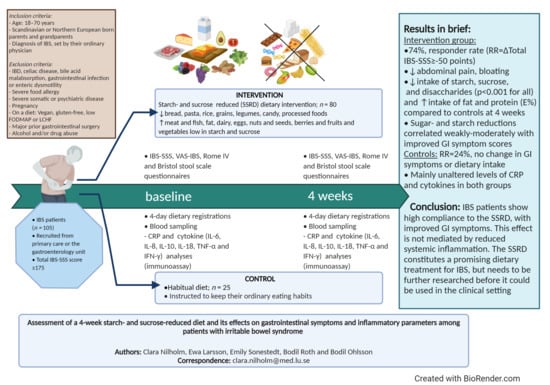
Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Dietary Advice
2.3. Questionnaires
2.3.1. Study Questionnaire
2.3.2. Food Diary Registrations
2.3.3. Rome IV Questionnaire
2.3.4. The Irritable Bowel Syndrome-Symptom Severity Score Questionnaire
2.3.5. The Visual Analog Scale for Irritable Bowel Syndrome Questionnaire
2.3.6. Bristol Stool Form Scale
2.4. CRP and Cytokine Analyses
2.5. Statistical Analyses
2.5.1. Study Group Size
2.5.2. Non-Parametric Statistical Analyses
2.5.3. Parametric Statistical Analyses
2.5.4. Effect Size Calculations
3. Results
3.1. Participant Characteristics
3.2. Gastrointestinal Symptoms and IBS and Non-IBS FGID Subgroups at Baseline
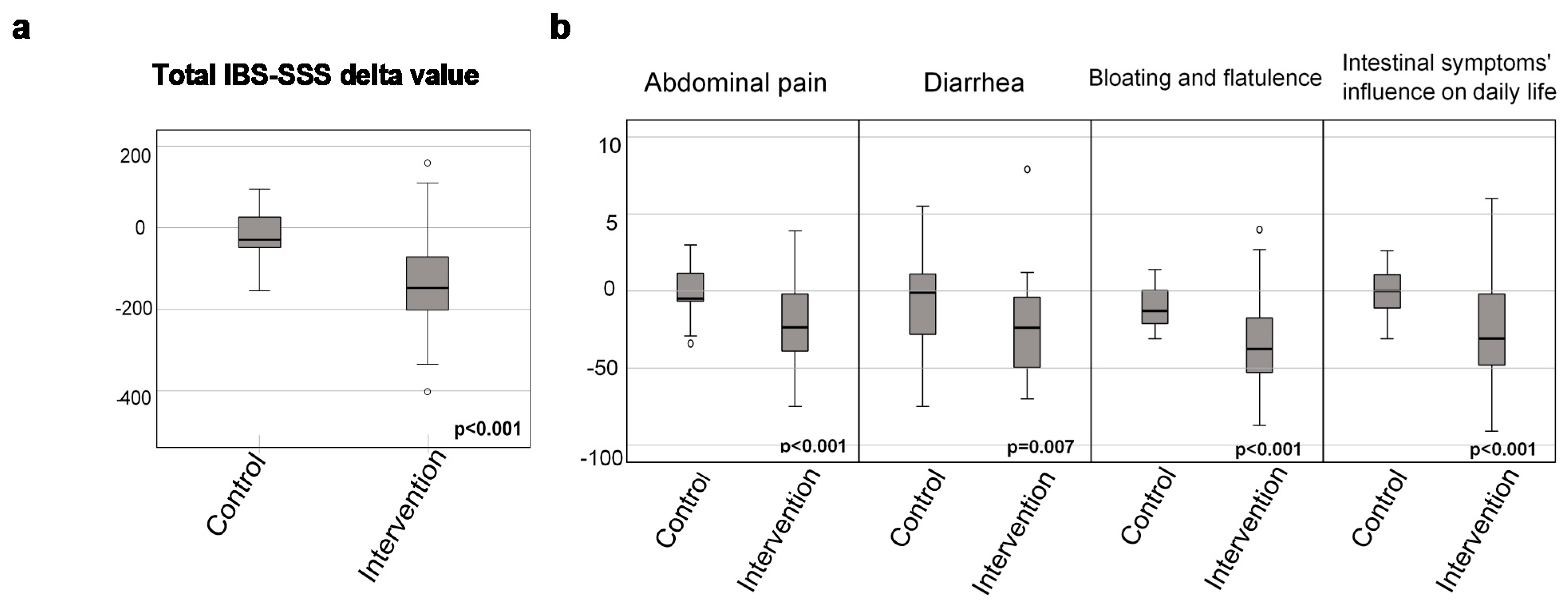
3.3. Gastrointestinal Symptoms after the 4-Week Dietary Intervention
3.4. Dietary Intake
3.4.1. Evaluation of Compliance
3.4.2. Dietary Intake in Non-IBS Controls
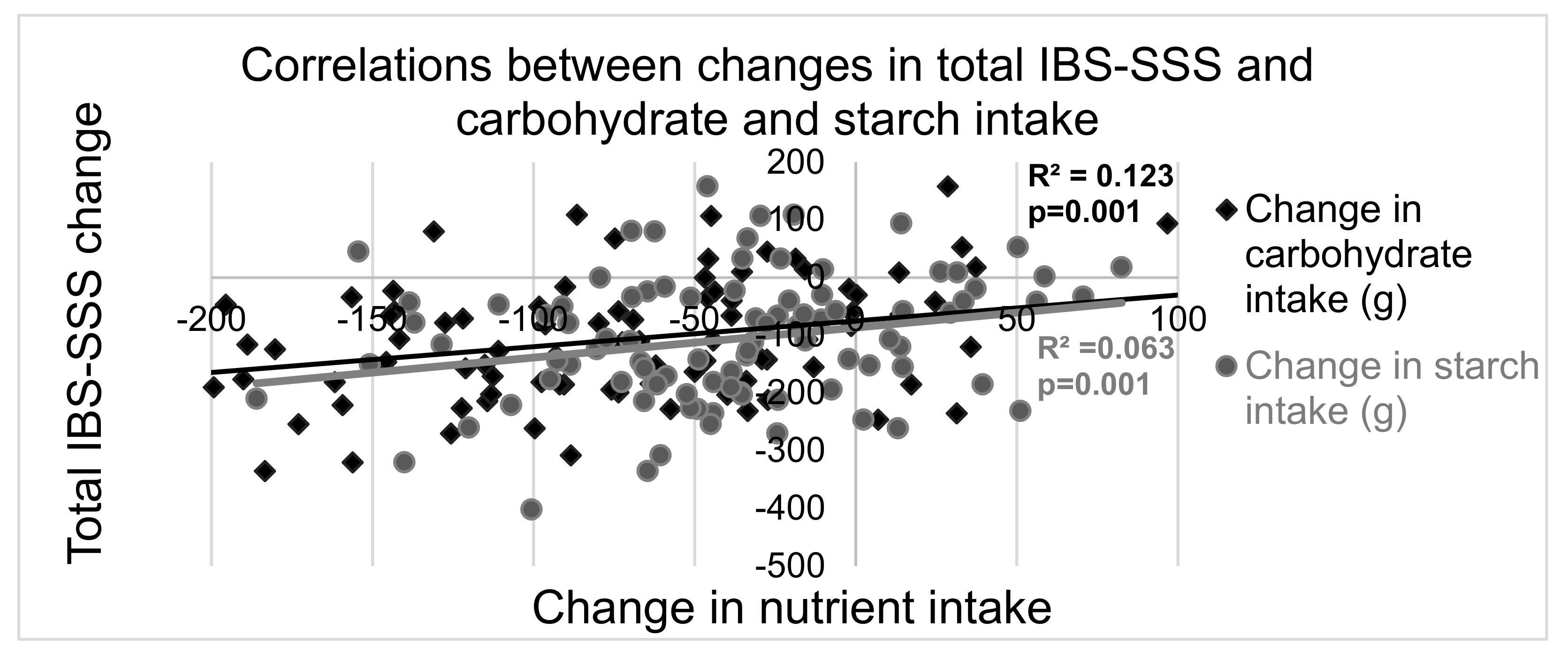
3.5. Correlations between Changes in Gastrointestinal Symptoms and Dietary Intakes
3.6. Plasma Concentrations of CRP and Cytokines
3.7. Subgroup Analysis of GI Symptoms
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Clinical Trials Registration
References
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e3. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164–174. [Google Scholar]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Dietet. 2016, 29, 549–575. [Google Scholar] [CrossRef]
