Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis
Abstract
1. Introduction
1.1. Disruption of the Microbiota-Gut Brain Axis
1.2. Nutrient-Microbiota Interactions and Gut-Brain Axis
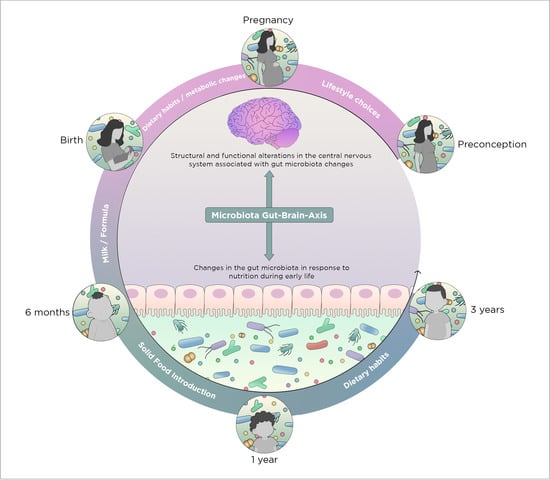
2. Early Nutrition-Microbiota Crosstalk in Sensitive Time Windows of Development
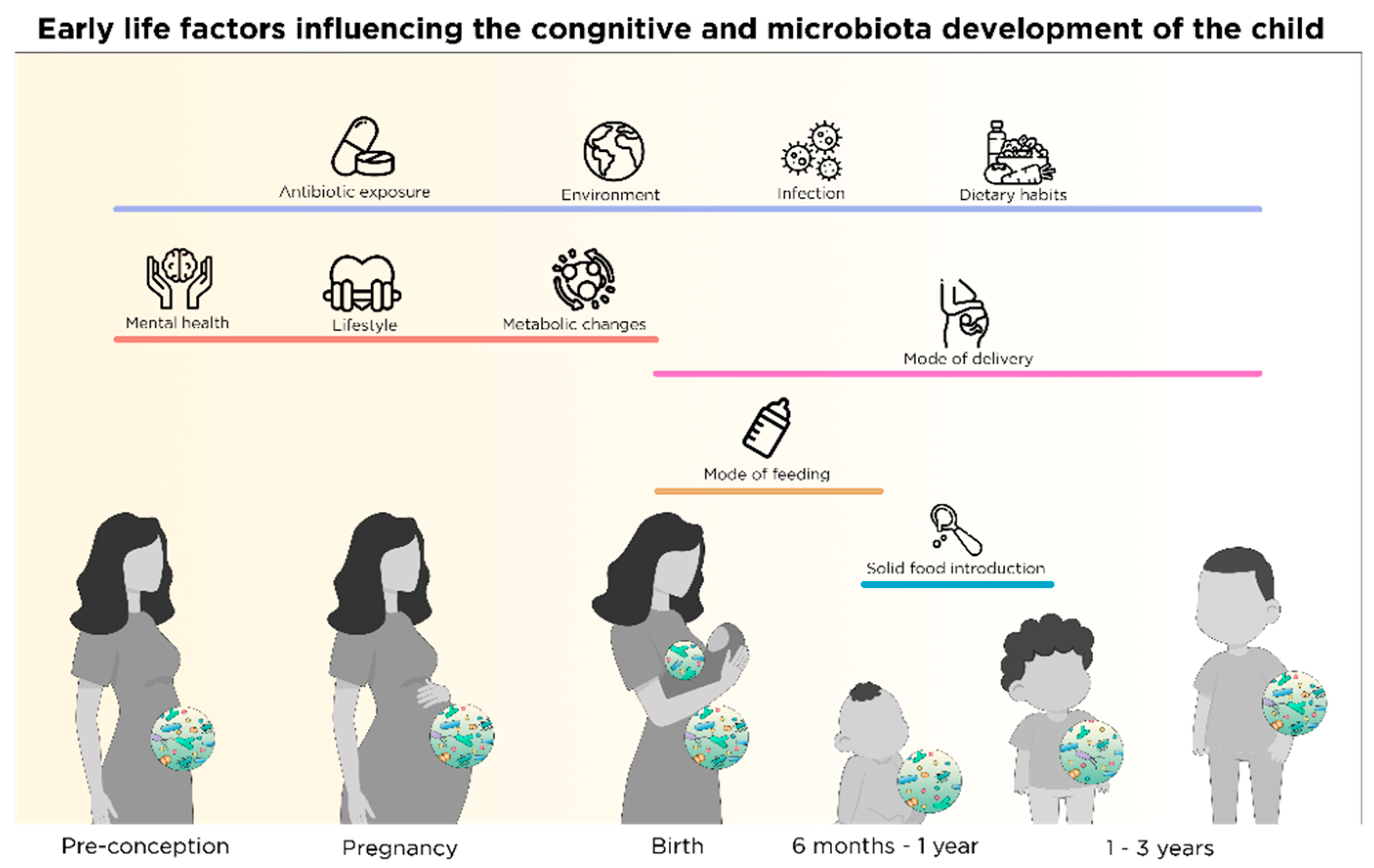
2.1. Nutritional and Microbial Regulation in Pre-Conception
2.2. Nutrition-Microbial Input on Neurodevelopment in Pregnancy
2.2.1. Maternal Nutrition and Fetal Neurodevelopment
2.2.2. Maternal Nutrition and Offspring Gut Microbiota Development
2.2.3. Maternal Microbial Signatures in Pregnancy Prime Fetal CNS
2.3. Mode of Birth and Microbiota
2.3.1. Vaginal Delivery and Vertical Transmission of Microbiota
2.3.2. Caesarean Section and the Missing Microbes
2.3.3. C-Section-Related Risks and Adversities
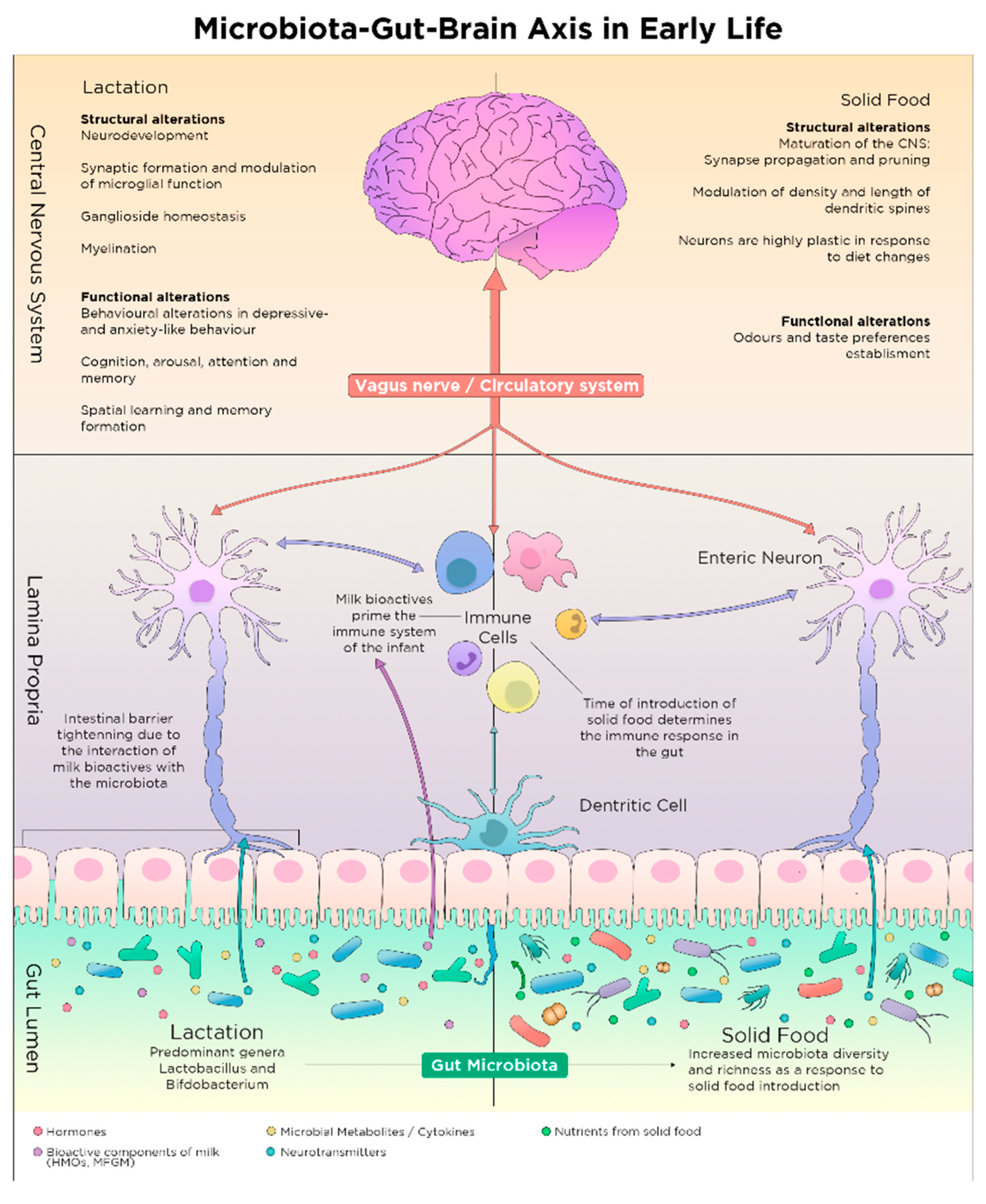
2.4. Shaping of Microbiota Composition and Neurodevelopment via Postnatal Early Life Nutrition
2.4.1. The Microbiota Expansion and the First Food after Birth
2.4.2. Breastfeeding and Composition of Breast Milk
Breastfeeding
Maternal Characteristics, Breast Milk and Child Development
Macro- and Micronutrient Composition of Breast Milk
Bioactive Components of Breast Milk
Milk Fat Globule Membrane
Human Milk Oligosaccharides and Sialic Acid
Breast Milk Microbiota
2.4.3. Infant Formula
Macronutrients in Infant Formula
Prebiotics, Probiotics and Synbiotics in Infant Formula
Infant Formula, Risks and Health Concerns
3. Microbiota Changes and Neurodevelopment: From Infancy to Childhood
3.1. Microbiota Maturation and Brain Development during Weaning
3.2. Solid Food Introduction and Establishment of Nutritional Habits
4. Early Life Microbiota: Another Component in the Vicious Cycle of Malnutrition
4.1. Undernutrition and CNS Development
4.2. Undernutrition and Microbiota
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Dierikx, T.; Visser, D.; Benninga, M.; Van Kaam, A.; De Boer, N.; De Vries, R.; Van Limbergen, J.; De Meij, T. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: A systematic review. J. Infect. 2020, 81, 190–204. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.G.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Genet. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Morais, L.H.; Iv, H.L.S.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Genet. 2020, 1–15. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2004, 55, 137–154. [Google Scholar]
- Berthoud, H.-R. Vagal and hormonal gut-brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic Gastrointestinal Inflammation Induces Anxiety-Like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterolgy 2010, 139, 2102–2112.e2101. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Clarke, G.; Quigley, E.M.; Groeger, J.A.; Dinan, T.G.; Cryan, J.F. Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 2012, 36, 310–340. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e593. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Spichak, S.; Guzzetta, K.E.; O’Leary, O.F.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Without a bug’s life: Germ-free rodents to interrogate microbiota-gut-neuroimmune interactions. Drug Discov. Today Dis. Model. 2018, 28, 79–93. [Google Scholar] [CrossRef]
- Verdu, E.F.; Bercik, P.; Verma-Gandhu, M.; Huang, X.-X.; Blennerhassett, P.; Jackson, W.; Mao, Y.; Wang, L.; Rochat, F.; Collins, S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006, 55, 182–190. [Google Scholar] [CrossRef]
- O’Mahony, S.; Felice, V.; Nally, K.; Savignac, H.; Claesson, M.; Scully, P.; Woznicki, J.; Hyland, N.; Shanahan, F.; Quigley, E.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef]
- Keogh, C.E.; Kim, D.H.; Pusceddu, M.M.; Knotts, T.A.; Rabasa, G.; Sladek, J.A.; Hsieh, M.T.; Honeycutt, M.; Brust-Mascher, I.; Barboza, M.; et al. Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav. Immun. 2021, 91, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kumpu, M.; Kekkonen, R.A.; De Vos, W.M. Lactobacillus rhamnosus GG Intake Modifies Preschool Children’s Intestinal Microbiota, Alleviates Penicillin-Associated Changes, and Reduces Antibiotic Use. PLoS ONE 2016, 11, e0154012. [Google Scholar] [CrossRef] [PubMed]
- Firestein, M.R.; Myers, M.M.; Austin, J.; Stark, R.I.; Barone, J.L.; Ludwig, R.J.; Welch, M.G. Perinatal antibiotics alter preterm infant EEG and neurobehavior in the Family Nurture Intervention trial. Dev. Psychobiol. 2019, 61, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra381. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2016, 595, 489–503. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nat. Cell Biol. 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Allais, L.; Kerckhof, F.-M.; Verschuere, S.; Bracke, K.R.; De Smet, R.; Laukens, D.; Abbeele, P.V.D.; De Vos, M.; Boon, N.; Brusselle, G.G.; et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. 2015, 18, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Sitarik, A.R.; Havstad, S.L.; Fujimura, K.E.; Wegienka, G.; Cassidy-Bushrow, A.E.; Kim, H.; Zoratti, E.M.; Lukacs, N.W.; Boushey, H.A.; et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci. Rep. 2016, 6, 31775. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, S.; Zeng, Z.; Ota, T. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Heal. Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: Comorbidity or causative mechanisms? BioEssays 2014, 36, 933–939. [Google Scholar] [CrossRef]
- Needham, B.D.; Tang, W.; Wu, W.-L. Searching for the gut microbial contributing factors to social behavior in rodent models of autism spectrum disorder. Dev. Neurobiol. 2018, 78, 474–499. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Lavebratt, C.; Yang, L.L.; Giacobini, M.; Forsell, Y.; Schalling, M.; Partonen, T.; Gissler, M. Early exposure to antibiotic drugs and risk for psychiatric disorders: A population-based study. Transl. Psychiatry 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Persaud, R.R.; Azad, M.B.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Kozyrskyj, A.L.; Laprise, C. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: A population-based study. J. Matern. Neonatal Med. 2015, 28, 1190–1195. [Google Scholar] [CrossRef]
