additional effects on inflammation and insulin resistance in older adults: A pilot randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 38, 94–98. [Google Scholar] [CrossRef]
| Reference | Model | Creatine Protocol | Main Findings |
|---|---|---|---|
| Ferrante et al. [17] | Transgenic mice model of Huntington’s disease | Diet supplemented with 1, 2, or 3% of Cr for 21 d | ↑ glucose tolerance; ↑ neuroprotective effect; ↑ body weight; ↑ motor performance on the rotarod test. |
| Op’t Eijnde et al. [9] | Male Wistar rats | Powdered rat chow with 5% of Cr for 5 d | ↑ Cr and PCr muscle content; ↔ muscle GLUT-4 content; ↔ glucose transport rate; ↔ plasma insulin; ↔ blood glucose. |
| Young and Young, [48] | Male Sprague Dawley rats | 300 mg∙kg−1∙d−1 for 5 wk | ↑ Cr and PCr muscle content; ↔ basal rates of glucose uptake; ↔ insulin-stimulated rates of glucose uptake. |
| Rooney et al. [31] | Male Wister rats | Chow containing 2% of Cr for 2, 4, or 8 wk | ↔ fasting plasma glucose; ↔ plasma glucose after oral glucose load; ↑ fasting plasma insulin levels; ↑ pancreatic TCr content. |
| Ju et al. [10] | Female Wistar rats | Chow containing 2% of Cr for 3 wk | ↑ glycogen content; ↑ muscle GLUT-4 content; ↑ GLUT-4 mRNA; ↑ AMPK phosphorylation; ↑ Acetyl-Coa carboxylase phosphorylation. |
| Op’t Eijnde et al. [32] | Male Goto-Kakizaki rats | Pallets enriched with 2% of Cr for 8 wk | ↑ muscle Cr content only in young rats (but not in older rats); ↓ plasma insulin concentration; ↔ Blood D-glucose concentration after OGTT; ↓ insulinogenic index. |
| Nicastro et al. [49] | Male Wistar rats | 5 g∙Kg−1∙d−1 of Cr for 7 d + 5 mg∙kg−1∙d−1 of DXM | ↑ serum glucose and insulin after Cr + DXM; ↑ HOMA-IR after Cr + DXM; ↓ GLUT-4 translocation after Cr + DXM |
| Reference | Sample (n) | Study Design | Creatine Protocol | Main Findings |
|---|---|---|---|---|
| Newman et al. [16] | Healthy, active, untrained, male adults (17) | Sigle-blind, placebo-controlled trial | Loading phase: 20 g∙d−1 (4 × 5 g) of Cr for 5 d + Maintenance phase: 3 g∙d−1 for 28 d | ↑ muscle TCr; ↔ muscle glycogen content; ↔ plasma glucose and insulin during OGTT; ↔ glucose-insulin index; ↔ index of insulin sensitivity. |
| Rooney et al. [51] | Healthy, vegetarian adults (14) | Controlled-trial | 5 g∙d−1 of Cr for 42 d | ↑ plasma total Cr concentration; ↑ plasma glucose response; ↔ plasma insulin. |
| Van Loon et al. [33] | Young, nonvegetarians adults (20) | Double-blind placebo-controlled trial | Loading phase: 20 g∙d−1 (4 × 5 g) of Cr for 5 d + Maintenance phase: 2 g∙d−1 for 6 wk | ↑ muscle glycogen, Cr and PCr after loading phase, with a decline in maintenance phase; ↔ GLUT-4 mRNA; ↔ total muscle GLUT-4 protein content. |
| Safdar et al. [34] | Young, healthy, nonobese men (12) | Double-blind, crossover, randomized, placebo-controlled trial | Loading phase: 20 g∙d−1 (4 × 5 g) of Cr for 3 d + Maintenance phase: 5 g∙d−1 for 7 d | ↑ muscle total Cr ↑ PKB/Akt1 expression and protein; ↑ MAPK expression; ↔ GLUT-4 mRNA |
| Rocic et al. [52] | Recently diagnosed T2DM patients, without anti-diabetic treatment (30) | Open-label, cross-over | 6 g∙d−1 of Cr or 1000 mg∙d−1 of metformin for 5 d | ↓ glucose concentration in both groups; ↔ insulin, C-peptide and, HbA1c. |
| Reference | Model | Creatine and Training Protocol | Main Findings |
|---|---|---|---|
| Souza et al. [59] | Male Wistar rats | Loading phase: 5 g∙kg−1 body weight of Cr for 7 d + Maintenance phase: 1 g∙d−1 for 8 wk Training: swimming | ↓ plasma glucose levels during 1–4 wk after Cr alone; ↓ plasma glucose levels during 1–8 wk after Cr and exercise protocol. |
| Freire et al. [61] | Male Wistar rats | Pallets enriched with 2% of Cr for 4 or 8 wk Training: swimming | ↔ glucose uptake; ↔ glucose AUC during OGTT; ↔ liver and quadriceps glycogen content. |