- Algera, J.; Colomier, E.; Simrén, M. The dietary management of patients with irritable bowel syndrome: A narrative review of the existing and emerging evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef]
- Burke, M. Carbohydrate intolerance and disaccharidase measurement—A mini-review. Clin. Biochem. Rev. 2019, 40, 167–174. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Zheng, T.; Bonfiglio, F.; Bujanda, L.; Dlugosz, A.; Lindberg, G.; Schmidt, P.T.; Karling, P.; Ohlsson, B.; Simren, M.; et al. Increased prevalence of rare sucrase-isomaltase pathogenic variants in irritable bowel syndrome patients. Clin. Gastroenterol. Hepatol. 2018, 16, 1673–1676. [Google Scholar] [CrossRef]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.-M.; Von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase–isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef]
- Treem, W.R. Congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 1–14. [Google Scholar] [CrossRef]
- Mack, A.; Bobardt, J.; Haß, A.; Nichols, K.; Schmid, R.; Stein-Thoeringer, C. Changes in gut microbial metagenomic pathways associated with clinical outcomes after the elimination of malabsorbed sugars in an IBS cohort. Gut Microbes 2019, 11, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Calmet, F.H.; Garrido, J. Sucrase-isomaltase deficiency as a potential masquerader in irritable bowel syndrome. Digest. Dis. Sci. 2020, 65, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J. Effect of dietary sugar intake on biomarkers of subclinical inflammation: A systematic review and meta-analysis of intervention studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Ramne, S.; Drake, I.; Ericson, U.; Nilsson, J.; Orho-Melander, M.; Engström, G.; Sonestedt, E. Identification of inflammatory and disease-associated plasma proteins that associate with intake of added sugar and sugar-sweetened beverages and their role in type 2 diabetes risk. Nutrients 2020, 12, 3129. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jö Nsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity commissioned by the ILSI Europe Metabolic Syndrome and Diabetes Task Force. Br. J. Nutr. 2011, 106 (Suppl. 3), S1–S78. [Google Scholar]
- Nilholm, C.; Larsson, E.; Roth, B.; Gustafsson, R.J.; Ohlsson, B. Irregular dietary habits with a high intake of cereals and sweets are associated with more severe gastrointestinal symptoms in IBS patients. Nutrients 2019, 11, 1279. [Google Scholar] [CrossRef]
- Ohlsson, B.; Orho-Melander, M.; Nilsson, P.M. Higher levels of serum zonulin may rather be associated with increased risk of obesity and hyperlipidemia, than with gastrointestinal symptoms or disease manifestations. Int. J. Mol. Sci. 2017, 18, 582. [Google Scholar] [CrossRef]
- Brunkwall, L.; Jönsson, D.; Ericson, U.; Hellstrand, S.; Kennbäck, C.; Östling, G.; Jujic, A.; Melander, O.; Engström, G.; Nilsson, J.; et al. The Malmö Offspring Study (MOS): Design, methods and first results. Eur. J. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Choosing Your Foods—A Basic Dietary Guide for People with CSID. 2020. Available online: https://www.sucroseintolerance.com/choosing-your-foods/ (accessed on 21 September 2020).