- Delara, M.; McMillan, D.E.; Nickel, N.C.; Jong, G.W.; Seitz, D.P.; Mignone, J. Early life exposure to antibiotics and the risk of mood and anxiety disorders in children and adolescents: A population-based cohort study. J. Psychiatr. Res. 2020. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.G.; Stanton, C.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Trajectories: Role of Maternal and Early-Life Nutrition. Ann. Nutr. Metab. 2019, 74, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nat. Cell Biol. 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Jahromi, M.F.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; McCoy, K.D. Maternal microbiota in pregnancy and early life. Science 2019, 365, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Grote, V.; Kirchberg, F.F.; Prell, C.; Rzehak, P.; Uhl, O.; Weber, M. Long-Term Health Impact of Early Nutrition: The Power of Programming. Ann. Nutr. Metab. 2017, 70, 161–169. [Google Scholar] [CrossRef]
- Hullar, M.A.J.; Fu, B.C. Diet, the Gut Microbiome, and Epigenetics. Cancer J. 2014, 20, 170–175. [Google Scholar] [CrossRef]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef]
- Burggren, W.; Mueller, C.A. Developmental Critical Windows and Sensitive Periods as Three-Dimensional Constructs in Time and Space. Physiol. Biochem. Zool. 2015, 88, 91–102. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Dinan, T.G.; Cryan, J.F. Annual Research Review: Critical windows—The microbiota–gut–brain axis in neurocognitive development. J. Child Psychol. Psychiatry 2019, 61, 353–371. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Goyal, M.S.; Venkatesh, S.; Milbrandt, J.; Gordon, J.I.; Raichle, M.E. Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development. Proc. Natl. Acad. Sci. USA 2015, 112, 14105–14112. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.Y.; Fatemi, A.; Johnston, M.V. Cerebral plasticity: Windows of opportunity in the developing brain. Eur. J. Paediatr. Neurol. 2017, 21, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Huus, K.E.; Bauer, K.C.; Brown, E.M.; Bozorgmehr, T.; Woodward, S.E.; Serapio-Palacios, A.; Boutin, R.C.; Petersen, C.; Finlay, B.B. Commensal Bacteria Modulate Immunoglobulin A Binding in Response to Host Nutrition. Cell Host Microbe 2020, 27, 909–921.e905. [Google Scholar] [CrossRef] [PubMed]
- Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991, 338, 131–137. [CrossRef]
- Kinoshita, M.; Kayama, H.; Kusu, T.; Yamaguchi, T.; Kunisawa, J.; Kiyono, H.; Sakaguchi, S.; Takeda, K. Dietary Folic Acid Promotes Survival of Foxp3+ Regulatory T Cells in the Colon. J. Immunol. 2012, 189, 2869–2878. [Google Scholar] [CrossRef]
- Matok, I.; Gorodischer, R.; Koren, G.; Landau, D.; Wiznitzer, A.; Levy, A. Exposure to folic acid antagonists during the first trimester of pregnancy and the risk of major malformations. Br. J. Clin. Pharmacol. 2009, 68, 956–962. [Google Scholar] [CrossRef]
- Shere, M.; Bapat, P.; Nickel, C.; Kapur, B.; Koren, G. Association Between Use of Oral Contraceptives and Folate Status: A Systematic Review and Meta-Analysis. J. Obstet. Gynaecol. Can. 2015, 37, 430–438. [Google Scholar] [CrossRef]
- Feinberg, J.I.; Bakulski, K.M.; Jaffe, A.E.; Tryggvadottir, R.; Brown, S.C.; Goldman, L.R.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Fallin, M.D.; et al. Paternal sperm DNA methylation associated with early signs of autism risk in an autism-enriched cohort. Int. J. Epidemiol. 2015, 44, 1199–1210. [Google Scholar] [CrossRef]
- Short, A.K.; Yeshurun, S.; Powell, R.; Perreau, V.M.; Fox, A.; Kim, J.H.; Pang, T.Y.; Hannan, A.J. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl. Psychiatry 2017, 7, e1114. [Google Scholar] [CrossRef]
- Tyebji, S.; Hannan, A.J.; Tonkin, C.J. Pathogenic Infection in Male Mice Changes Sperm Small RNA Profiles and Transgenerationally Alters Offspring Behavior. Cell Rep. 2020, 31, 107573. [Google Scholar] [CrossRef]
- Marques, A.H.; O’Connor, T.G.; Eroth, C.; Susser, E.; Ebjørke-Monsen, A.-L. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front. Neurosci. 2013, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Wu, R.Y.; Jenmalm, M.C. Gut microbiota and allergy: The importance of the pregnancy period. Pediatr. Res. 2015, 77, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, A.J.; De Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Rackaityte, E.; Halkias, J.; Fukui, E.M.; Mendoza, V.F.; Hayzelden, C.; Crawford, E.D.; Fujimura, K.E.; Burt, T.D.; Lynch, S.V. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020, 26, 599–607. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A Structural MRI Study of Human Brain Development from Birth to 2 Years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2009, 35, 147–168. [Google Scholar] [CrossRef]
- Knudsen, E.I. Sensitive Periods in the Development of the Brain and Behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef]
- Brunton, P.J. Effects of maternal exposure to social stress during pregnancy: Consequences for mother and offspring. Reproduction 2013, 146, R175–R189. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.J.; Korosi, A.; Layé, S.; Shukitt-Hale, B.; Barrientos, R.M. Food for thought: How nutrition impacts cognition and emotion. npj Sci. Food 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee on Military Nutrition. Food Components to Enhance Performance: An Evaluation of Potential Performance-Enhancing Food Components for Operational Rations; Marriott, B.M., Ed.; National Academies Press: Washington, DC, USA, 1940. [Google Scholar] [CrossRef]
- Tran, T.D.; Biggs, B.-A.; Tran, T.; Simpson, J.A.; Hanieh, S.; Dwyer, T.; Fisher, J. Impact on Infants’ Cognitive Development of Antenatal Exposure to Iron Deficiency Disorder and Common Mental Disorders. PLoS ONE 2013, 8, e74876. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Ars, C.L.; Nijs, I.M.; Marroun, H.E.; Muetzel, R.; Schmidt, M.; Graaff, J.S.-D.; Van Der Lugt, A.; Jaddoe, V.W.; Hofman, A.; Steegers, E.A.; et al. Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: The Generation R Study. Br. J. Nutr. 2016, 122, S1–S9. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef]
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef]
- Collado, M.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Shin, H.; Pizoni, A.; Werlang, I.C.; Matte, U.; Goldani, M.Z.; Goldani, H.A.S.; Dominguez-Bello, M.G. Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci. Rep. 2016, 6, 23133. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H.M.; Daya, S.; Berns, K.I. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed]