| Vaisy et al. [62] | Male Wistar rats | Cafeteria diet enriched with 2.5% of Cr for 12 wk Training: swimming | ↔ fasting blood glucose concentration; ↓ fasting plasma insulin level after training and training + creatine; ↓ whole body insulin level. |
| Araújo et al. [60] | Male Wistar rats | Loading phase: Chow containing 13% of Cr for 7d + Maintenance phase: Chow containing 2% of Cr for 55 d Training: high intensity treadmill running | ↔ glucose uptake; ↓ glucose AUC during OGTT after Cr and exercise protocol. |
| Reference | Sample (n) | Study Design | Creatine and Training Protocol | Main Findings |
|---|---|---|---|---|
| Op’t Eijnde et al. [9] | Young, healthy subjects (22) | Double-blind placebo-controlled trial | Loading phase: 20 g∙d−1 during immobilization period (2 wk) + Maintenance phase: 15 g∙d−1 for 3 wk followed by 5 g∙d−1 for 7 wk during rehabilitation training Program training: knee-extensor resistance training (3 times∙wk−1) | Immobilization period: ↓ 20% GLUT-4 in placebo group, but not in Cr group; ↔ glycogen and Cr muscle content in both groups. Rehabilitation period: ↑ 40% GLUT-4 in Cr group; ↑ glycogen and Cr muscle content after Cr. |
| Derave et al. [63] | Young, healthy subjects (22) | Double-blind, placebo-controlled trial | Loading phase: 15 g∙d−1 during immobilization period (2 wk) combined or not with protein supplementation + Maintenance phase: 2.5 g∙d−1 for 6 wk during rehabilitation training Program training: knee-extensor resistance training (3 times∙wk−1) | Immobilization period: ↓ GLUT-4 in placebo and Cr group, but not in Cr + protein group; ↔ glycogen and Cr muscle content in all groups. Rehabilitation period: ↑ 24% GLUT-4 in Cr group and ↑ 33% in Cr + protein group; ↑ glycogen and Cr muscle content after Cr and Cr + protein supplementation. |
| Gualano et al. [18] | Healthy, sedentary male (22) | Double-blind, randomized-placebo-controlled trial | Loading phase: 0.3 g∙kg−1∙d−1 of Cr for 1 wk + Maintenance phase: 0.15 g∙kg−1∙d−1 for 11 wk Program training: aerobic training at 70% of the VO2max | ↓ glucose AUC after OGTT; ↔ fasting insulin; ↔ HOMA-IR. |
| Gualano et al. [19] | T2DM patients (25) | Double-blind, randomized-placebo-controlled trial | 5 g∙d−1 of Cr for 12 wk Program training: Aerobic training and resistance training | ↓ HbA1c in Cr group; ↓ glycemia during MTT (0, 30 and 60 min) in Cr group; ↑ muscle PCr content in Cr group; ↑ muscle strength and function in Cr group |
| Alves et al. [38] | T2DM patients (25) | Double-blind, randomized-placebo-controlled trial | 5 g∙d−1 of Cr for 12 wk Program training: Aerobic training and resistance training | ↑ AMPK protein expression; ↔ IR-β, Akt1 and MAPK. |
| Oliveira et al. [64] | Healthy, older adults (32) | randomized, double-blind, placebo-controlled, parallel-group clinical trial | 5 g∙d−1 of Cr for 12 wk Program training: resistance training | ↔ inflammatory biomarkers ↔ fasting blood glucose; ↔ fasting insulin; ↔ HOMA-IR. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis, M.Y.; Artioli, G.G.; Gualano, B. Potential of Creatine in Glucose Management and Diabetes. Nutrients 2021, 13, 570. https://doi.org/10.3390/nu13020570
Solis MY, Artioli GG, Gualano B. Potential of Creatine in Glucose Management and Diabetes. Nutrients. 2021; 13(2):570. https://doi.org/10.3390/nu13020570
Chicago/Turabian StyleSolis, Marina Yazigi, Guilherme Giannini Artioli, and Bruno Gualano. 2021. "Potential of Creatine in Glucose Management and Diabetes" Nutrients 13, no. 2: 570. https://doi.org/10.3390/nu13020570
APA StyleSolis, M. Y., Artioli, G. G., & Gualano, B. (2021). Potential of Creatine in Glucose Management and Diabetes. Nutrients, 13(2), 570. https://doi.org/10.3390/nu13020570