- The National Food Agency S. The AIVO Diet Computer Program from the National Food Agency. Available online: https://www.mashie.com/sv/loesningar/vaara-produkter/aivo/ (accessed on 30 November 2020).
- Nordic Council of Ministers. Nordic Nutrition Recommendations. 2012. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/broschyrer-foldrar/nordiska-naringsrekommendationer-2012-svenska.pdf (accessed on 23 October 2020).
- Palsson, O.S.; Whitehead, W.E.; van Tilburg, M.A. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology 2016. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Persson, J.; Sjölund, K.; Ohlsson, B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol. Nurs. 2013, 36, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Meso Scale Diagnostics. Mesoscale Discovery® V-Plex Plus Proinflammatory Panel 1 Human Kit. Available online: https://www.mesoscale.com/en/products/v-plex-plus-proinflammatory-panel-1-human-kit-k15049g/ (accessed on 26 April 2020).
- Meso Scale Diagnostics. Mesoscale Discovery® U-PLEX Human IL-18 Assay. Available online: https://www.mesoscale.com/en/products/u-plex-human-il-18-assay-k151vjk/ (accessed on 26 April 2020).
- Medical Service, Lab Medicine Skåne. Analysportalen. Available online: http://analysportalen-labmedicin.skane.se/pics//Labmedicin/Verksamhetsomr%C3%A5den/Klinisk%20kemi/Analyser/Skane/P-CRP%20p%C3%A5%20Cobas%20(NPU19748).pdf (accessed on 26 April 2020).
- Darwiche, G.; Höglund, P.; Roth, B.; Larsson, E.; Sjöberg, T.; Wohlfart, B.; Steen, S.; Ohlsson, B. An Okinawan-based Nordic diet improves anthropometry, metabolic control, and health-related quality of life in Scandinavian patients with type 2 diabetes: A pilot trial. Food Nutr. Res. 2016, 60, 32594. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, C.; Roth, B.; Höglund, P.; Blennow, K.; Englund, E.M.; Hansson, O.; Zetterberg, H.; Ohlsson, B. Dietary intervention with an Okinawan-based Nordic diet in type 2 diabetes renders decreased interleukin-18 concentrations and increased neurofilament light concentrations in plasma. Nutr. Res. 2018, 60, 13–25. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 21, 19–25. [Google Scholar]
- Macbeth, G.; Razumiejczyk, E.; Ledesma, R.D. Cliff´s delta calculator: A non-parametric effect size program for two groups of observations. Univ. Psychol. 2010, 10, 545–555. [Google Scholar] [CrossRef]
- Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Hercberg, S.; Benamouzig, R.; Julia, C. Western dietary pattern is associated with irritable bowel syndrome in the French NutriNet Cohort. Nutrients 2017, 9, 986. [Google Scholar] [CrossRef]