- Al Rubaye, H.; Adamson, C.C.; Jadavji, N.M. The role of maternal diet on offspring gut microbiota development: A review. J. Neurosci. Res. 2021, 99, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Hallam, M.C.; Barile, D.; Meyrand, M.; German, J.B.; Reimer, R.A. Maternal high-protein or high-prebiotic-fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity 2014, 22, 2344–2351. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Popkov, V.A.; Silachev, D.N.; Jankauskas, S.S.; Zorova, L.D.; Pevzner, I.B.; Babenko, V.A.; Plotnikov, E.Y.; Zorov, D.B. Molecular and cellular interactions between mother and fetus. Pregnancy as a rejuvenating factor. Biochemistry 2016, 81, 1480–1487. [Google Scholar] [CrossRef]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocr. 2019, 55, 100797. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- De Goffau, M.C.; Charnock-Jones, D.S.; Smith, G.C.S.; Parkhill, J. Batch effects account for the main findings of an in utero human intestinal bacterial colonization study. Microbiome 2021, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Hornef, M.W. A philosophical perspective on the prenatal in utero microbiome debate. Microbiome 2021, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics Modulate Host-Microbe Interaction in the Placenta and Fetal Gut: A Randomized, Double-Blind, Placebo-Controlled Trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nat. Cell Biol. 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Morrison, K.E.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef]
- Girchenko, P.; Lahti-Pulkkinen, M.; Heinonen, K.; Reynolds, R.M.; Laivuori, H.; Lipsanen, J.; Villa, P.M.; Hämäläinen, E.; Kajantie, E.; Lahti, J.; et al. Persistently High Levels of Maternal Antenatal Inflammation Are Associated With and Mediate the Effect of Prenatal Environmental Adversities on Neurodevelopmental Delay in the Offspring. Biol. Psychiatry 2020, 87, 898–907. [Google Scholar] [CrossRef]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Göpel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. BioMed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Ream, M.A.; Lehwald, L. Neurologic Consequences of Preterm Birth. Curr. Neurol. Neurosci. Rep. 2018, 18, 48. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nat. Cell Biol. 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Maternal Vertical Transmission Affecting Early-life Microbiota Development. Trends Microbiol. 2020, 28, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F.; Filion, K.B.; et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010, 86 (Suppl. 1), 13–15. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. Can. Med Assoc. J. 2013, 185, 385–394. [Google Scholar] [CrossRef]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 1–18. [Google Scholar] [CrossRef]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.-A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast Milk Microbiota Is Shaped by Mode of Delivery and Intrapartum Antibiotic Exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, S.K.; Oliva-Hemker, M. Fecal microbiota transplantation in children: A brief review. Pediatr. Res. 2016, 80, 2–6. [Google Scholar] [CrossRef]
- Korpela, K.; Helve, O.; Kolho, K.-L.; Saisto, T.; Skogberg, K.; Dikareva, E.; Stefanovic, V.; Salonen, A.; Andersson, S.; De Vos, W.M. Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 2020, 183, 324–334.e325. [Google Scholar] [CrossRef] [PubMed]
- McCann, A.; Ryan, F.J.; Stockdale, S.R.; Dalmasso, M.; Blake, T.; Ryan, C.A.; Stanton, C.; Mills, S.; Ross, P.R.; Hill, C. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ 2018, 6, e4694. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Rodgers, R.; Rodriguez, C.; Handley, S.A.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Lim, E.S.; Holtz, L.R. Discordant transmission of bacteria and viruses from mothers to babies at birth. Microbiome 2019, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Scholtens, S.; De Jongste, J.C.; Wijga, A.H.; Gerritsen, J.; Postma, D.S.; Brunekreef, B.; Hoekstra, M.O.; Aalberse, R.; Smit, H.A. Asthma at 8 years of age in children born by caesarean section. Thorax 2009, 64, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e641-6455. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.A.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef]
- Blustein, J.; Attina, T.M.; Liu, M.; Ryan, A.M.; Cox, L.M.; Blaser, M.J.; Trasande, L. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int. J. Obes. 2013, 37, 900–906. [Google Scholar] [CrossRef]
- Montoya-Williams, D.; Lemas, D.J.; Spiryda, L.; Patel, K.; Carney, O.O.; Neu, J.; Carson, T.L. The Neonatal Microbiome and Its Partial Role in Mediating the Association between Birth by Cesarean Section and Adverse Pediatric Outcomes. Neonatology 2018, 114, 103–111. [Google Scholar] [CrossRef]
- Al Khalaf, S.Y.; O’Neill, S.M.; O’Keeffe, L.M.; Henriksen, T.B.; Kenny, L.C.; Cryan, J.F.; Khashan, A.S. The impact of obstetric mode of delivery on childhood behavior. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Curran, E.A.; Kenny, L.C.; Dalman, C.; Kearney, P.M.; Cryan, J.F.; Dinan, T.G.; Khashan, A.S. Birth by caesarean section and school performance in Swedish adolescents- a population-based study. BMC Pregnancy Childbirth 2017, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Slykerman, R.F.; Li, E.; Milne, B.J. Birth by caesarean section and educational achievement in adolescents. Aust. N. Zealand J. Obstet. Gynaecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, M.; McCarthy, F.P.; O’Keeffe, G.W.; Khashan, A.S. The association between caesarean section and cognitive ability in childhood. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 55, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ruiz, A.; Mosley, M.; Jacobs, A.J.; Hoffiz, Y.C.; Forger, N.G. Birth delivery mode alters perinatal cell death in the mouse brain. Proc. Natl. Acad. Sci. USA 2018, 115, 11826–11831. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.; Guimond, D.; Tyzio, R.; Pons-Bennaceur, A.; Lozovaya, N.; Burnashev, N.; Ferrari, D.C.; Ben-Ari, Y. Term or Preterm Cesarean Section Delivery Does Not Lead to Long-term Detrimental Consequences in Mice. Cereb. Cortex 2019, 29, 2424–2436. [Google Scholar] [CrossRef]
- Morais, L.H.; Golubeva, A.V.; Moloney, G.M.; Moya-Pérez, A.; Ventura-Silva, A.P.; Arboleya, S.; Bastiaanssen, T.F.; O’Sullivan, O.; Rea, K.; Borre, Y.; et al. Enduring Behavioral Effects Induced by Birth by Caesarean Section in the Mouse. Curr. Biol. 2020, 30, 3761–3774.e3766. [Google Scholar] [CrossRef]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Denning, P.W. Probiotic Bacteria Induce Maturation of Intestinal Claudin 3 Expression and Barrier Function. Am. J. Pathol. 2012, 180, 626–635. [Google Scholar] [CrossRef]
- Bergmann, K.R.; Liu, S.X.; Tian, R.; Kushnir, A.; Turner, J.R.; Li, H.-L.; Chou, P.M.; Weber, C.R.; De Plaen, I.G. Bifidobacteria Stabilize Claudins at Tight Junctions and Prevent Intestinal Barrier Dysfunction in Mouse Necrotizing Enterocolitis. Am. J. Pathol. 2013, 182, 1595–1606. [Google Scholar] [CrossRef]
- Luck, B.; Engevik, M.A.; Ganesh, B.P.; Lackey, E.P.; Lin, T.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Sillitoe, R.V.; et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 2020, 10, 7737. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; Van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Beidelman, A.I.; Schanler, R.J. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Valentine, C.J.; Wagner, C.L. Nutritional Management of the Breastfeeding Dyad. Pediatr. Clin. N. Am. 2013, 60, 261–274. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Neville, M.C.; Anderson, S.M.; McManaman, J.L.; Badger, T.M.; Bunik, M.; Contractor, N.; Crume, T.; Dabelea, D.; Donovan, S.M.; Forman, N.; et al. Lactation and Neonatal Nutrition: Defining and Refining the Critical Questions. J. Mammary Gland. Biol. Neoplasia 2012, 17, 167–188. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 2012, CD003517. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med Clin. N. Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and immunological factors in breast milk: A role in the intergenerational transmission from maternal psychopathology to child development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef]
- Emmett, P.M.; Rogers, I.S. Properties of human milk and their relationship with maternal nutrition. Early Hum. Dev. 1997, 49, S7–S28. [Google Scholar] [CrossRef]
- Butte, N.F.; King, J.C. Energy requirements during pregnancy and lactation. Public Heal. Nutr. 2005, 8, 1010–1027. [Google Scholar] [CrossRef]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lönnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- Horta, B.L.; De Sousa, B.A.; De Mola, C.L. Breastfeeding and neurodevelopmental outcomes. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Heron, J.; Francomb, H.; Oke, S.; Golding, J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001, 323, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: A cross-national, ecological analysis. J. Affect. Disord. 2002, 69, 15–29. [Google Scholar] [CrossRef]
- Keim, S.A.; Daniels, J.L.; Siega-Riz, A.M.; Dole, N.; Herring, A.H.; Scheidt, P.C. Depressive Symptoms during Pregnancy and the Concentration of Fatty Acids in Breast Milk. J. Hum. Lact. 2012, 28, 189–195. [Google Scholar] [CrossRef]
- Thibeau, S.; D’Apolito, K.; Minnick, A.F.; Dietrich, M.S.; Kane, B.; Cooley, S.; Groer, M. Relationships of Maternal Stress with Milk Immune Components in African American Mothers of Healthy Term Infants. Breastfeed. Med. 2016, 11, 6–14. [Google Scholar] [CrossRef]
- Garofalo, R.P. Cytokines in Human Milk. J. Pediatr. 2010, 156, S36–S40. [Google Scholar] [CrossRef]
- Dewey, K.G.; Heinig, M.J.; A Nommsen, L.; Lonnerdal, B. Maternal versus infant factors related to breast milk intake and residual milk volume: The DARLING study. Pediatrics 1991, 87, 829–837. [Google Scholar]
- Picciano, M.F. Pregnancy and Lactation: Physiological Adjustments, Nutritional Requirements and the Role of Dietary Supplements. J. Nutr. 2003, 133, 1997S–2002S. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A. Human Milk for the Premature Infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288.e1275. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef]
- Jackson, J.G.; Janszen, D.B.; Lonnerdal, B.; Lien, E.L.; Pramuk, K.P.; Kuhlman, C.F. A multinational study of α-lactalbumin concentrations in human milk. J. Nutr. Biochem. 2004, 15, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, A.; Rueda, M.S.; Turin, C.G.; Ochoa, T.J. Factors affecting lactoferrin concentration in human milk: How much do we know? Biochem. Cell Biol. 2017, 95, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Karmaus, W.; Davis, S.R.; Gangur, V. Review: Immune Markers in Breast Milk and Fetal and Maternal Body Fluids: A Systematic Review of Perinatal Concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Siziba, L.P.; Lorenz, L.; Stahl, B.; Mank, M.; Marosvölgyi, T.; Décsi, T.; Rothenbacher, D.; Genuneit, J. Mank Changes in Human Milk Fatty Acid Composition during Lactation: The Ulm SPATZ Health Study. Nutrients 2019, 11, 2842. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef]
- Hernell, O.; Timby, N.; Domellöf, M.; Lönnerdal, B. Clinical Benefits of Milk Fat Globule Membranes for Infants and Children. J. Pediatr. 2016, 173, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, F.; Yang, H.; Li, J.; Li, Y.; Ding, X.; Xiong, X.; Yin, Y. Effects of dietary supplementation with epidermal growth factor on nutrient digestibility, intestinal development and expression of nutrient transporters in early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 618–625. [Google Scholar] [CrossRef]
- Khailova, L.; Dvorak, K.; Arganbright, K.M.; Williams, C.S.; Halpern, M.D.; Dvorak, B. Changes in Hepatic Cell Junctions Structure During Experimental Necrotizing Enterocolitis: Effect of EGF Treatment. Pediatr. Res. 2009, 66, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.; Klotz, M.; Hirschberg, D.L.; Waldura, B.; Schofer, O.; Ehnert, S.; Schwarz, L.K.; Van Ginneken, C.; Schäfer, K.-H. Breast milk contains relevant neurotrophic factors and cytokines for enteric nervous system development. Mol. Nutr. Food Res. 2011, 55, 1592–1596. [Google Scholar] [CrossRef]