- Austin, G.L.; Dalton, C.B.; Hu, Y. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2009, 7, 706–708.e1. [Google Scholar] [CrossRef]
- Holmes, R. Carbohydrate digestion and absorption. J. Clin. Pathol. Suppl. R. Coll. Pathol. 1971, 5, 10–13. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Takakura, W.; Pimentel, M. Small intestinal bacterial overgrowth and irritable bowel syndrome—An update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small intestinal bacterial overgrowth and irritable bowel syndrome: A bridge between functional organic dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Verdu, E.F. Non-celiac gluten or wheat sensitivity: It’s complicated! Neurogastroenterol. Motil. 2018, 30, 1. [Google Scholar] [CrossRef]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and wheat-sensitive Irritable Bowel Syndrome (IBS): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020.e4. [Google Scholar] [CrossRef]
- Polster, A.V.; Palsson, O.S.; Törnblom, H.; Öhman, L.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. Subgroups of IBS patients are characterized by specific, reproducible profiles of GI and non-GI symptoms and report differences in healthcare utilization: A population-based study. Neurogastroenterol. Motil. 2019, 31, e13483. [Google Scholar] [CrossRef]
- Martin-Viñas, J.J.; Quigley, E.M. Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. J. Dig. Dis. 2016, 17, 572–581. [Google Scholar] [CrossRef]
- Bashashati, M.; Rezaei, N.; Shafieyoun, A.; McKernan, D.P.; Chang, L.; Öhman, L.; Quigley, E.M.; Schmulson, M.; Sharkey, K.A.; Simrén, M. Cytokine imbalance in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2014, 26, 1036–1048. [Google Scholar] [CrossRef]
| Intervention n = 80 * (60 Women) | Control n = 25 ** (22 Women) | Intervention | Control | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | p | Median of Differences (IQR) | Median of Differences (IQR) | p | |
| Age (years) | 48 (37–57) | 35 (29–50) | 0.028 | |||
| Disease duration (years) | 19 (9.5–29) | 12 (10–30) | 0.53 | |||
| Weight (kg) | −2.1 (−3.0–(−1.0) | 0 (−0.5–1.1) | <0.001 | |||
| Baseline | 72 (64–85) | 68 (57–75) | 0.035 | |||
| 4 weeks | 71 (64–82) | 68 (61–77) | 0.301 | |||
| IBS-SSS total score | −148 (−203–(−72)) | −30 (−54–33) | <0.001 | |||
| Baseline | 306 (250–356) | 310 (247–351) | 0.82 | |||
| 4 weeks | 156 (88–250) | 300 (233–331) | <0.001 | |||
| Abdominal pain Ref value: 5 (1–13) | −24 (−40–(−2.0)) | −5.0 (−7.0–12) | <0.001 | |||
| Baseline | 52 (37–65) | 49 (27–63) | 0.44 | |||
| 4 weeks | 24 (5.8–43) | 50 (32–63) | <0.001 | |||
| Diarrhea Ref value: 3 (0–10) | −24 (−49–(−1.8)) | −1.0 (−34–12) | 0.007 | |||
| Baseline | 57 (18–76) | 47 (5.0–71) | 0.32 | |||
| 4 weeks | 14 (1.0–33) | 24 (1.0–49) | 0.27 | |||
| Constipation Ref value: 6 (2–16) | −13 (−43–0) | −12 (−40–6.0) | 0.41 | |||
| Baseline | 47 (1.0–73) | 54 (30–69) | 0.76 | |||
| 4 weeks | 18 (0.0–36) | 28 (1.0–68) | 0.15 | |||
| Bloating and flatulence Ref value: 10 (2–23) | −39 (−53–(−18)) | −13.0 (−22–1.0) | <0.001 | |||
| Baseline | 77 (59–85) | 78 (68–89) | 0.38 | |||
| 4 weeks | 28 (11–54) | 69 (56–80) | <0.001 | |||
| Vomiting and nausea Ref value: 2 (0–4) | −3.5 (−14–1.0) | −4.0 (−16–0) | 0.95 | |||
| Baseline | 12 (1.0–37) | 29 (5.5–51) | 0.13 | |||
| 4 weeks | 3.0 (0.0–24) | 12 (2.0–56) | 0.04 | |||
| Psychological well-being Ref value: 5 (2–15) | −8.0 (−23–2.8) | −1.0 (−14–8) | 0.08 | |||
| Baseline | 50 (24–69) | 47 (24–71) | 0.95 | |||
| 4 weeks | 36 (13–53) | 48 (32–60) | 0.09 | |||
| Intestinal symptoms’ influence on daily life Ref value: 2 (0–14) | −31 (−48–(−2.0)) | 0 (−12–12) | <0.001 | |||
| Baseline | 72 (52–86) | 68 (53–79) | 0.54 | |||
| 4 weeks | 35 (20–63) | 65 (51–82) | <0.001 | |||
| BSFS Average daily stool frequency | −0.1 (−0.5–0.3) | −0.01 (−0.4–0.3) | 0.56 | |||
| 10 day-run-in | 1.9 (1.2–2.7) | 2.0 (1.6–2.4) | 0.97 | |||
| Intervention | 1.6 (1.1–2.6) | 1.9 (1.2–2.5) | 0.66 |
| Intervention n = 80 * | Control n = 25 ** | Intervention | Control | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | p | Median of Differences (IQR) | Median of Differences (IQR) | p | |
| Energy (kcal) | −137 (−662–212) | −105 (−389–222) | 0.39 | |||
| Baseline | 1660 (1393–2107) | 1387 (1202–2027) | 0.19 | |||
| 4 weeks | 1472 (1139–1969) | 1640 (1216–2169) | 0.40 | |||
| Carbohydrates (g) | −91 (−142–(−40)) | −16 (−73–27) | 0.001 | |||
| Baseline | 185 (144–223) | 177 (112–208) | 0.40 | |||
| 4 weeks | 88 (66–128) | 182 (89–224) | <0.001 | |||
| Carbohydrates (E%) | −17 (−30–(−5.5)) | −6.7 (−18–6.8) | 0.007 | |||
| Baseline | 43 (38–49) | 43 (37–52) | 0.76 | |||
| 4 weeks | 25 (18–36) | 42 (32–49) | <0.001 | |||
| Protein (g) | 8.6 (−16–33) | −0.1 (−11–20) | 0.64 | |||
| Baseline | 72 (55–83) | 59 (46–71) | 0.046 | |||
| 4 weeks | 82 (58–99) | 65 (53–82) | 0.13 | |||
| Protein (E%) | 4.7 (0.4–9.8) | 1.2 (−1.3–4.7) | 0.068 | |||
| Baseline | 16 (14–19) | 16 (12–19) | 0.35 | |||
| 4 weeks | 21 (19–26) | 17 (13–20) | 0.006 | |||
| Fat (g) | 13 (−21–47) | 0.3 (−14–39) | 0.55 | |||
| Baseline | 65 (45–94) | 61 (46–72) | 0.39 | |||
| 4 weeks | 72 (56–104) | 69 (46–97) | 0.26 | |||
| Fat (E%) | 13 (2.1–21) | 7.6 (−7.7–13) | 0.018 | |||
| Baseline | 36 (29–43) | 34 (27–43) | 0.83 | |||
| 4 weeks | 47 (39–55) | 38 (31–45) | 0.002 | |||