- Boesmans, W.; Gomes, P.; Janssens, J.; Tack, J.; Berghe, P.V. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut 2008, 57, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shiou, S.-R.; Yu, Y.; Chen, S.; Ciancio, M.J.; Petrof, E.O.; Sun, J.; Claud, E.C. Erythropoietin Protects Intestinal Epithelial Barrier Function and Lowers the Incidence of Experimental Neonatal Necrotizing Enterocolitis. J. Biol. Chem. 2011, 286, 12123–12132. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Woo, J.G.; Morrow, A.L. Characteristics and Potential Functions of Human Milk Adiponectin. J. Pediatr. 2010, 156, S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.G.; Guerrero, M.L.; Guo, F.; Martin, L.J.; Davidson, B.S.; Ortega, H.; Ruiz-Palacios, G.M.; Morrow, A.L. Human Milk Adiponectin Affects Infant Weight Trajectory During the Second Year of Life. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Dündar, N.O.; Dundar, B.; Cesur, G.; Yılmaz, N.; Sutcu, R.; Ozguner, F.; Yilmaz, N. Ghrelin and adiponectin levels in colostrum, cord blood and maternal serum. Pediatr. Int. 2010, 52, 622–625. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Gordon, I.; Zagoory-Sharon, O.; Leckman, J.F.; Feldman, R. Oxytocin, cortisol, and triadic family interactions. Physiol. Behav. 2010, 101, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, X.; Maasen, S. Oxytocin: From a Hormone for Birth to a Social Hormone: The Hormonal Governance of Sociability aka Society. Ntm 2018, 26, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Gura, T. Nature’s first functional food. Science 2014, 345, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; DiFilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size: Structure-activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef]
- Lemaire, M.; Le Huërou-Luron, I.; Blat, S. Effects of infant formula composition on long-term metabolic health. J. Dev. Orig. Heal. Dis. 2018, 9, 573–589. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2’-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Somasundaram, I.; Dhanasekaran, M.; Rajkumar, J.S.; Sudarsanam, D. Exploring the stem cell and non-stem cell constituents of human breast milk. Cytotechnology 2012, 65, 385–393. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C.M. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef]
- Peterson, J.A.; Hamosh, M.; Scallan, C.D.; Ceriani, R.L.; Henderson, T.R.; Mehta, N.R.; Armand, M.; Hamosh, P. Milk Fat Globule Glycoproteins in Human Milk and in Gastric Aspirates of Mother’s Milk-Fed Preterm Infants. Pediatr. Res. 1998, 44, 499–506. [Google Scholar] [CrossRef]
- Pacheco, A.R.; Barile, D.; Underwood, M.A.; Mills, D.A. The impact of the milk glycobiome on the neonate gut microbiota. Annu. Rev. Anim. Biosci. 2015, 3, 419–445. [Google Scholar] [CrossRef]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Bharatharaj, J.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Mahony, S.M.O.; Neufeld, K.M.; Waworuntu, R.V.; Pusceddu, M.M.; Manurung, S.; Murphy, K.; Strain, C.; Laguna, M.C.; Peterson, V.L.; Stanton, C.; et al. The enduring effects of early-life stress on the microbiota–gut–brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur. J. Neurosci. 2020, 51, 1042–1058. [Google Scholar] [CrossRef]
- Nishimaru, H.; Restrepo, C.E.; Ryge, J.; Yanagawa, Y.; Kiehn, O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc. Natl. Acad. Sci. USA 2005, 102, 5245–5249. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Schverer, M.; O’Mahony, S.M.; O’Riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The Human Milk Metabolome Reveals Diverse Oligosaccharide Profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Stuebe, A.; Seed, P.C.; LaTuga, M. A Review of the Source and Function of Microbiota in Breast Milk. Semin. Reprod. Med. 2014, 32, 068–073. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Aakko, J.; Kumar, H.; Rautava, S.; Wise, A.; Autran, C.; Bode, L.; Isolauri, E.; Salminen, S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef. Microbes 2017, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Schols, H.A.; Van Zoeren, D.; Van Lingen, R.A.; Jebbink, L.J.G.; Heuvel, E.G.V.D.; Voragen, A.G.; Gruppen, H. Oligosaccharides in feces of breast- and formula-fed babies. Carbohydr. Res. 2011, 346, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal Microbiota Composition of Breast-Fed Infants Is Correlated With Human Milk Oligosaccharides Consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef]
- Borewicz, K.; Gu, F.; Saccenti, E.; Hechler, C.; Beijers, R.; De Weerth, C.; Van Leeuwen, S.S.; Schols, H.A.; Smidt, H. The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Sci. Rep. 2020, 10, 4270. [Google Scholar] [CrossRef]
- Bruggencate, S.J.M.T.; Bovee-Oudenhoven, I.M.J.; Feitsma, A.L.; Van Hoffen, E.; Schoterman, M.H.C. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef]
- Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Martínez-Lara, E.; Blanco, S.; Martín, M.J.; Castanys, E.; Buck, R.; et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J. Nutr. Biochem. 2015, 26, 455–465. [Google Scholar] [CrossRef]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2′-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Human and Bovine Milk Oligosaccharides Elicit Improved Recognition Memory Concurrent With Alterations in Regional Brain Volumes and Hippocampal mRNA Expression. Front. Neurosci. 2020, 14, 770. [Google Scholar] [CrossRef]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 5–510. [Google Scholar] [CrossRef] [PubMed]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated Oligosaccharides and Glycoconjugates of Human Milk. The Impact on Infant and Newborn Protection, Development and Well-Being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brandmiller, J.C. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Obelitz-Ryom, K.; Bering, S.B.; Overgaard, S.H.; Eskildsen, S.F.; Ringgaard, S.; Olesen, J.L.; Skovgaard, K.; Pankratova, S.; Wang, B.; Brunse, A.; et al. Bovine Milk Oligosaccharides with Sialyllactose Improves Cognition in Preterm Pigs. Nutrients 2019, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Fleming, S.A.; Labhart, B.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary Sialyllactose Influences Sialic Acid Concentrations in the Prefrontal Cortex and Magnetic Resonance Imaging Measures in Corpus Callosum of Young Pigs. Nutrients 2017, 9, 1297. [Google Scholar] [CrossRef]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramirez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef]
- Roger, L.C.; Costabile, A.; Holland, D.T.; Hoyles, L.; McCartney, A.L. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology 2010, 156, 3329–3341. [Google Scholar] [CrossRef]