| Fiber (g) | −0.6 (−11–4.9) | 0.7 (−4.4–3.1) | 0.56 | |||
| Baseline | 18 (13–26) | 16 (12–22) | 0.32 | |||
| 4 weeks | 18 (12–23) | 15 (11–22) | 0.42 | |||
| Fiber (E%) | 0.2 (−0.7–0.7) | 0 (−0.7–0.3) | 0.45 | |||
| Baseline | 2.1 (1.6–2.6) | 1.9 (1.4–2.8) | 0.89 | |||
| 4 weeks | 2.2 (1.5–2.9) | 1.8 (1.4–2.2) | 0.052 |
| Intervention n = 80 * | Control n = 25 ** | Intervention | Control | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | p | Median of Differences (IQR) | Median of Differences (IQR) | p | |
| Sucrose (g) | −14 (−32–(−3.5)) | −3.8 (−22–4.4) | 0.029 | |||
| Baseline | 23 (13–38) | 21 (13–43) | 0.82 | |||
| 4 weeks | 5.4 (2.4–13) | 20 (4.5–36) | 0.001 | |||
| Sucrose (E%) | −3.8 (−5.8–(−0.8)) | −0.9 (−3.8–2.8) | 0.008 | |||
| Baseline | 5.5 (4.0–8.8) | 6.2 (3.8–11) | 0.65 | |||
| 4 weeks | 1.4 (0.8–3.1) | 6.3 (2.0–9.0) | <0.001 | |||
| Monosaccharides (g) | −5.6 (−18–12) | −6.8 (−18–9) | 0.77 | |||
| Baseline | 21 (13–31) | 22 (12–31) | 0.88 | |||
| 4 weeks | 21 (9.7–29) | 17 (6.7–31) | 0.80 | |||
| Monosaccharides (E%) | −0.8 (−3.2–2.7) | −1.5 (−4.5–2.7) | 0.32 | |||
| Baseline | 5.0 (3.3–7.5) | 5.1 (3.9–8.2) | 0.58 | |||
| 4 weeks | 4.7 (2.5–8.2) | 4.4 (2.2–7.6) | 0.46 | |||
| Disaccharides (g) | −20 (−35–(−4.6)) | −10 (−18–7.0) | 0.02 | |||
| Baseline | 33 (22–48) | 32 (20–51) | 0.61 | |||
| 4 weeks | 16 (7.8–27) | 28 (18–55) | 0.002 | |||
| Disaccharides (E%) | −4.4 (−6.1–(−0.9)) | −1.8 (−4.8–3.4) | 0.042 | |||
| Baseline | 8.1 (6.3–11) | 7.3 (5.9–13) | 0.86 | |||
| 4 weeks | 4.2 (2.4–7.0) | 9.1 (3.9–12) | 0.001 | |||
| Total sugar (g) | −26 (−54–(−1.0)) | −15 (−35–17) | 0.13 | |||
| Baseline | 63 (41–89) | 58 (42–74) | 0.63 | |||
| 4 weeks | 41 (28–58) | 58 (21–86) | 0.10 | |||
| Total sugar (E%) | −4.9 (−9.3–1.4) | −3.0 (−6.5–4.5) | 0.34 | |||
| Baseline | 14 (11–19) | 14 (11–21) | 0.84 | |||
| 4 weeks | 11 (7.5–16) | 16 (9.4–20) | 0.053 | |||
| Starch (g) | −50 (−78–(−23)) | −8.3 (−34–34) | <0.001 | |||
| Baseline | 77 (49–116) | 71 (43–91) | 0.44 | |||
| 4 weeks | 22 (2.6–48) | 82 (37–101) | <0.001 | |||
| Starch (E%) | −12 (−18–(−0.6)) | −1.6 (−9.4–13) | 0.001 | |||
| Baseline | 19 (12–25) | 17 (14–23) | 0.83 | |||
| 4 weeks | 6.1 (0.7–15) | 18 (13–26) | <0.001 |
| Intervention n = 80 | Controls n = 25 | |||||
|---|---|---|---|---|---|---|
| Median | IQR | p | Median | IQR | p | |
| CRP Ref: <3.0 mg/L | ||||||
| Baseline | 0.7 | 0.6–2.2 | 0.7 | 0.6–1.0 | ||
| 4 weeks | 0.8 | 0.6–2.0 | 0.07 | 0.8 | 0.6–1.0 | 0.62 |
| TNF-α Ref: 0.82 (0.63–1.05) | ||||||
| Baseline | 2.5 | 2.1–3.0 | 2.1 | 1.9–2.8 | ||
| 4 weeks | 2.5 | 2.1–3.3 | 0.40 | 2.5 | 2.2–2.9 | 0.003 |