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and Bifidobacteria in Human Breast Milk: Influence of Antibiotherapy and Other Host and Clinical Factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nat. Cell Biol. 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.E.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the Diversity and Temporal Stability of Bacterial Communities in Human Milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Kent, J.C.; Owens, R.A.; Hartmann, P.E. Ultrasound Imaging of Milk Ejection in the Breast of Lactating Women. Pediatrics 2004, 113, 361–367. [Google Scholar] [CrossRef]
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons From Maternal Cells? Pediatr. 2007, 119, e724–e732. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc. Nutr. Soc. 2010, 69, 407–415. [Google Scholar] [CrossRef]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of Bifidobacteria within the Infant Gut Microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; De Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e324. [Google Scholar] [CrossRef]
- Moschen, A.R.; Wieser, V.; Tilg, H. Dietary Factors: Major Regulators of the Gut’s Microbiota. Gut Liver 2012, 6, 411–416. [Google Scholar] [CrossRef]
- Shively, C.A.; Register, T.C.; Appt, S.E.; Clarkson, T.B.; Uberseder, B.; Clear, K.Y.; Wilson, A.S.; Chiba, A.; Tooze, J.A.; Cook, K.L. Consumption of Mediterranean versus Western Diet Leads to Distinct Mammary Gland Microbiome Populations. Cell Rep. 2018, 25, 47–56.e3. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services; World Health Organization: Geneva, Switzerland, 2017; 120p. [Google Scholar]
- Koletzko, B.; Von Kries, R.; Monasterolo, R.C.; Subías, J.E.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Anton, B.; Gruszfeld, D.; et al. Can infant feeding choices modulate later obesity risk? Am. J. Clin. Nutr. 2009, 89, 1502S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Luque, V.; Ferré, N.; Mendez-Riera, G.; Koletzko, B.; Grote, V.; Demmelmair, H.; Bluck, L.; Wright, A.; Closa-Monasterolo, R.; et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. Int. J. Obes. 2012, 36, 548–553. [Google Scholar] [CrossRef]
- Ailhaud, G.P.; Massiera, F.; Weill, P.; Legrand, P.; Alessandri, J.-M.; Guesnet, P. Temporal changes in dietary fats: Role of n−6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 2006, 45, 203–236. [Google Scholar] [CrossRef]
- Oosting, A.; Kegler, D.; Wopereis, H.J.; Teller, I.C.; Van De Heijning, B.J.M.; Verkade, H.J.; Van Der Beek, E.M. Size and phospholipid coating of lipid droplets in the diet of young mice modify body fat accumulation in adulthood. Pediatr. Res. 2012, 72, 362–369. [Google Scholar] [CrossRef]
- Lasekan, J.; Jacobs, J.; Reisinger, K.S.; Montalto, M.B.; Frantz, M.P.; Blatter, M.M. Lactose-Free Milk Protein-Based Infant Formula: Impact on Growth and Gastrointestinal Tolerance in Infants. Clin. Pediatr. 2011, 50, 330–337. [Google Scholar] [CrossRef]
- Einerhand, A. Infant formula brought closer to breast milk thanks to prebiotic oligosaccharides. Agro Food Ind. Hi-Tech 2016, 27, 20–24. [Google Scholar]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The Influence of Milk Oligosaccharides on Microbiota of Infants: Opportunities for Formulas. Annu. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Książyk, J.; Lagström, H.; Sánchez-Luna, M.; Migacheva, N.B.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mugambi, M.N.; Musekiwa, A.; Lombard, M.; Young, T.; Blaauw, R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: A systematic review. Nutr. J. 2012, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.E.; Stahl, B.; Fanaro, S.; Jelinek, J.; Boehm, G.; Coppa, G.V. Dietary prebiotic oligosaccharides are detectable in the faeces of formula-fed infants. Acta Paediatr. 2005, 94, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ben, X.-M.; Li, J.; Feng, Z.-T.; Shi, S.-Y.; Lu, Y.-D.; Chen, R.; Zhou, X.-Y. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J. Gastroenterol. 2008, 14, 6564–6568. [Google Scholar] [CrossRef]
- Scholtens, P.A.M.J.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.J.; Knol, J.; Vandenplas, Y. Fecal Secretory Immunoglobulin A Is Increased in Healthy Infants Who Receive a Formula with Short-Chain Galacto-Oligosaccharides and Long-Chain Fructo-Oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J. Probiotics and Prebiotics in Infant Formulae. Prebiotics Probiotics Potential Benefits Nutr. Health 2019. [Google Scholar] [CrossRef]
- Lee, L.Y.; Bharani, R.; Biswas, A.; Lee, J.; Tran, L.-A.; Pecquet, S.; Steenhout, P. Normal growth of infants receiving an infant formula containing Lactobacillus reuteri, galacto-oligosaccharides, and fructo-oligosaccharide: A randomized controlled trial. Matern. Health Neonatol. Perinatol. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brüssow, H.; Szajewska, H.; et al. Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I -3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczyński, M.; Szymanski, H.; Sadowska-Krawczenko, I.; Piwowarczyk, A.; Rasmussen, P.B.; Kristensen, M.B.; E West, C.; Hernell, O. Effects of infant formula supplemented with prebiotics compared with synbiotics on growth up to the age of 12 mo: A randomized controlled trial. Pediatr. Res. 2017, 81, 752–758. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Sailajanandan, P.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nat. Cell Biol. 2017, 548, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Alterio, A.; Bugianesi, E.; Ciampalini, P.; Mariani, P.; Finocchi, M.; Agostoni, C.; Nobili, V. Insulin Dynamics of Breast- or Formula-Fed Overweight and Obese Children. J. Am. Coll. Nutr. 2011, 30, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Subias, J.E.; Scaglioni, S.; Verduci, E.; Dain, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Oddy, W.H.; Mearin, M.L.; Grote, V.; Mori, T.A.; Szajewska, H.; Shamir, R.; Koletzko, S.; Weber, M.; Beilin, L.J.; et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am. J. Clin. Nutr. 2017, 106, 568–580. [Google Scholar] [CrossRef]
- Alvisi, P.; Brusa, S.; Alboresi, S.; Amarri, S.; Bottau, P.; Cavagni, G.; Corradini, B.; Landi, L.; Loroni, L.; Marani, M.; et al. Recommendations on complementary feeding for healthy, full-term infants. Ital. J. Pediatr. 2015, 41, 36. [Google Scholar] [CrossRef]
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First Foods and Gut Microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef]
- Nicklaus, S.; Remy, E. Early Origins of Overeating: Tracking Between Early Food Habits and Later Eating Patterns. Curr. Obes. Rep. 2013, 2, 179–184. [Google Scholar] [CrossRef]
- Johnson, E.W.; Eller, P.; Jafek, B. Distribution of OMP-, PGP 9.5 and CaBP-like immunoreactive chemoreceptor neurons in the developing human olfactory epithelium. Anat. Embryol. 2004, 191, 311–317. [Google Scholar] [CrossRef]
- Lipchock, S.V.; Reed, D.R.; Mennella, J.A. The Gustatory and Olfactory Systems During Infancy: Implications for Development of Feeding Behaviors in the High-Risk Neonate. Clin. Perinatol. 2011, 38, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2019, 78, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Dörsam, A.F.; Preissl, H.; Micali, N.; Lörcher, S.B.; Zipfel, S.; Giel, K. The Impact of Maternal Eating Disorders on Dietary Intake and Eating Patterns during Pregnancy: A Systematic Review. Nutrients 2019, 11, 840. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, X.; Li, J.; He, L.; Xu, M. Effects of early-life malnutrition on neurodevelopment and neuropsychiatric disorders and the potential mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 83, 64–75. [Google Scholar] [CrossRef]
- Wells, J.C.K. The capacity–load model of non-communicable disease risk: Understanding the effects of child malnutrition, ethnicity and the social determinants of health. Eur. J. Clin. Nutr. 2018, 72, 688–697. [Google Scholar] [CrossRef]
- Ozkale, M.; Sipahi, T. Hematologic and Bone Marrow Changes in Children with Protein-Energy Malnutrition. Pediatr. Hematol. Oncol. 2013, 31, 349–358. [Google Scholar] [CrossRef]
- Rytter, M.J.H.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The Immune System in Children with Malnutrition—A Systematic Review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Diallo, A.; Raoult, D. Gut microbiota and malnutrition. Microb. Pathog. 2017, 106, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Antonow-Schlorke, I.; Schwab, M.; Cox, L.A.; Li, C.; Stuchlik, K.; Witte, O.W.; Nathanielsz, P.W.; McDonald, T.J. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc. Natl. Acad. Sci. USA 2011, 108, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.C.; De Tomás, M.E.; Serres, C.; Mercuri, O. Protein-energy malnutrition during gestation and lactation in rats affects growth rate, brain development and essential fatty acid metabolism. J. Nutr. 1995, 125, 1017–1024. [Google Scholar] [PubMed]
- Wang, L.; Xu, R.-J. The effects of perinatal protein malnutrition on spatial learning and memory behaviour and brain-derived neurotrophic factor concentration in the brain tissue in young rats. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1.), 467–472. [Google Scholar] [PubMed]
- Scrimshaw, N.S. Malnutrition, brain development, learning, and behavior. Nutr. Res. 1998, 18, 351–379. [Google Scholar] [CrossRef]
- De Souza, A.S.; Spreafico, F.; Carmo, M.D.G.T.D. Effects of maternal malnutrition and postnatal nutritional rehabilitation on brain fatty acids, learning, and memory. Nutr. Rev. 2011, 69, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nat. Cell Biol. 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Smythe, P. Changes in intestinal bacterial flora and role of infection in kwashiorkor. Lancet 1958, 272, 724–727. [Google Scholar] [CrossRef]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Blanton, L.V.; Barratt, M.J.; Charbonneau, M.R.; Ahmed, T.; Gordon, J.I. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016, 352, 1533. [Google Scholar] [CrossRef]
- Gehrig, J.L.; Venkatesh, S.; Chang, H.-W.; Hibberd, M.C.; Kung, V.L.; Cheng, J.; Chen, R.Y.; Subramanian, S.; Cowardin, C.A.; Meier, M.F.; et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 2019, 365, eaau4732. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.V.; Dinh, D.M.; Ward, H.D. Childhood malnutrition and the intestinal microbiome. Pediatr. Res. 2015, 77, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.H.N.; Ramdath, D. Free Radicals in the Pathogenesis of Kwashiorkor. Proc. Nutr. Soc. 1987, 46, 53–68. [Google Scholar] [CrossRef] [PubMed]
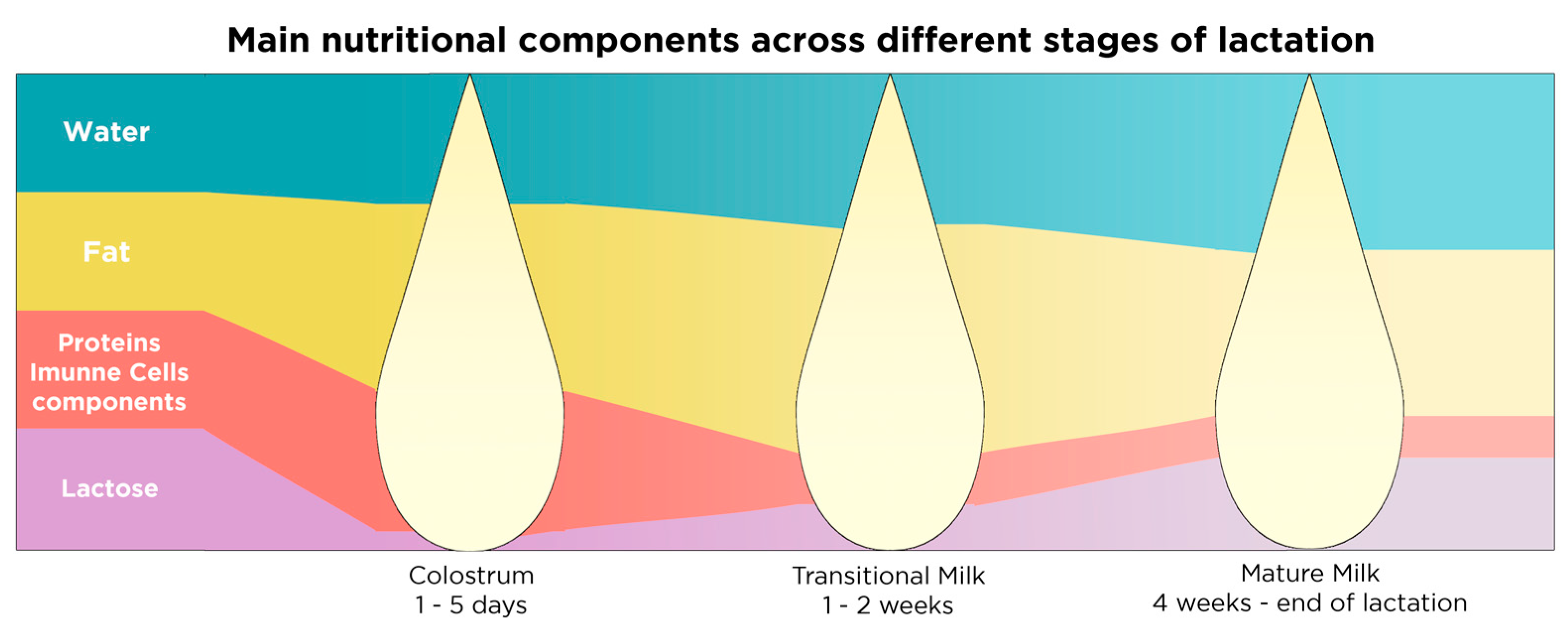
| Stages of Lactation | Duration | Components | Effect on | References |
|---|---|---|---|---|
| Stage 1: Colostrum | 1–5 days postnatally | Very rich in immunoglobulins, lactoferrin, leukocytes, growth factors, vitamins A and E, proteins and fat low quantities of lactose | Immune system development | [147,151] |
| Stage 2: Transitional milk | 1–2 weeks postnatally | Richer in lactose and fat compared to colostrum, richer in proteins and fat compared to mature milk | Nutritional needs of the baby | [147,151] |
| Stage 3: Mature milk | 1st month–end of lactation | Richer in lactose and water compared to colostrum and transitional milk | Nutritional needs of the baby | [151] |
| Richer in vitamins B1 and B6 compared to colostrum and transitional milk | [147,151] |
| Category of Molecule | Breastmilk Component | Subcategory of Molecule | Effect on | References | Population | Highly Present in which Stage of Lactation |
|---|---|---|---|---|---|---|
| Macronutrients | Casein | Proteins | Immune system development, general growth and development | [166] | Humans: multiple | More abundant in colostrum compared to mature milk |
| α-Lactalbumin | [167] | Humans: multiple | ||||
| Lactoferrin | [168] | Humans: multiple | ||||
| Immunoglobulins | [169] | In vitro, mice & humans | ||||
| Lysozyme | [147] | Humans: multiple | ||||
| Serum albumin | [166] | Humans: multiple | ||||
| Palmitic and oleic acid | Heterogenous mixture of proteins, lipids, fatty acids and cholesterol | General growth and development | [170] | Humans: multiple | Richer in colostrum compared to mature milk and richer in evening compared to morning feedings | |
| Milk fat globule membranes: MFGM | Cognition, neurodevelopment, boost immune system against infections | [165,171,172] | In vitro, mice and humans | |||
| Lactose | Carbohydrates | Growth and development | [147] | Humans: multiple | More abundant in mature milk and when milk is expressed more frequently | |