| IFN-γ Ref: 5.54 (3.79–8.83) | ||||||
| Baseline | 2.7 | 2.1–4.6 | 2.4 | 1.5–3.8 | ||
| 4 weeks | 2.8 | 1.9–4.2 | 0.89 | 3.5 | 2.1–4.5 | 0.21 |
| IL-6 Ref: 0.70 (0.49–1.06) | ||||||
| Baseline | 0.6 | 0.4–0.9 | 0.5 | 0.4–0.8 | ||
| 4 weeks | 0.6 | 0.4–0.9 | 0.68 | 0.5 | 0.4–0.8 | 0.68 |
| IL-8 Ref: 9.79 (7.99–13.21) | ||||||
| Baseline | 12 | 9.2–14 | 11 | 8.1–14 | ||
| 4 weeks | 12 | 9.4–16 | 0.017 | 10 | 8.4–13 | 0.29 |
| IL-10 Ref: 0.23 (0.17–0.29) | ||||||
| Baseline | 0.2 | 0.2–0.3 | 0.2 | 0.1–0.4 | ||
| 4 weeks | 0.3 | 0.2–0.3 | 0.42 | 0.2 | 0.1–0.3 | 0.07 |
| IL-18 Ref: 946 (585–1640) * | n0 = 75; n4 = 73 | n = 22 | ||||
| Baseline | 986 | 781–1308 | 978 | 805–1189 | ||
| 4 weeks | 962 | 741–1313 | 0.34 | 1024 | 816–1101 | 0.57 |
| Δ Mean ± Standard Deviation | n | ANOVA with Bonferroni for IBS Subgroup/Non-IBS FGID (p) | |||
|---|---|---|---|---|---|
| IBS-D | IBS-M | Non-IBS FGID | |||
| Total IBS-SSS | |||||
| IBS-C | −87 ± 110 | 13 | 1 | 0.054 | 1 |
| IBS-D | −123 ± 103 | 21 | 0.39 | 1 | |
| IBS-M | −177 ± 101 | 27 | 1 | ||
| non-IBS FGID | −139 ± 53 | 10 | |||
| Diarrhea | |||||
| IBS-C | 24 ± 6.6 | 13 | 0.001 | <0.001 | 0.28 |
| IBS-D | −31 ± 25 | 21 | 1 | 0.88 | |
| IBS-M | −39 ± 21 | 27 | 0.09 | ||
| non-IBS FGID | −18 ± 32 | 11 | |||
| Constipation | |||||
| IBS-C | −30 ± 27 | 13 | <0.001 | 1 | 0.75 |
| IBS-D | 5.6 ± 16 | 21 | <0.001 | 0.09 | |
| IBS-M | −39 ± 24 | 27 | 0.033 | ||
| non-IBS FGID | −16 ± 24 | 11 | |||
| Bloating and flatulence | |||||
| IBS-C | −30 ± 29 | 13 | 1 | 0.12 | 1 |
| IBS-D | −29 ± 28 | 21 | 0.022 | 1 | |
| IBS-M | −51 ± 22 | 27 | 0.009 | ||
| non-IBS FGID | −20 ± 24 | 10 | |||
| Intestinal symptoms’ influence on daily life | |||||
| IBS-C | −8.2 ± 33 | 13 | 0.49 | 0.032 | 1 |
| IBS-D | −27 ± 29 | 21 | 1 | 1 | |
| IBS-M | −37 ± 26 | 27 | 0.67 | ||
| non-IBS FGID | −19 ± 38 | 10 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilholm, C.; Larsson, E.; Sonestedt, E.; Roth, B.; Ohlsson, B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 416. https://doi.org/10.3390/nu13020416
Nilholm C, Larsson E, Sonestedt E, Roth B, Ohlsson B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients. 2021; 13(2):416. https://doi.org/10.3390/nu13020416
Chicago/Turabian StyleNilholm, Clara, Ewa Larsson, Emily Sonestedt, Bodil Roth, and Bodil Ohlsson. 2021. "Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome" Nutrients 13, no. 2: 416. https://doi.org/10.3390/nu13020416
APA StyleNilholm, C., Larsson, E., Sonestedt, E., Roth, B., & Ohlsson, B. (2021). Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients, 13(2), 416. https://doi.org/10.3390/nu13020416