| Micronutrient | Vitamins A and E | Vitamins | Growth and development | [147,151] | Humans: multiple, mice and rats | Higher in colostrum compared to mature milk |
| B complex vitamins | Micronutrients | Epigenetic potential, neurotransmitter synthesis, neurodevelopment, protection from neural tube defect | [15] | In vitro, mice and humans | B1 and B6 higher in mature milk compared to colostrum | |
| Iron, copper and zinc | Metals | Neurodevelopment, haematopoiesis | [147,151] | In vitro, mice and humans | Higher in colostrum compared to mature milk |
| Breastmilk Component | Subcategory of Molecule | Effect on | References | Population | Stage of Lactation |
|---|---|---|---|---|---|
| Epidermal growth factor (EGF) | Growth factors | Intestinal maturation, immune system imprinting during weaning | [164] | Mice | Present as long as the lactation lasts |
| Enterocyte stimulation for nutrient absorption | [173] | Pigs, human | |||
| Tight junction and cell death regulation in response to gut inflammation | [174] | Rats | |||
| Brain-derived neurotrophic factor (BDNF) | Normal growth, development and function of neurons in the CNS* and PNS* | [175] | Rats | Present in breast milk up to 90 days postnatally | |
| Increase intestinal motility by stimulation of the ENS* | [176] | In vitro and rats (ex vivo) | |||
| Glial-derived neurotrophic factor (GDNF) | Normal growth, development and function of glial cells in the CNS* and PNS*, supports neuronal health and development | [175] | Mice and rats | ||
| Erythropoietin (EPO) | Hormones | Protective effect on intestinal tight junction, prevent anaemia and reduces the risk of necrotizing enterocolitis | [177] | In vitro | |
| Adiponectin | Regulates metabolism and suppresses inflammation | [178,179] | Humans: Hispanic, mice and humans, humans: Hispanic | ||
| Regulates body weight later in life | [178,179] | Mice and humans, humans: Hispanic | |||
| Ghrelin and leptin | Control appetite, body composition and metabolism | [180,181] | Humans: multiple, Humans: Caucasians, humans: multiple | ||
| Prolactin | Stimulating milk production | [151] | Humans: multiple, mice and rats | ||
| Oxytocin | Production stimulated in PVN* by skin to skin contact with the mother | [151,182] | Humans: multiple | Low levels in breast milk | |
| Sociability | [183] | Mice and Humans: multiple | |||
| Breast milk microbiota | Microbiota | Modulate the gut-brain axis, boost gut barrier function, improve the development of intestinal diseases, are able to rescue behavioural deficits, as well as anxiety-like and depressive-like behaviour, in preclinical models, regulate cytokine and tryptophan levels in mice, shape neurodevelopment, promote synaptic formation and microglial action | [141,142,143] | Rats, mice, humans: multiple, mice | Through the course of lactation |
| Human-milk oligosaccharides (HMOs) (lacto-N-tetraoze, 2-Fucusyllactose) | Carbohydrates + prebiotics (concentration varies depending on stage of lactation) | Modulate the microbiota-gut-brain axis | [151,184,185,186,187] | In vitro, mice, rats and humans: multiple | Through the course of lactation |
| Protect from infection in the gut by reducing colonization of pathogens and promoting the viability and diversity of commensals | [184] | Mice, rats and humans: multiple | |||
| Improve cognitive development | [187] | Humans: Hispanic | |||
| Inducing maturity of epithelial cells and improve gut barrier function | [151,184,185,186,187] | In vitro, mice, rats and humans: multiple | |||
| Macrophages | Cells | Protection against infection, T-cell activation | [169] | Humans: Indo-Aryan and In vitro, mice | More abundant in colostrum than mature milk |
| Stem cells | Regeneration and repair | [188] | Humans: Caucasian, Indo-Aryan |
| Category of Molecule | Formula Component | Effect on | Associated with | References | Population |
|---|---|---|---|---|---|
| Protein | Whey protein and casein (cow-based formula) | General growth and development | Weight gain in infancy and higher adiposity later in life | [237] | Humans: Caucasian |
| Allergies in milk protein | [60] | Humans: multiple, mice and rats | |||
| Higher secretion of insulin due to stimulation of β-pancreatic cells by amino-acids | [237] | Humans: Caucasian | |||
| Fat | Mixed vegetable oils (DHA & ARA) | Adipocyte stimulation and fat storage | High consumption of ω-6 fatty acids might induce adiposity in early life | [186,238] | Humans: multiple, mice and rats |
| MFGM (cow-based formula) | Fat storage and general metabolism | Decreased fat accumulation, concentrations of leptin, glucose and lipids in plasma | [239] | Mice | |
| Cognitive development | Improves cognition of formula-fed infants | [171] | Humans: Caucasians | ||
| Carbohydrates | Lactose | General growth and development | Low gastrointestinal tolerance | [240] | Humans: multiple |
| Category of Molecule | Formula Component | Effect on | Associated with | References | Population |
|---|---|---|---|---|---|
| HMOs | Short-chain galacto-oligosaccharides (scGOS) and long-chain fructo-oligosaccharides (lcFOS) | Highly metabolized by Bifidobacteria, contrary to the HMOs present in human milk that are only partly metabolized by Bifidobacteria | Weight gain but had no effect on height or head circumference, significantly altered microbiota composition in infants | [242] | Humans: multiple |
| 2-fucosyllactose (2’FL) and Lacto-N-neotetraoze (LNnT) | Similar composition of faecal microbiota between formula-fed and breastfed infants at 3 months of age | [243] | Humans: multiple, mice and In vitro | ||
| Synbiotics | GOS and FOS together with strains of Bifidobacteria and Lactobacilli | No effect on child development | [250,251,252] | Humans: Asian, Caucasian |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 423. https://doi.org/10.3390/nu13020423
Ratsika A, Codagnone MC, O’Mahony S, Stanton C, Cryan JF. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients. 2021; 13(2):423. https://doi.org/10.3390/nu13020423
Chicago/Turabian StyleRatsika, Anna, Martin C. Codagnone, Siobhain O’Mahony, Catherine Stanton, and John F. Cryan. 2021. "Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis" Nutrients 13, no. 2: 423. https://doi.org/10.3390/nu13020423
APA StyleRatsika, A., Codagnone, M. C., O’Mahony, S., Stanton, C., & Cryan, J. F. (2021). Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients, 13(2), 423. https://doi.org/10.3390/nu13020423